Abstract
Predatory reef fishes regularly visit mutualistic cleaner fish (Labroides dimidiatus) to get their ectoparasites removed but show no interest in eating them. The concept of compensated trait loss posits that characters can be lost if a mutualistic relationship reduces the need for a given trait. Thus, selective pressures on escape performance might have relaxed in L. dimidiatus due to its privileged relationship with predators. However, the cost of failing to escape a predatory strike is extreme even if predation events on cleaners are exceptionally rare. Additionally, cleaners must escape from non-predatory clients that regularly punish them for eating mucus instead of parasites. Therefore, strong escape capabilities might instead be maintained in cleaner fish because they must be able to flee when in close proximity to predators or dissatisfied clients. We compared the fast-start escape performance of L. dimidiatus with that of five closely related wrasse species and found that the mutualistic relationship that cleaners entertain with predators has not led to reduced escape performance. Instead, conflicts in cleaning interactions appear to have maintained selective pressures on this trait, suggesting that compensated trait loss might only evolve in cases of high interdependence between mutualistic partners that are not tempted to cheat.
Keywords: cooperation, coral reef fish, escape performance, fast-start, mutualism, predation
1. Introduction
Evolutionary theory predicts that anti-predator traits should decay and resources be reallocated elsewhere if predation pressure relaxes on a given species [1–3]. A classic example involves the loss of anti-predator behaviour in species that have colonized isolated islands devoid of their natural predators [2]. Recent studies also suggest that trait loss can be driven by non-predatory interspecific interactions [4]. For example, mutualistic partners can perform the same function as a lost trait, a phenomenon termed compensated trait loss [4]. Several studies have linked compensated trait loss to mutualistic partners that provide protection against predators: for example, fungal endophytes providing grass hosts with chemical defences against herbivores [5] and ants protecting acacia trees from herbivores in exchange for nesting sites [6]. Similar to defences against predators, defences against pathogens or competitors can also be reduced due to protection by mutualistic partners, such as ants that protect fungus [7] and damselfish that garden algae [8]. Here, we ask whether reduced predation pressure on fish that provide cleaning services to predators can lead to decreased escape performance via compensated trait loss.
Labroides dimidiatus is a small coral reef fish (Labridae) referred to as an ‘obligate cleaner’ because it feeds almost exclusively on the ectoparasites of other reef fishes (hereafter ‘clients’). Every day, dozens of client species visit the territory of a single cleaner fish and get their ectoparasites removed [9], including both predatory and non-predatory species [10,11]. While this is considered a mutualistic interaction, cleaners prefer to eat client mucus over ectoparasites [12], creating an important conflict of interest: clients have to make cleaners eat against their preference not to be cheated. Non-predatory clients use partner control strategies such as partner switching (i.e. change partners if dissatisfied with the service) and punishment (i.e. chase the cleaner to ram or bite it) [13] to achieve cheating frequencies that are low enough to yield overall net benefits [14–16]. Unlike non-predatory clients, piscivores can cheat cleaners via predation attempts, and it has been proposed that the threat of reciprocity (i.e. reciprocating cheating by trying to eat a cleaner) enforces cooperative behaviour by cleaners [17]. In fact, cleaners never appear to cheat predators and provide high levels of tactile stimulation (i.e. a ‘massage’ with the pelvic fins) [11,18], which reduces cortisol levels in clients [19]. Such unconditional high service quality has been argued to make it self-serving for predators to refrain from eating cleaners [20,21]. Indeed, cleaners readily approach predators and enter their mouth without getting eaten (electronic supplementary material, video S1). To date, only anecdotal evidence of predation on cleaners exists and there are no observations of a predation event during a cleaning interaction despite extensive field observations by numerous researchers [22–24]. Predatory clients have even been documented to reduce predatory activities near cleaning stations [25]. Rather than hiding from predators, cleaners instead advertise their presence to prospective clients via a characteristic oscillating ‘dance’ [10,22]. Finally, L. dimidiatus has evolved some of the most conspicuous colours and patterns in the marine environment [26], which some species of scale-eating fangblennies mimic, apparently decreasing their own predation risk [27]. Therefore, cleaner wrasse appears to experience dramatically reduced predation risks compared with other reef fishes. Contrary to obligate cleaners such as L. dimidiatus, facultative cleaners do not exclusively rely on cleaning for food acquisition, and tend to shift away from cleaning as adults. Cleaning interactions involving facultative cleaners appear to be free of conflict because facultative cleaners do not cheat clients and are not known to interact with predators [28]. Thus, selective pressures on escape performance might be more similar among facultative- and non-cleaners than for obligate cleaners.
Based on the concept of compensated trait loss and known trade-offs in resource allocation between defence and foraging/reproduction, one could predict that a reduction in predation pressure has relaxed selection on escape performance in L. dimidiatus. Cleaners might therefore provide a unique example because: (i) there are few examples of compensated trait loss for vertebrates and none for fish [4]; (ii) the lost (or reduced) trait would be a behavioural response (i.e. escape response) and (iii) the loss is not compensated for in the cleaners' phenotype but rather by the mutualist predators refraining from eating cleaners. However, there are two arguments for why such an effect might be absent or even reversed. First, the relative safety from predation might be annulled by cleaners approaching predators and entering their mouth, making any predation attempt by the client in these contexts a late-stage, high-risk encounter. If sufficiently frequent, such events may select for high escape performance, so cleaners can flee from a predator in close proximity or out of a closing mouth. Second, cleaners regularly have to flee from non-predatory clients that chase them in response to mucus feeding [11,12]. Cleaners must evade these chases to avoid injuries from being rammed or bitten (examples of punishment in electronic supplementary material, video S1). Here again, successful fleeing might rely on high escape performance due to the physical proximity of the punisher.
Given that a cleaner's escape performance relies heavily on quick reactions, we examined the fast-start escape response, the main behaviour used by fishes to escape from a predatory attack. This behaviour consists of a rapid swimming burst (lasting tens of milliseconds) in which a fish bends its body into a characteristic ‘C’ shape and performs a high-energy propulsive stroke [29,30]. In nature, fast-start performance appears to be tightly linked with predation pressure. Namely, predator-induced morphology has been shown to improve fast-start performance in carp [31] and mosquitofish [32]. Conversely, various components of fast-starts tend to decline if other anti-predator strategies are used, such as body armour or schooling [33]. In some species that have protective features, such as the spiny eel [34] or the lionfish [35], researchers have failed to elicit fast-starts. Fast-start escape performance thus appears to respond flexibly to ecological demands both within and across species.
Here, we use a comparative approach to contrast the fast-start performance of L. dimidiatus with that of two non-cleaner and three facultative-cleaner fish species.
By comparing closely related species that differ in their dependency on cleaning for food, we aimed to understand whether selective pressures on fast-start performance in cleaners have (i) relaxed because of reduced predation by mutualist predators or (ii) been maintained or even increased because of the necessity to escape from conflicts. As facultative cleaners generally shift away from cleaning as adults, selective pressures on escape performance in this group might also shift throughout ontogeny. If fast-start responses are critical in the context of cleaning interactions, even when this behaviour occurs at low frequencies, we would expect the performance of obligate and facultative cleaners to diverge more at the adult than at the juvenile stage.
2. Material and methods
(a). Study species
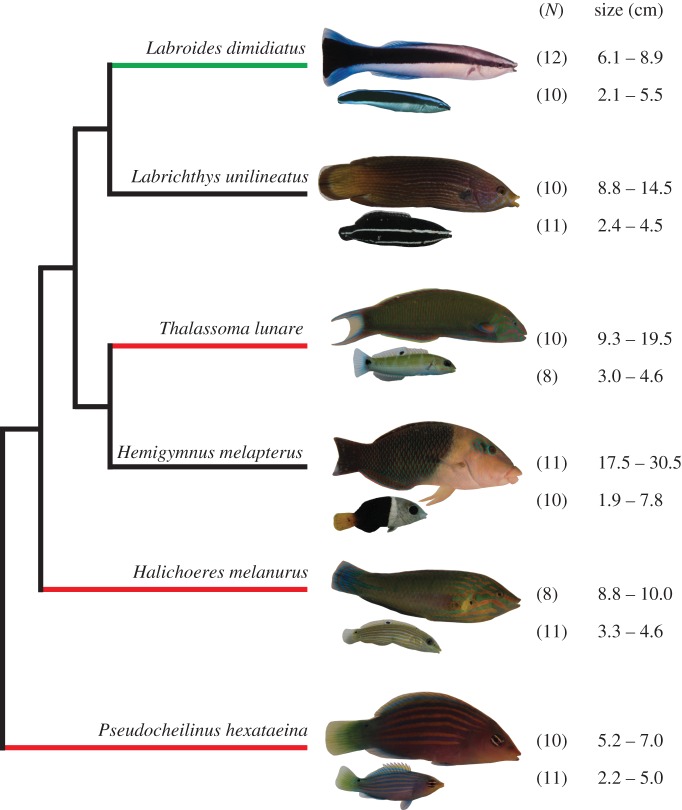
We examined 122 individuals belonging to six species of wrasses (Labridae) that co-occur on the Great Barrier Reef (figure 1). Labroides dimidiatus is an obligate cleaner, i.e. all of its energy input comes from cleaning interactions; three species (Pseudocheilinus hexataenia, Thalassoma lunare and Halichoeres melanurus) are facultative cleaners, i.e. they might occasionally clean, primarily as juveniles; and two species (Labroides unilineatus and Hemygimnus melapterus) are non-cleaners. We chose these species because they are locally abundant, represent a diversity of trophic niches present in the Labridae [38], and have been used in previous studies aimed at identifying specific selective pressures on L. dimidiatus (e.g. [37]). Fish were classified as juveniles or adults based on body coloration [39,40] (figure 1). As P. hexataenia does not exhibit an ontogenetic colour shift, adults were distinguished from juveniles based on size differences. Adults were greater than 5 cm total length (TL), which corresponds to two-thirds of the maximum size for this species [40]. Fish were collected on reefs surrounding Lizard Island, Australia (14°40′ S 145°28′ E), and captured using a barrier net and small hand nets. We used a 10% clove oil solution to momentarily sedate species that enter the reef matrix when chased (P. hexataenia and some juveniles of other species). Fish were transported to the Lizard Island Research Station immediately following capture and housed in individual aquaria with flow-through seawater pumped directly from the reef. We allowed a minimum acclimation time of 24 h prior to experimentation (mean = 22.5; s.d. = 14.9 days). Fish were last fed the day before the experiments so they were all tested in a standardized, post-absorptive state.
Figure 1.
Schematic of the phylogenetic relationships between study species, based on the phylogeny of Cowman & Bellwood [36] (branch lengths are not scaled). The colour of the branch indicates dependency on cleaning: green, obligate cleaner; red, facultative cleaner; black, non-cleaner. The range of body size (TL) of the fish tested in the fast-start experiments is given for each species, for adults and juveniles. The number of individuals tested (N) is indicated in parentheses. Pictures are not to scale. Figure modified from [37].
(b). Fast-start experiments
Juveniles were tested between August 2012–September 2012, and adults, January 2013–February 2013. Daily average water temperatures at Lizard Island varied between 23.5°C and 26°C when the juveniles were tested, and between 26°C and 30.5°C for the adults (source: Australian Institute of Marine Science). The experimental set-up consisted of an acrylic tank (70 × 60 × 35 cm) mounted on a wooden structure, with a flow-through system with water pumped directly from the reef. We filmed the fish from below the tank, through a mirror placed at a 45° angle, at 420 frames s−1 (fps) using a high-speed camera (Casio Exilim EX-FH100, Casio Computer Co., Tokyo, Japan). Escape responses were triggered by releasing a 50 ml cylindrical plastic vial filled with lead weights (165 g) suspended above the tank with an electromagnet. The vial fell inside an opaque PVC tube (10 cm diameter) suspended 1 cm above the water surface, which prevented visual stimulation before contact with the water. The tube was positioned approximately 10 cm from the centre of the arena, so the fish could be startled while in the centre. The water level was kept between 10 and 20 cm (depending on the size of the fish tested) to minimize vertical displacement while allowing full extension of the anal and dorsal fins. Lighting was provided by three 150 W halogen work lights, positioned approximately 75 cm above the sides of the tank, at a 45° angle. A 5 cm scale was affixed to the bottom of the tank for distance measurements (see Video analysis). Prior to an experiment, a focal fish was transferred from its holding tank to the experimental tank; its TL was measured, and it was allowed a minimum of 30 min of acclimation time. To minimize variation in performance due to differences in positioning relative to the stimulus, we tried to stimulate fish when they were at an angle of approximately 90° relative to the stimulus (mean = 93.9°, s.d. = 34.6°) and approximately 10 cm from the stimulus (mean = 11.3 cm, s.d. = 4.3 cm) (see the electronic supplementary material, video S2 for examples). Each fish was tested three times with a minimum time interval of 30 min between trials. If a test fish did not respond or moved and considerably changed its position immediately prior to the stimulation, additional trials were conducted. Each fish experienced on average 3.85 (s.d. = 1.75) trials. Some individuals frequently swam to the centre of the arena, whereas others tended to remain near the edges. In the latter case, we gently moved a PVC pipe along the walls of the arena, encouraging fish to move away from the edges. Following the experiments, one juvenile and one adult of each species were euthanized with an overdose of Aqui-S (100 mg l−1, New Zealand Ltd) to measure their centre of mass (CoM). The position of the CoM relative to the tip of the snout was obtained for each species and age class and used in subsequent video analyses. All other fishes were returned to the reef upon completion of the experiments.
(c). Field observations
Some coral reef fishes spend most of their time in close proximity to the reef, refuging in shelters such as branching corals, holes and overhangs [41], whereas others tend to occupy the water column [42]. Differences in habitat use can affect the ability of prey to detect approaching predators, with likely implications for selection on escape performance. For example, physical structures such as branching corals, rocks or weeds diminish the field of view, making predator detection more likely in the late stages of a predator–prey sequence, when escape is the only remaining option [43]. To examine differences in habitat use among our study species, we conducted field observations in July–September 2014 on SCUBA or snorkel. Eight adults per species were observed for a 15 min period and we noted, every 30 s, whether an individual was in sight or inside the reef matrix (i.e. in the dead reef structure or sheltering in live coral). We also estimated fish TL to the nearest 0.5 cm, and recorded the number of cleaning interactions.
(d). Video analysis
The typical fast-start escape response used by fishes, the C-start, can be categorized into two types. Double-bend C-starts comprise a first and second stage separated by the change in direction of the anterior body midline [29], whereas single-bend C-starts consist of only the first stage. We used the software ImageJ 1.48v [44] and the plugin MTrackJ [45] to extract behavioural and kinematic variables from escape response videos. Two experimenters performed the video analysis. For each trial, we measured a fish's escape latency (the time from the onset of the stimulus to the first head movement of the fish), the duration of stages 1 and 2, and the location of the fish's CoM every 2.4 ms (i.e. every frame) during the escape response. We used these data to compute the following variables: (i) stage 1 turning rate (calculated as the angle between the segment joining the CoM and the tip of the snout at the beginning and end of stage 1 divided by the duration of stage 1); and distance–time variables including (ii) cumulative escape distance (Desc), (iii) maximum velocity (Umax) and (iv) maximum acceleration (Amax) calculated over the mean escape response duration (i.e. stages 1 + 2) across all trials and species (29 ± 14 ms; mean ± s.d.) [46]. We also measured the distance from the snout of the fish to the stimulus and the angle between the snout, the CoM and the stimulus to control for the variation in the position of the fish when startled [47]. Umax and Amax were smoothed using a five-point quadratic polynomial regression [48].
(e). Statistical analysis
We analysed escape response trials only when the angle of the fish's body relative to the stimulus was above 25° or below 155° to reduce potential biases due to the stimulus falling frontally or dorsally. The final data set comprised 271 trials, corresponding to a mean of 2.22 (s.d. = 0.89; min = 1; max = 4) trials per individual. We tested for interspecific differences in five measures of escape performance using linear mixed-effects models (LMM): escape latency (ms), Umax (cm s−1), Amax (m s−2), Desc (cm) and turning rate (° ms−1). In addition to these five standard kinematic variables, we computed the cumulative distance travelled in 34 ms (Desc_stim), which corresponded to the time between the onset of the stimulus to the end of stage 2 (approximately). This metric includes a fish's response latency and therefore provides an ecologically relevant measure of how far a fish can escape because it captures the actual distance covered from the onset of a threat. We controlled for any effect of observer, distance to the stimulus, angle relative to stimulus, trial number and fish identity by including the following terms in the models: response.variable ∼ sin(angle.stim) + dist.stim + trial.number + observer + species + (1|Individual). Distance and angle to the stimulus were centred prior to the analysis. We assessed normality and homoscedasticity of the residuals with qqplots and plots of residuals versus fitted values. Latency was log transformed to comply with model assumptions. Juvenile and adult fishes were tested at different times of the year, when water temperatures differ. Since water temperature is known to influence escape performance [29], each group was analysed separately. We did not perform pairwise comparisons due to the large number of tests required. Rather, we interpret pairwise differences between species by referring to the overlap of the confidence intervals (CI) of the means predicted by the linear models. With sample sizes above 10, p-values are significant (less than 0.05) if the fraction of the CI arm overlapping between two groups is smaller than 0.5 [49].
Body length can have an effect on Desc and Umax [30,50], and researchers often control for size by reporting relative values (i.e. in body lengths and body lengths s−1 [29]). In our analysis, we did not control for body length for three reasons: (i) size and fish species were collinear (see the electronic supplementary material, figure S1), (ii) Desc and Umax are thought to be size-independent when measured in a fixed time interval [51] and (iii) we were interested in absolute (i.e. in terms of centimetres) rather than relative performance (i.e. body lengths). Escape success depends on how fast and how far a fish can move away from a threat, irrespective of its body size.
Nevertheless, we ran two supplementary analyses to investigate whether the differences we observed could be due to size differences only. First, we used a standard way to control for size by dividing Desc, Desc-stim, Umax and Amax by an individual's TL, which provides relative measures of escape performance. Second, we included TL as a covariate in the models to control for body size. The script for these analyses is available online [52] and a summary of the results is included in the electronic supplementary material.
Data from our field observations did not meet the assumptions of an analysis of variance (ANOVA). Therefore, we used the non-parametric equivalent of a one-way ANOVA, the Kruskall–Wallis test, to investigate differences in time spent by the six species inside the reef matrix [53]. All analyses were done in R v. 3.2.2 [54].
3. Results
(a). Adults
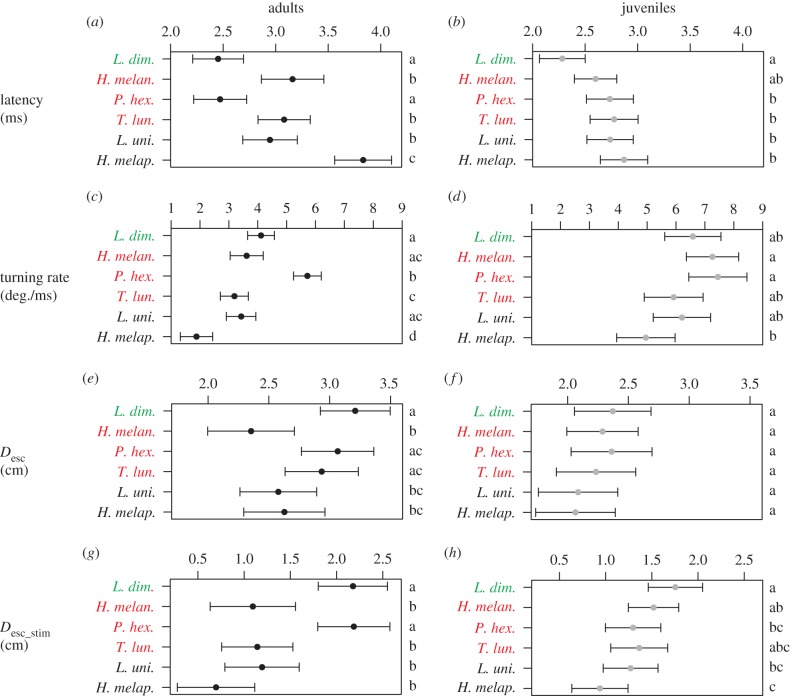
We found significant interspecific differences in escape latency (table 1 and figure 2a), turning rate (table 1 and figure 2c), Desc (table 1 and figure 2e), Dest_stim (table 1 and figure 2g) and Umax (electronic supplementary material, table S1.1 and figure S2.1). The only measure that did not differ among species for adults was Amax (electronic supplementary material, table S1.2 and figure S2.3).
Table 1.
Results from the LMMs with escape latency, turning rate, cumulative distance from the onset of the escape response (Desc) and cumulative distance from the onset of the stimulus (Desc-stim) as response variables, for both adults and juveniles. d.f., degrees of freedom; sum sq., sum of squares.
| response | predictor | adults |
juveniles |
|||||
|---|---|---|---|---|---|---|---|---|
| d.f. | sum sq. | F | p-value | sum sq. | F | p-value | ||
| latency | sin(angle) | 1 | 1 × 10−4 | 0.00 | 0.985 | 0.04 | 0.42 | 0.517 |
| dist. stim. | 1 | 6.24 | 32.2 | 7.45 × 10−8 *** | 4.67 | 47.8 | 8.48 × 10−10 *** | |
| trial | 1 | 0.05 | 0.26 | 0.610 | 0.02 | 0.17 | 0.684 | |
| observer | 1 | 0.19 | 1.00 | 0.322 | 0.12 | 1.18 | 0.282 | |
| species | 5 | 16.9 | 17.5 | 3.42 × 10−10 *** | 2.03 | 4.14 | 3.02 × 10−3 ** | |
| turning rate | sin(angle) | 1 | 1.92 | 1.56 | 0.213 | 5.07 | 2.33 | 0.130 |
| dist. stim. | 1 | 4.63 | 3.76 | 5.43 × 10−2 | 55.5 | 25.5 | 2.49 × 10−6 *** | |
| trial | 1 | 2.34 | 1.90 | 0.170 | 0.45 | 0.21 | 0.651 | |
| observer | 1 | 0.68 | 0.55 | 0.458 | 0.65 | 0.30 | 0.588 | |
| species | 5 | 199 | 32.3 | 2 × 10−16 *** | 47.7 | 4.38 | 2.38 × 10−3 ** | |
| Desc | sin(angle) | 1 | 0.04 | 0.08 | 0.774 | 0.70 | 2.38 | 0.126 |
| dist. stim. | 1 | 0.02 | 0.04 | 0.843 | 2.79 | 9.46 | 2.75 × 10−3 *** | |
| trial | 1 | 0.09 | 0.19 | 0.661 | 0.38 | 1.28 | 0.260 | |
| observer | 1 | 0.18 | 0.37 | 0.544 | 0.34 | 1.16 | 0.287 | |
| species | 5 | 12.3 | 5.07 | 2.53 × 10−4 *** | 1.27 | 0.86 | 0.513 | |
| Desc_stim | sin(angle) | 1 | 0.65 | 1.46 | 0.230 | 0.49 | 2.88 | 9.30 × 10−2 |
| dist. stim. | 1 | 11.9 | 26.5 | 8.42 × 10−7 *** | 11.6 | 68.3 | 1.62 × 10−12 *** | |
| trial | 1 | 9.8 × 10−3 | 0.02 | 0.883 | 0.16 | 0.97 | 0.328 | |
| observer | 1 | 0.02 | 0.05 | 0.816 | 0.10 | 0.60 | 0.442 | |
| species | 5 | 22.42 | 10.9 | 2.18 × 10−7 *** | 3.36 | 3.97 | 3.71 × 10−3 ** | |
*p < 0.05; **p < 0.01; ***p < 0.001.
Figure 2.
Performance of the six study species in escape latency (a,b), turning rate (c,d), cumulative distance from the onset of the escape response (Desc; e,f) and cumulative distance from the onset of the stimulus (Desc-stim; g,h). Plots display the mean and 95% CI predicted by the LMMs. The obligate cleaner is displayed in green, facultative cleaners in red and the non-cleaners in black. Different letters indicate that the CI do not overlap for more than half of the error bar length (i.e. significant differences below α = 0.05 [49]). Plots were created with the R package ‘effects’ [55]. Species names are abbreviated (see figure 1 for full names).
Pseudocheilinus hexataenia and L. dimidiatus were the two species that had the shortest response latency: their performance was significantly better than that of the four other species (no overlap of CI; figure 2a). Pseudocheilinus hexataenia outperformed all the other species in the turning rate (figure 2c). The turning rate of L. dimidiatus was also very high and significantly exceeded that of T. lunare and H. melapterus (figure 2c). Halichoeres melapterus preformed significantly worse than all other species for both escape latency and turning rate (figure 2a,c).
With regard to Desc, L. dimidiatus covered significantly more distance than H. melanurus, L. unilineatus and H. melapterus (figure 2e). Pseudocheilinus hexataenia and T. lunare also performed very well and covered a significantly larger distance than H. melanurus, while L. unilineatus and H. melapterus reached intermediate distances (figure 2e). Results for Umax produced a similar clustering of species (electronic supplementary material, figure S2.1). When cumulative distance was measured from the onset of the stimulus (Desc_stim) rather than from the first head movement (Desc), L. dimidiatus and P. hexataenia outperformed all other species (figure 2g). This pattern was similar to the one observed for escape latency (figure 2a).
(b). Juveniles
We found significant differences across species in response latency (table 1 and figure 2b), turning rate (table 1 and figure 2d) and Desc_stim (table 1; figure 2h). There were no differences among species in Desc (table 1 and figure 2f), Umax and Amax (electronic supplementary material, table S1; figures S2.2 and S2.4). Labroides dimidiatus had a significantly shorter escape latency than all other species (figure 2b). The turning rate of P. hexataenia and H. melanurus was the highest, but only significantly higher than H. melapterus. The turning rate of juveniles was generally less variable than in adults (figure 2d). Once the stimulus hit the water surface, L. dimidiatus covered a significantly larger distance (Desc_stim) than P. hexataenia, L. unilineatus and H. melapterus (figure 2h). The patterns observed for Desc_stim reflected those observed for escape latency (figure 2b), as in the adults.
(c). Field observations
Species differed significantly in the percentage of time spent, out of sight, inside the reef matrix (Kruskal–Wallis, d.f. = 5, χ2 = 18.420, p = 0.0024). Pseudocheilinus hexataenia spent over 20% of its time inside the reef, which was significantly more than any other species (electronic supplementary material, table S2). The other species all spent less than 2.5% of their time inside the reef. We recorded 469 cleaning interactions across the eight L. dimidiatus (observed for 15 min each). Facultative-cleaner species participated in few cleaning interactions: one P. hexataenia (6 cm) interacted with a client twice and two H. melanurus (6.5 and 7 cm) were involved in four and one cleaning interactions, respectively. We note that facultative cleaning occurs predominantly in juveniles; thus, our observations of adult facultative cleaners underestimate the occurrence of cleaning behaviour in these species.
4. Discussion
We asked whether the cleaner wrasse L. dimidiatus has evolved reduced fast-start escape performance due to lower predation risk resulting from mutualist predatory clients that refrain from hunting it. Our results do not support this hypothesis. Instead, they provide evidence to the contrary: compared with five closely related wrasse species that are facultative- or non-cleaners, L. dimidiatus consistently scored among the top two performers. Below, we discuss the implications of our results for our understanding of marine cleaning mutualisms and links between ecology and fast-start performance.
(a). Conflict in a mutualism selects for high fast-start performance
Fast-start escape performance has often been linked to predation pressure [31–33,56,57]. Currently, we do not know to what extent predation risk explains the high fast-start performance observed in L. dimidiatus. While cleaner fish entertain a privileged relationship with predatory clients [20,22,23,25], the close physical proximity of these interactions might lead to rare, high-risk predation attempts, either due to mistakes (i.e. predators inadvertently eating cleaners) or due to cheating by predators. Currently, there is no evidence that such events occur in nature [22–24] and we do not know whether cleaners can escape predators under these circumstances. By contrast, various benthic sit-and-wait predators such as hawkfish and lizardfish are not regular clients and would readily eat cleaners. Unsuccessful predation attempts from these two species have been observed by R.B. Such predation risk may be important enough to inhibit any compensated trait loss, or even select for increased escape performance. However, given the low frequency of these predation attempts, we hypothesize that chases used by non-predatory clients to punish cheating cleaners [11] might be the driving factor selecting for high escape performance. Field observations indicate that cleaners are chased about 1.4 times h−1 (194 times during 144 h of observations) [58]. Thus, cleaners regularly need to escape non-predatory clients to avoid injuries. On extremely rare occasions, the chaser might be a predator (observed once by R.B. over hundreds of hours of observations) where the cleaner potentially risks being eaten [17].
The relative importance of predation risk and punishment in influencing the fast-start performance of L. dimidiatus cannot be determined from our results. However, Caribbean cleaning gobies (Elacatinus spp.) would provide an ideal system to tease apart these effects. Unlike L. dimidiatus, Elacatinus spp. are not tempted to cheat because they prefer eating ectoparasites over mucus [59], and clients do not use punishment to enforce cooperation [60]. Since Elacatinus spp. also clean predatory clients, comparing the fast-start performance of co-occurring cleaning and non-cleaning gobies would enable the determination of whether punishment or the threat of predation is the most likely driver of cleaners' high escape performance.
Three of the wrasse species we examined are facultative cleaners (H. melanurus, P. hexataenia and T. lunare). Since facultative cleaners engage in few cleaning interactions, mostly with non-predatory clients [28], there might be insufficient conflict with clients to create significant positive selection on escape performance. We also found less variation in escape performance across species at the juvenile than the adult stage. Predation pressure on juvenile coral reef fishes is high, and decreases throughout ontogeny [61]. While facultative cleaners mainly engage in cleaning at the juvenile stage [23], the need to escape client chases remains constant throughout L. dimidiatus' life history because it cleans both as a juvenile and as an adult [28]. Differences in selective pressures throughout ontogeny could thus explain why we found more variation across species for adults than juveniles.
(b). Linking ecology and fast-start performance
Two species exhibited very high escape performance in our experiments: L. dimidiatus and P. hexataenia. Both are smaller in size compared with the other four species we examined (figure 1; electronic supplementary material, figure S1). However, L. dimidiatus and P. hexataenia differ strongly in their habitat use, with P. hexataenia being the only species that spent considerable amounts of time (>20%) inside the reef matrix (electronic supplementary material, table S2). Below, we discuss potential implications of size and habitat use for escape performance.
Size can affect a fish's escape performance, with larger fish achieving a greater absolute distance and velocity than small species during fast-starts [29,30]. This is mainly due to the fact that larger fishes take more time to complete a fast-start [50,51]. However, when comparing fishes of different sizes in a fixed time period (as was done here), absolute distance and velocity are size-independent [35,51]. Two additional analyses indicated that size alone does not explain the performance differences we observed among species (see the electronic supplementary material). In addition, the two species that performed the best in our experiments were the two smallest, which contrasts with known effects of size on absolute measures of escape performance [29,30,50,51]. We note that the size of our experimental set-up might have restricted the maximum performance of the largest individuals tested (adult H. melapterus). Nevertheless, juvenile H. melapterus also performed relatively poorly, suggesting that our data are robust.
Trade-offs between various aspects of swimming performance such as steady and burst swimming are often linked to habitat use and predation pressure [62]. Pseudocheilinus hexataenia occupies a small territory inside branching corals and spends most of its time within centimetres of the reef. Navigating in the narrow interstices between coral branches requires high manoeuvrability, which might explain why adults of this species exhibited very rapid turning rates (figure 2). The ecology of this species therefore shares some similarities with that of L. dimidiatus: living in a highly structured environment means that many encounters with predators do not involve the early stages of a predator–prey interaction (i.e. detection, approach) because predators typically become visible only at close range [43]. Therefore, L. dimidiatus and P. hexataenia might have experienced strong selective pressures on short-range escape performance.
(c). Conclusions/outlook
Our study of fast-start escape performance complements other research aiming to identify how interactions with clients affect the behaviour and cognitive abilities of cleaner fish [28,37,63,64]. The goal of these studies is to understand the evolutionary consequences of interspecific interactions on phenotypic traits, both with respect to the evolution of novel traits and the loss of ancestral traits. The absence of evidence for compensated trait loss in L. dimidiatus has interesting implications for future research. It suggests that the evolutionary dynamics of traits differ between mutualisms characterized by high interdependence and low conflicts of interest on the one hand, and by low interdependence and high conflicts interest on the other hand. Only in the former case can interacting partners rely on each other and hence experience relaxed selection on traits that one of the partners compensates for. When conflicts exist and individuals are tempted to cheat and increase their benefits at the expense of others [65,66], compensated trait loss may occur only in cases of high interdependence, such as in specialized host–parasite systems [4].
Supplementary Material
Acknowledgements
We thank the members of the University of Neuchâtel's eco-ethology laboratory for help during fieldwork and useful comments and discussions, the Lizard Island Research Station staff for field support and R. A. Slobodeanu for statistical advice. We particularly thank Hadrien Raggenbass for his help with video analysis, and Océane Krattinger and Maïwenn Jornod for data collection in the field.
Ethics
All experiments were carried out in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, and under the approval of a Queensland Government (Australia) Animal Ethics Committee.
Data accessibility
The data and code for this study are archived in the repository figshare [52] following best practices [67] (doi:10.6084/m9.figshare.3398740).
Authors' contributions
S.G. and D.G.R. collected the data. S.G., D.G.R. and R.B. developed the experimental design and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
S.G. was supported by a grant from the Fonds des Donations of the University of Neuchâtel for fieldwork, R.B. by the Swiss Science Foundation 31003A_153067 and D.G.R. by the Fonds de Recherche du Québec Nature et Technologies.
References
- 1.van Damme R, Castilla AM. 1996. Chemosensory predator recognition in the lizard Podarcis hispanica: effects of predation pressure relaxation. J. Chem. Ecol. 22, 13–22. ( 10.1007/BF02040196) [DOI] [PubMed] [Google Scholar]
- 2.Blumstein DT, Daniel JC. 2005. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 272, 1663–1668. ( 10.1098/rspb.2005.3147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Steen S, Cullum AJ, Bennett AF. 2002. Rapid evolution of escape ability in Trinidadian guppies (Poecilia reticulata). Evolution 56, 776–784. ( 10.1111/j.0014-3820.2002.tb01388.x) [DOI] [PubMed] [Google Scholar]
- 4.Ellers J, Toby Kiers E, Currie CR, McDonald BR, Visser B. 2012. Ecological interactions drive evolutionary loss of traits. Ecol. Lett. 15, 1071–1082. ( 10.1111/j.1461-0248.2012.01830.x) [DOI] [PubMed] [Google Scholar]
- 5.Müller CB, Krauss J. 2005. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 8, 450–456. ( 10.1016/j.pbi.2005.05.007) [DOI] [PubMed] [Google Scholar]
- 6.Janzen DH. 1966. Coevolution of mutualism between ants and acacias in Central America. Evolution 20, 249–275. ( 10.2307/2406628) [DOI] [PubMed] [Google Scholar]
- 7.Currie CR, Stuart AE. 2001. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. Lond. B 268, 1033–1039. ( 10.1098/rspb.2001.1605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hata H, Kato M. 2006. A novel obligate cultivation mutualism between damselfish and Polysiphonia algae. Biol. Lett. 2, 593–596. ( 10.1098/rsbl.2006.0528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grutter AS. 1996. Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Mar. Ecol. Prog. Ser. 130, 61–70. ( 10.3354/meps130061) [DOI] [Google Scholar]
- 10.Potts GW. 1973. The ethology of Labroides dimidiatus (cuv. & val.) (Labridae, Pisces) on Aldabra. Anim. Behav. 21, 250–291. ( 10.1016/S0003-3472(73)80068-5) [DOI] [Google Scholar]
- 11.Bshary R. 2001. The cleaner fish market. In Economics in nature: social dilemmas, mate choice and biological markets (eds Noë R, Van Hooff JARAM, Hammerstein P), pp. 146–172. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Grutter AS, Bshary R. 2003. Cleaner wrasse prefer client mucus: support for partner control mechanisms in cleaning interactions. Proc. R. Soc. Lond. B 270, S242–S244. ( 10.1098/rsbl.2003.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bshary R, Grutter AS. 2005. Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol. Lett. 1, 396–399. ( 10.1098/rsbl.2005.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clague GE, Cheney KL, Goldizen AW, McCormick MI, Waldie PA, Grutter AS. 2011. Long-term cleaner fish presence affects growth of a coral reef fish. Biol. Lett. 7, 863–865. ( 10.1098/rsbl.2011.0458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ros AFH, Lusa J, Meyer M, Soares M, Oliveira RF, Brossard M, Bshary R. 2011. Does access to the bluestreak cleaner wrasse Labroides dimidiatus affect indicators of stress and health in resident reef fishes in the Red Sea? Horm. Behav. 59, 151–158. ( 10.1016/j.yhbeh.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 16.Waldie PA, Blomberg SP, Cheney KL, Goldizen AW, Grutter AS. 2011. Long-term effects of the cleaner fish Labroides dimidiatus on coral reef fish communities. PLoS ONE 6, e21201 ( 10.1371/journal.pone.0021201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bshary R, Bronstein JL. 2004. Game structures in mutualistic interactions: what can the evidence tell us about the kind of models we need? Adv. Study Behav. 34, 59–102. ( 10.1016/S0065-3454(04)34002-7) [DOI] [Google Scholar]
- 18.Grutter AS. 2004. Cleaner fish use tactile dancing behavior as a preconflict management strategy. Curr. Biol. 14, 1080–1083. ( 10.1016/j.cub.2004.05.048) [DOI] [PubMed] [Google Scholar]
- 19.Soares MC, Oliveira RF, Ros AFH, Grutter AS, Bshary R.. 2011. Tactile stimulation lowers stress in fish. Nat. Commun. 2, 534 ( 10.1038/ncomms1547) [DOI] [PubMed] [Google Scholar]
- 20.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 21.Bshary R, Bronstein JL. 2011. A general scheme to predict partner control mechanisms in pairwise cooperative interactions between unrelated individuals. Ethology 117, 271–283. ( 10.1111/j.1439-0310.2011.01882.x) [DOI] [Google Scholar]
- 22.Feder HM. 1966. Cleaning symbiosis in the marine environment. In Symbiosis (ed. Henry SM.), pp. 327–380. New York, NY: Academic Press. [Google Scholar]
- 23.Côté IM. 2000. Evolution and ecology of cleaning symbioses in the sea. Oceanogr. Mar. Biol. Annu. Rev. 38, 311–355. [Google Scholar]
- 24.Bshary R, Côté IM. 2008. New perspectives on marine cleaning mutualism. In Fish behaviour (eds Magnhagen C, Braithwaite VA, Forsgren E, Kapoor BG), pp. 563–592. Enfield: Science Publishers. [Google Scholar]
- 25.Cheney KL, Bshary R, Grutter AS. 2008. Cleaner fish cause predators to reduce aggression toward bystanders at cleaning stations. Behav. Ecol. 19, 1063–1067. ( 10.1093/beheco/arn067) [DOI] [Google Scholar]
- 26.Cheney KL, Grutter AS, Blomberg SP, Marshall NJ. 2009. Blue and yellow signal cleaning behavior in coral reef fishes. Curr. Biol. 19, 1283–1287. ( 10.1016/j.cub.2009.06.028) [DOI] [PubMed] [Google Scholar]
- 27.Cheney KL. 2013. Cleaner fish coloration decreases predation risk in aggressive fangblenny mimics. Behav. Ecol. 24, 1161–1165. ( 10.1093/beheco/art043) [DOI] [Google Scholar]
- 28.Barbu L, Guinand C, Bergmüller R, Alvarez N, Bshary R. 2011. Cleaning wrasse species vary with respect to dependency on the mutualism and behavioural adaptations in interactions. Anim. Behav. 82, 1067–1074. ( 10.1016/j.anbehav.2011.07.043) [DOI] [Google Scholar]
- 29.Domenici P, Blake R. 1997. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 200, 1165–1178. [DOI] [PubMed] [Google Scholar]
- 30.Domenici P. 2011. Fast Start. In Encyclopedia of fish physiology: from genome to environment (eds Farrell AP, Stevens ED, Cech JJ, Richards JG). San Diego, CA: Academic Press. [Google Scholar]
- 31.Domenici P, Turesson H, Brodersen J, Bronmark C. 2008. Predator-induced morphology enhances escape locomotion in crucian carp. Proc. R. Soc. B 275, 195–201. ( 10.1098/rspb.2007.1088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langerhans RB. 2009. Morphology, performance, fitness: functional insight into a post-Pleistocene radiation of mosquitofish. Biol. Lett. 5, 488–491. ( 10.1098/rsbl.2009.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domenici P. 2010. Context-dependent variability in the components of fish escape response: integrating locomotor performance and behavior. J. Exp. Zool. Part Ecol. Genet. Physiol. 313A, 59–79 ( 10.1002/jez.580) [DOI] [PubMed] [Google Scholar]
- 34.Eaton RC, Bombardieri RA, Meyer DL. 1977. The Mauthner-initiated startle response in teleost fish. J. Exp. Biol. 66, 65–81. [DOI] [PubMed] [Google Scholar]
- 35.Webb PW. 1978. Fast-start performance and body form in seven species of teleost fish. J. Exp. Biol. 74, 211–226. [Google Scholar]
- 36.Cowman PF, Bellwood DR. 2011. Coral reefs as drivers of cladogenesis: expanding coral reefs, cryptic extinction events, and the development of biodiversity hotspots: cladogenesis on coral reefs. J. Evol. Biol. 24, 2543–2562. ( 10.1111/j.1420-9101.2011.02391.x) [DOI] [PubMed] [Google Scholar]
- 37.Gingins S, Bshary R. 2016. The cleaner wrasse outperforms other labrids in ecologically relevant contexts, but not in spatial discrimination. Anim. Behav. 115, 145–155. ( 10.1016/j.anbehav.2016.02.022) [DOI] [Google Scholar]
- 38.Cowman PF, Bellwood DR, van Herwerden L. 2009. Dating the evolutionary origins of wrasse lineages (Labridae) and the rise of trophic novelty on coral reefs. Mol. Phylogenet. Evol. 52, 621–631. (doi:16/j.ympev.2009.05.015) [DOI] [PubMed] [Google Scholar]
- 39.Randall JE, Allen GR, Steene RC. 1997. Fishes of the Great Barrier Reef and Coral Sea. Honolulu, HI: University of Hawaii Press. [Google Scholar]
- 40.Allen G, Steene R, Humann P, DeLoach N.. 2005. Reef fish identification—tropical Pacific. Illustrated edn Jacksonville, FL: New World Publications. [Google Scholar]
- 41.Ménard A, Turgeon K, Roche DG, Binning SA, Kramer DL. 2012. Shelters and their use by fishes on fringing coral reefs. PLoS ONE 7, e38450 ( 10.1371/journal.pone.0038450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mora C. 2015. Ecology of fishes on coral reefs, 1 edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 43.Kelley JL, Magurran AE. 2011. Learned defences and counterdefences in predator–prey interactions. In Fish cognition and behavior (eds Brown C, Laland K, Krause J), pp. 36–58. New York, NY: Wiley-Blackwell. [Google Scholar]
- 44.Rasband WS. 1997. ImageJ. Bethesda, MD: National Institutes of Health; (http://imagej.nih.gov/ij/) [Google Scholar]
- 45.Meijering E, Dzyubachyk O, Smal I. 2012. Methods for cell and particle tracking. Methods Enzym. 504, 183–200. ( 10.1016/B978-0-12-391857-4.00009-4) [DOI] [PubMed] [Google Scholar]
- 46.Domenici P, Blake RW. 1991. The kinematics and performance of the escape response in the angelfish (Pterophyllum eimekei). J. Exp. Biol. 156, 187–205. [Google Scholar]
- 47.Jornod M, Roche DG. 2015. Inter- vs intra-individual variation and temporal repeatability of escape responses in the coral reef fish Amblyglyphidodon curacao. Biol. Open 4, 1395–1399. ( 10.1242/bio.013508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanczos C. 1956. Applied analysis. Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- 49.Cumming G, Fidler F, Vaux DL. 2007. Error bars in experimental biology. J. Cell Biol. 177, 7–11. ( 10.1083/jcb.200611141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb PW. 1976. The effect of size on the fast-start performance of rainbow trout Salmo cairdneri, and a consideration of piscivorous predator–prey interactions. J. Exp. Biol. 65, 157–177. [DOI] [PubMed] [Google Scholar]
- 51.Domenici P, Blake RW. 1993. The effect of size on the kinematics and performance of angelfish (Pterophyllum eimekei) escape responses. Can. J. Zool. 71, 2319–2326. ( 10.1139/z93-325) [DOI] [Google Scholar]
- 52.Gingins S, Roche D, Bshary R.. 2017. Data and supplementary material for: mutualistic cleaner fish maintains high escape performance despite privileged relationship with predators. ( 10.6084/m9.figshare.3398740.v1) [DOI] [PMC free article] [PubMed]
- 53.de Mendiburu F. 2015. agricolae: Statistical procedures for agricultural research. See http://CRAN.R-project.org/package=agricolae [Google Scholar]
- 54.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 55.Fox J. 2003. Effect displays in R for generalised linear models. J. Stat. Softw. 8, 1–27. ( 10.18637/jss.v008.i15) [DOI] [Google Scholar]
- 56.Andraso G. 1997. A comparison of startle response in two morphs of the brook stickleback (Culaea inconstans): further evidence for a trade-off between defensive morphology and swimming ability. Evol. Ecol. 11, 83–90. ( 10.1023/A:1018487529938) [DOI] [Google Scholar]
- 57.Beck JL, Rooker JR. 2011. Effect of predator exposure on the performance and survival of red drum (Sciaenops ocellatus). Environ. Biol. Fishes 93, 267–276. ( 10.1007/s10641-011-9912-1) [DOI] [Google Scholar]
- 58.Bshary R, Grutter AS. 2002. Asymmetric cheating opportunities and partner control in a cleaner fish mutualism. Anim. Behav. 63, 547–555. ( 10.1006/anbe.2001.1937) [DOI] [Google Scholar]
- 59.Soares MC, Côté IM, Cardoso SC, Oliveira RF, Bshary R. 2010. Caribbean cleaning gobies prefer client ectoparasites over mucus. Ethology 116, 1244–1248. ( 10.1111/j.1439-0310.2010.01838.x) [DOI] [Google Scholar]
- 60.Soares MC, Côté IM, Cardoso SC, Bshary R. 2008. The cleaning goby mutualism: a system without punishment, partner switching or tactile stimulation. J. Zool. 276, 306–312. ( 10.1111/j.1469-7998.2008.00489.x) [DOI] [Google Scholar]
- 61.Hixon MA. 1991. Predation as a process structuring coral-reef fish communities. In The ecology of fishes on coral reefs (ed. Sale PF.), pp. 475–508. San Diego, CA: Academic Press. [Google Scholar]
- 62.Domenici P. 2003. Habitat, body design and the swimming performance of fish. Vertebr. Biomech. Evol. 1, 137–160. ( 10.1111/j.0022-1112.2004.00568.x) [DOI] [Google Scholar]
- 63.Salwiczek LH, et al. 2012. Adult cleaner wrasse outperform capuchin monkeys, chimpanzees and orang-utans in a complex foraging task derived from cleaner–client reef fish cooperation. PLoS ONE 7, e49068 ( 10.1371/journal.pone.0049068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gingins S, Werminghausen J, Johnstone RA, Grutter AS, Bshary R. 2013. Power and temptation cause shifts between exploitation and cooperation in a cleaner wrasse mutualism. Proc. R. Soc. B 280, 20130553 ( 10.1098/rspb.2013.0553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Axelrod R, Hamilton WD. 1981. The evolution of cooperation. Science 211, 1390–1396. ( 10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 66.West SA, Griffin AS, Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672. ( 10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 67.Roche DG, Kruuk LEB, Lanfear R, Binning SA. 2015. Public data archiving in ecology and evolution: how well are we doing? PLoS Biol. 13, e1002295 ( 10.1371/journal.pbio.1002295) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gingins S, Roche D, Bshary R.. 2017. Data and supplementary material for: mutualistic cleaner fish maintains high escape performance despite privileged relationship with predators. ( 10.6084/m9.figshare.3398740.v1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and code for this study are archived in the repository figshare [52] following best practices [67] (doi:10.6084/m9.figshare.3398740).