Abstract
The high extinction risk of small populations is commonly explained by reductions in fecundity and breeder survival associated with demographic and environmental stochasticity. However, ecological theory suggests that population extinctions may also arise from reductions in the number of floaters able to replace the lost breeders. This can be particularly plausible under harsh fragmentation scenarios, where species must survive as small populations subjected to severe effects of stochasticity. Using a woodpecker study in fragmented habitats (2004–2016), we provide here empirical support for the largely neglected hypothesis that floaters buffer population extirpation risks. After controlling for population size, patch size and the intrinsic quality of habitat, populations in patches with floaters had a lower extinction probability than populations in patches without floaters (0.013 versus 0.131). Floaters, which often replace the lost breeders, were less likely to occur in small and low-quality patches, showing that population extirpations may arise from unnoticed reductions in floater numbers in poor-quality habitats. We argue that adequate pools of the typically overlooked floaters may buffer extirpation risks by reducing the detrimental impacts of demographic and environmental stochasticity. However, unravelling the influence of floaters in buffering stochastic effects and promoting population stability require additional studies in an ample array of species and stochastic scenarios.
Keywords: buffer effect, demographic and environmental stochasticity, habitat degradation, habitat loss and fragmentation, non-breeding, population extirpation
1. Introduction
Small populations are subjected to high extinction risks associated with demographic and environmental stochasticity [1–5]. However, despite massive research in recent decades, the mechanisms underlying the ability of small local populations to persist in spite of severe impacts of stochastic processes are poorly known. Small populations are likely to arise as a result of habitat loss and fragmentation, where species often occur in small discrete habitat patches and are subjected to negative impacts of stochasticity that restrict their probability of survival [6–8]. As major drivers of eco-evolutionary dynamics [9], habitat loss and fragmentation provide a suitable framework for the study of the persistence of small populations. In addition, given that habitat loss, fragmentation and degradation are widespread phenomena that structure ecological populations, understanding how local populations persist, or become extinct, under those habitat changes is a central issue in ecology and conservation biology. In this paper, we examine the extirpation of populations under habitat fragmentation and degradation scenarios as a model framework to assess how small populations subjected to severe impacts of stochasticity are able to persist.
So far, most research on the persistence and dynamics of populations has focused on the impact of the variation in fecundity and survival of breeders. In particular, the high extinction risk of small populations has been usually associated either with reductions in the fecundity and survival of the breeders under stochastic scenarios or with reductions in those parameters as the population size decreases (i.e. the Allee effect [10,11]). The impact of a reduction in the number of floaters (i.e. non-breeding but sexually mature and behaviourally dispersing individuals able to enter the breeding population when a vacancy becomes available) on the persistence and dynamics of populations has received considerably less attention [11–14]. Ecological theory, however, suggests that floaters constitute pools of non-breeding individuals that may rescue small populations from extinction by replacing the breeders that die or disperse out of the populations [15–17]. Specifically, large pools of non-breeding individuals have been suggested to buffer the impact of environmental and demographic stochasticity on the viability of the breeding population [18,19]. While empirical evidence of the influence of floaters in buffering the extinction risk of local populations is lacking, empirically testing this hypothesis would provide solid support for the connection between floaters and the persistence of small populations subjected to negative impacts of stochasticity.
Habitat loss, fragmentation and degradation decrease the overall quality of the habitats by reducing the size of the habitat patches and the intrinsic quality of the local patches that remain in the landscape [8]. Because recent studies show that floaters actively search for good-quality habitats [20], the pool of floaters is expected to decrease dramatically under severe reductions in habitat quality associated with habitat loss, fragmentation and degradation, which in turn may compromise the persistence of the breeding population. Empirical support for the hypothesis that habitat loss, fragmentation and degradation reduce the pool of floaters is, however, scarce. Bayne & Hobson [21] provided one exception by examining the occurrence of floaters in the ovenbird (Seiurus auricapillus), as they experimentally demonstrated the presence of floaters in contiguous forests but rarely in forest fragments. A better understanding of the factors that influence the occurrence of floaters in fragmented and degraded habitats, where populations are subjected to negative impacts of stochastic processes, is essential to understanding the distribution and persistence of breeding populations.
In a 13-year study (2004–2016) of middle spotted woodpeckers (Dendrocopos medius) in the southern Cantabrian Mountains (northwest Spain), we (i) investigated the impact of habitat changes on the occurrence of floaters and (ii) assessed the influence of floaters in buffering the extinction risk of small local populations subjected to high impacts of stochasticity in fragmented habitats. The middle spotted woodpecker is a resident forest bird potentially sensitive to habitat loss, fragmentation and reduction in the local quality of habitat patches (e.g. [5,22–24]). Under severe fragmentation conditions, small local populations of woodpeckers are subjected to a high extinction risk associated with demographic and environmental stochasticity [5]. Previous research shows the existence of floaters that may replace the lost breeders in the Cantabrian Mountains [25,26], further indicating the suitability of our study system for testing floater-related hypotheses of population persistence and turnover.
We first examined the factors that may influence the occurrence of floaters. We built a set of predictions on the basis that floaters (i) are likely to choose high-quality habitats [20] and that (ii) habitat loss, fragmentation and degradation reduce the quality of the remaining habitat patches in the landscape. First, a reduction in patch size associated with habitat loss and fragmentation is expected to reduce the total amount of resources (e.g. food, shelter) in the habitat patches [27,28], which may lead floaters to disappear from small patches. Thus, we predict a positive relationship between floater occurrence and patch size. Regardless of patch geometry, in our second prediction, we expect a positive relationship between the occurrence of floaters and the intrinsic quality of the habitat in terms of the availability of resources within the patches, as individuals may prefer waiting for a vacancy in high-quality habitat patches than settling in low-quality patches where their fitness prospects are low [20]. Third, floaters may prefer to occupy highly populated patches resulting from low fragmentation conditions, as they may consider those patches to be of high quality in terms of the availability of resources such as food supply or mating opportunities (i.e. the conspecific attraction hypothesis, see [29]). Thus, we predict a positive relationship between floater occurrence and population size. Our last prediction of floater occurrence is grounded on the basis that an increase in isolation (inverse of connectivity) associated with habitat fragmentation may lead floaters to be less likely to reach isolated patches. Consequently, we predict a positive relationship between floater occurrence and connectivity. We then tested the hypothesis that floaters may buffer the extinction risk of populations by examining the relationship between the occurrence of floaters and the extinction of local populations in subsequent years. We predict that, after controlling for other factors that may influence colonization–extinction dynamics (i.e. population size, patch size and the intrinsic habitat quality of local patches; e.g. [5]), local populations will have low extirpation propensity in patches where floaters occur.
2. Material and methods
(a). Study species and area
The middle spotted woodpecker is a medium-sized (approx. 55 g) bird associated with old rough-bark deciduous forests of the western Palaearctic [30]. Its social system is characterized by territoriality [31] and monogamy [32]. Males and females share parental care [32] and raise 1 brood yr−1 [30]. These woodpeckers reach sexual maturity in their second year of life.
The study area covered approximately 770 km2 on the Cantabrian Mountains (northwest Spain, 42° N, 5° W) [5,26]. The Cantabrian population of middle spotted woodpeckers is placed at the southwestern edge of the species' geographic range and the southern edge of its distribution in Spain [33–35]. The study area is composed of interspersed patches of forest, pastures, scrubs, cereal crops, and urban and rocky areas, where this woodpecker breeds in old-growth deciduous oak forests dominated by Quercus pyrenaica [25,35]. Post-fledging juvenile woodpeckers are also associated with old-growth Pyrenean oak forests [24]. The species does not breed in heavily disturbed young oak patches and forests dominated by other species (Pinus pinaster, P. sylvestris, Fagus sylvatica, Populus sp., Salix sp., Fraxinus sp., Q. rotundifolia) [34–36]. We identified 104 habitat patches greater than or equal to 0.1 ha from georeferenced aerial photographs and field surveys. Habitat patches (i.e. old-growth deciduous oak forests) covered approximately 4% of the study area [5,26].
(b). General field methods
We monitored woodpecker occupancy and abundance in 99 habitat patches every spring from 2000 to 2016. In addition, we monitored the remaining five patches, located at the northern edge of the study area, for 3–9 years. We examined each patch three or four times on average in the pre-breeding season of each year (late February through early May), which coincides with the peak of intraspecific aggression [23]. We played kweek and rattle calls [30] to find the birds with territories as quickly as possible. We stopped every 100 m to alternate 30 s of species calls with 45 s of listening. If we did not detect woodpeckers, we repeated the playback and listening once more [25,35]. When we detected the presence of woodpeckers, we stopped playing the calls to avoid attracting birds from nearby territories [5]. We followed the birds and recorded their activities on 1 : 4000–1 : 10000 scale maps [23,25]. These methods allowed us to estimate accurately the number of territories (i.e. local population size) in the habitat patches [5,23]. After excluding a large patch that could not be entirely surveyed, 29 patches were occupied by one to three territories at least some years, five patches by 2–11 territories and the remaining 69 patches were not occupied by territorial woodpeckers in any year.
(c). Assessment of floater occurrence
Through regular monitoring (see §2b) conducted in 2000–2003, we acquired substantial field skills to identify the occurrence of floaters for each habitat patch in subsequent years. In 2004–2016, the combination of (i) colour-banding (20–30% of floaters and territorial individuals held rings annually), (ii) the low number of territories per patch (95% of patches with 0–2 territories), (iii) the use of multiple visits per year (see §2b) and (iv) the distinctive behaviour of floaters and territorial woodpeckers (see below) allowed us to assess annually the occurrence of floaters for each habitat patch. In addition, often two or three observers simultaneously inspected the highly populated patches (two or more territories), reducing the probability that floaters were misidentified as territorial birds of neighbouring couples.
Unlike unpaired territorial birds and helpers in cooperative-breeding species, floaters can be defined behaviourally as dispersers that do not hold a territory [11,37] but instead search for a breeding vacancy by exploring ranges that include both intrusions to the breeding sites and transient settlements in areas outside of established territories [11,31,38]. We confirmed these distinctive behaviours on the basis of observations to banded woodpeckers (35 floaters and 237 territorial birds) in our study system. Instead of defending fixed areas (i.e. territories), floaters roamed around one or a few breeding territories. They also occurred in habitat patches with no territorial birds, which is in accordance with previous observations of non-territorial woodpeckers intruding others' breeding territories and occurring temporarily in patches that lack territorial birds [23,25,26,30,31]. While territory holders stayed in a given patch the entire season, banded floaters occurred in up to three different patches, further supporting the idea that floaters lack territories and may use large vital ranges when searching for a vacancy [11,31]. Floaters often performed kweek-calls insistently, which is characteristic of pair-bonding behaviour but is sparsely used once woodpeckers mate in a territory ([30], authors' personal observation every year from 2000 to 2016). In response to kweek-calling, territorial woodpeckers typically performed rattle calls or scolding, occasionally followed by aggressive interactions with the intruders ([30], authors' personal observation every year from 2000 to 2016). Contrary to eight solitary unpaired males that defended territories throughout the pre-breeding season in patches lacking other conspecifics, floaters occupied patches with no territorial birds only temporarily. Floaters, but not territory owners, were attracted to our broadcasted vocalizations for up to several hundred metres. In addition, we performed up to nine visits to distinguish floaters from unpaired territorial birds in a given patch and year [23].
Most woodpeckers classified as territory holders or floaters maintained their status (270 of 272) throughout the pre-breeding season, as indicated by the fact that only two banded floaters became territory holders within a particular pre-breeding season. In addition, the number of territories was stable across successive visits to the habitat patches, and given that most replacements of territory owners by floaters occurred during or after the breeding season (16 of 18 replacements), we assume that the numbers of both floaters and territorial woodpeckers were reasonably stable during our annual surveys in the pre-breeding season. More importantly, the occurrence of floaters in the habitat patches was also fairly stable within each year (assessments in single visits yielded consistent estimations of floater occurrence in subsequent visits in 90–100% of the cases), even though floater occurrence varied in a few cases probably due to the mobility of floaters among patches (see above). To summarize, while we were unable to provide an exact estimation of the distribution and abundance of floaters, particularly in single visits to the habitat patches, we believe that our data represent a suitable approach to the occurrence of floaters in the patches for each year (i.e. the variable of interest in our analyses, see below) from 2004 to 2016.
(d). Patch-level covariates
Patch size and connectivity were calculated with ArcGIS 10.1 [5,24]. We calculated the connectivity of populations (sensu [39]) as
where Si is the connectivity of patch i to other patches with potential source populations j; pj is 1 for the occupied patches and 0 for the unoccupied patches in a given year; α scales the effect of distance to dispersal (1/α is the average dispersal distance); dij is the distance from patch i to j; and Aj is the size of the patch occupied by the potential source population j. Therefore, Si considers distances to all potential source populations and their patch sizes, and is based on the dispersal ability of individuals [40]. We estimated α as 0.192 in this study area [5].
We used the density of large oaks (greater than or equal to 37 cm dbh (diameter 1.3 m above ground)) in each patch as a measure of the intrinsic habitat quality. Large oaks provide forage for post-fledging juveniles and breeding adults [24,35]. The density of large oaks is inversely related to the size of home ranges of middle spotted woodpeckers [41], and positively related to the probability of patch occupancy and colonization of woodpeckers in our study area [5,35]. We calculated the density of large oaks in the patches by estimating the number of large oaks in 1645, 0.04 ha circular plots greater than or equal to 100 m apart [5,35], which represents an averaged sampling effort of 17 plots per patch.
(e). Factors that influence the occurrence of floaters
We used generalized linear mixed models (GLMMs) with binomial error distributions and logit link functions in the package lme4 [42] to assess the occurrence of floaters (floater occurrence = 1, no floaters = 0) in a given patch and year. Overall, we included in the models the data from 97 patches exhaustively monitored every year from 2004 to 2016. We excluded from the analyses seven patches whose areas were not exhaustively monitored (partly or entirely) every year. Local population size (number of territories in each local patch), the density of large oaks in the patches, patch size and connectivity of populations were fitted as explanatory variables. The correlations between these explanatory variables were low (rs < 0.44) to produce multicollinearity issues. All explanatory variables were standardized to a mean of zero and standard deviation of one to make model coefficients comparable. Patch and year identity were fitted as random terms to control for the potential dependence associated with the occurrence of multiple observations (n = 1261 patch-years) within the same patches and years.
We used an information-theoretic approach to assess which variables and models best explained the data [43]. We ran a set of 15 models that included combinations of the four explanatory variables that may influence the occurrence of floaters in the habitat patches. In addition, we ran a null model without explanatory variables (i.e. the intercept-only model). We used the MuMIn package [44] to rank the models according to Akaike's information criterion corrected for small sample sizes (AICc) and Akaike model weights [43,45]. Models with lower AICc values are better supported by the data. Akaike model weights quantify the support of every model by the data, where higher weights indicate better explanatory power and the sum of all model weights is 1 [43]. Because several models could explain the data (see Results), we calculated model-averaged parameter estimates and standard errors for each parameter. Model-averaging was conducted by considering the set of all candidate models. We used the MuMIn package to calculate the marginal R2-value of the best model as a measure of the variation explained by the fixed terms [46]. All statistical analyses were performed in R v. 3.2.2 [47].
(f). Floater occurrence and the extinction of small populations
We used a binomial GLMM to assess whether the probability of population extinction in a given patch and year (t) was related to the occurrence of floaters in the previous year (t − 1). Because the extinction probability of woodpeckers is related to patch population size [5], we used in the analysis only those patches occupied by one or two territories in a given year (i.e. only populations in patches with one or two territories became extinct in subsequent years, see [5]). Overall, 29 patches complied with this requirement for a total of 153 cases (patch-years). The binary response variable was the extinction of local populations in the habitat patches (population extinction in a patch = 1, population persistence = 0). The occurrence of floaters in each focal patch for the previous year was fitted as a fixed term. Patch and year identity were fitted as random terms to control for multiple observations within the same patches and years. In addition, we fitted the density of large oaks in the patches as a fixed term, as this habitat quality variable may influence patch-occupancy dynamics [5]. Finally, we fitted patch size and the number of territories (one or two) in the patches as fixed terms to control for their potential influence on the extinction of populations. Thus, this modelling approach allowed us to assess the influence of floaters on the extinction of local populations while controlling for other factors that may affect population persistence in the habitat patches (i.e. population size, patch size and a proxy of the intrinsic habitat quality).
3. Results
(a). Factors that influence the occurrence of floaters
We found floaters in 35 of the 97 patches inspected annually from 2004 to 2016. Overall, we identified floaters in 167 of 1261 cases (patch-years).
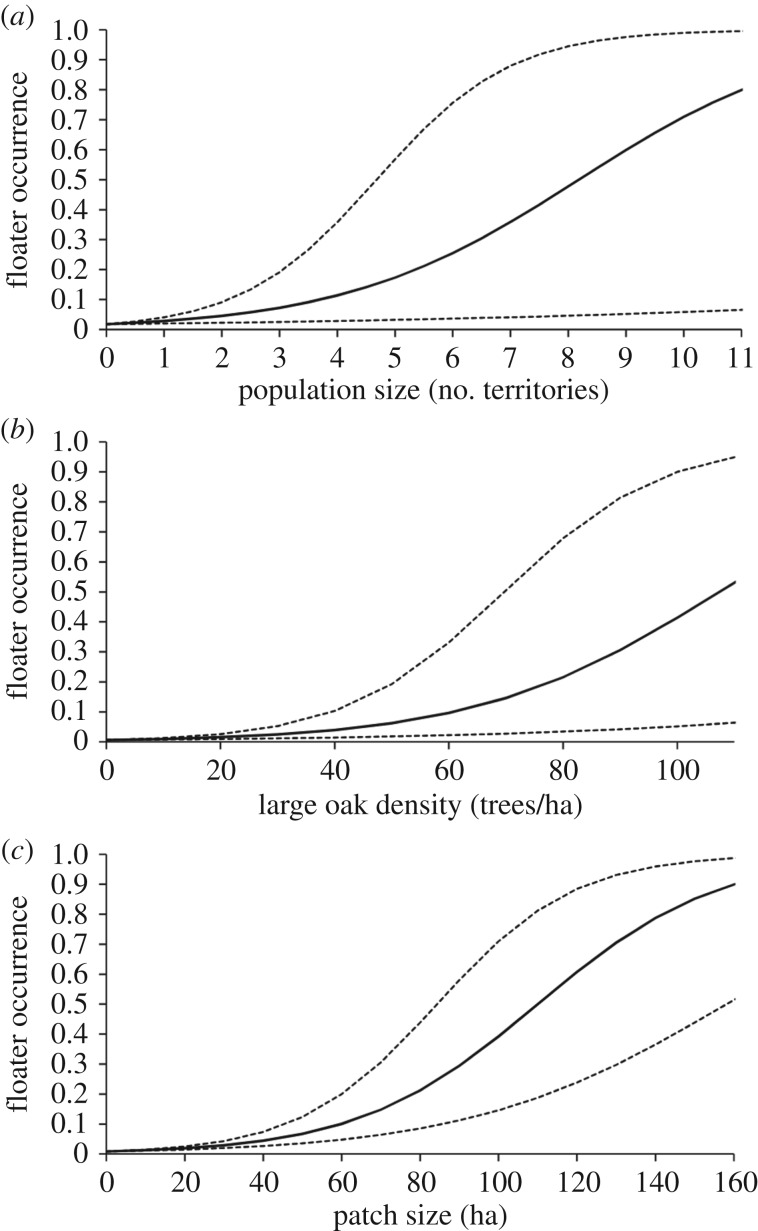
Model selection of analyses that examined the occurrence of floaters yielded two high-ranked models that accounted for most weight (0.91) in the candidate model set (table 1). Population size, the density of large oaks and patch size were included in the best model, which had a marginal R2 of 0.374 (i.e. 37.4% of the variation in the occurrence of floaters was associated with these three fixed terms). Floaters were more likely to inhabit highly populated patches (population size: standardized model-averaged parameter estimate ± s.e. = 0.609 ± 0.233, z = 2.620, p = 0.009; figure 1a), patches with high densities of large oaks (density of large oaks: standardized model-averaged parameter estimate ± s.e. = 1.220 ± 0.337, z = 3.624, p < 0.001; figure 1b) and large patches (patch size: standardized model-averaged parameter estimate ± s.e. = 1.180 ± 0.359, z = 3.285, p = 0.001; figure 1c). The addition of connectivity to the model with population size, large oak density and patch size did not improve model performance, as indicated by the higher AICc value and the similar log-likelihood value of the second ranked model compared with the best model (table 1). Indeed, the 95% confidence interval (CI) of the model-averaged parameter estimate for connectivity included zero (standardized model-averaged parameter estimate ± s.e. = −0.316 ± 0.324, 95% CI = −0.952 + 0.318, z = 0.978, p = 0.328), which suggests low influence of connectivity on the occurrence of floaters.
Table 1.
Model selection of analyses that examined the occurrence of floaters in the habitat patches. The occurrence of floaters was related to patch population size, the density of large oaks (greater than or equal to 37 cm dbh) in the habitat patches, patch size and connectivity of local populations. Patch and year identity were fitted as random terms. d.f., degrees of freedom; LogLik, log-likelihood; AICc, AIC corrected for small sample size; ΔAICc, difference in AICc to the best model. Models are ranked according to their Akaike weight (weight). N = 1261 cases (patch-years) in 97 patches inspected during the 13 study years (2004–2016).
| models | d.f. | LogLik | AICc | ΔAICc | weight |
|---|---|---|---|---|---|
| population size, large oak density, patch size | 6 | −278.45 | 568.97 | 0.00 | 0.56 |
| population size, large oak density, patch size, connectivity | 7 | −277.92 | 569.93 | 0.96 | 0.35 |
| large oak density, patch size | 5 | −281.59 | 573.23 | 4.26 | 0.06 |
| large oak density, patch size, connectivity | 6 | −281.53 | 575.13 | 6.17 | 0.03 |
| population size, large oak density | 5 | −286.02 | 582.09 | 13.12 | 0.00 |
| population size, patch size | 5 | −286.33 | 582.70 | 13.73 | 0.00 |
| population size, large oak density, connectivity | 6 | −285.58 | 583.22 | 14.25 | 0.00 |
| population size, patch size, connectivity | 6 | −286.26 | 584.59 | 15.62 | 0.00 |
| patch size | 4 | −290.87 | 589.76 | 20.80 | 0.00 |
| patch size, connectivity | 5 | −290.28 | 590.60 | 21.63 | 0.00 |
| large oak density | 4 | −292.41 | 592.86 | 23.89 | 0.00 |
| large oak density, connectivity | 5 | −292.41 | 594.86 | 25.89 | 0.00 |
| population size | 4 | −295.48 | 598.99 | 30.02 | 0.00 |
| population size, connectivity | 5 | −295.40 | 600.85 | 31.88 | 0.00 |
| connectivity | 4 | −302.67 | 613.37 | 40.44 | 0.00 |
| intercept-only model (null model) | 3 | −303.86 | 613.73 | 44.47 | 0.00 |
Figure 1.
Probability of occurrence of floaters in a patch in relation to population size and habitat variables. Floater occurrence probabilities were calculated as the predicted values (solid lines) and 95% CI (dashed lines) from the non-standardized model-averaged parameter estimates for local population size (a), large oak density (b) and patch size (c).
(b). Floater occurrence and the extinction of small populations
We observed 20 extinction events of 153 cases (patch-years) in 29 patches that hold one or two territories in a given year. We found floaters in 56 of 133 cases where populations persisted the following years (42.1%), but only in one of the 20 cases where populations became extinct in subsequent years (5.0%). Eighteen banded floaters replaced one of the breeders that disappeared or settled in a territory that became available (i.e. where both breeders disappeared).
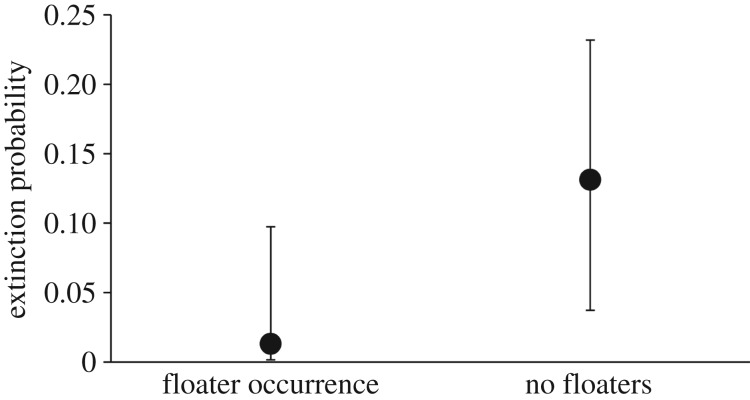
After controlling for population size (population size: parameter estimate ± s.e. = −0.881 ± 0.836, z = −1.055, p = 0.292), patch size (patch size: parameter estimate ± s.e. = −0.003 ± 0.298, z = −0.011, p = 0.991) and the density of large oaks (large oak density: parameter estimate ± s.e. = 0.327 ± 0.273, z = 1.197, p = 0.231), populations in patches with floaters were, on average, 10 times less likely to become extinct the following year than populations in patches with no floaters (occurrence of floaters in the previous year: parameter estimate ± s.e. = −2.439 ± 1.073, z = −2.273, p = 0.023; figure 2). A model that included only the occurrence of floaters as a fixed term had a marginal R2 of 0.330 (i.e. 33.0% of the variation in the extinction probability was associated with the occurrence of floaters in the previous year).
Figure 2.
Probability of extinction of local populations in relation to floater occurrence. The probability of extinction was defined as the probability that a population in a given patch became extinct the following year. Extinction probabilities are the predicted probabilities (±95% CI) calculated from the model that depicted the probability of population extinction in relation to the occurrence of floaters while controlling for population size, patch size and the density of large oaks in the local patches (see Results).
4. Discussion
Our results provide empirical support for the largely neglected hypothesis that floaters may buffer the extinction risk of small populations. After controlling for population size, patch size and the density of large oaks in the habitat patches (i.e. a proxy of the intrinsic habitat quality), populations with floaters were on average 10 times less likely to become extinct than populations with no floaters (0.013 versus 0.131; figure 2). The observations of banded floaters replacing one of the breeders that disappeared, or settling in a territory that became available, further support the poorly tested assumption that floaters constitute a pool of non-breeding individuals able to rescue small breeding populations from extinction by becoming part of the reproductive fraction of the population when given the chance [11,16].
Because the extinction risk of small populations is strongly associated with detrimental effects of demographic and environmental stochasticity [2], the pool of floaters is expected to buffer small populations against extinction by dampening the negative impacts of stochasticity. Indeed, buffer mechanisms have been suggested to reduce the variation in population growth rates associated with the environmental noise [19], which in turn decreases the time populations spend at low abundances levels [48] and, consequently, may reduce the extinction risk of small populations subjected to negative effects of stochasticity. This theoretical scenario is likely to occur in our study system, where floaters buffer the extinction risk of small local populations subjected to both demographic and environmental stochasticity [5]. In addition, our results bring empirical support to the growing body of theoretical studies suggesting that failure to account for the influence of non-breeders (e.g. floaters) on estimates of stochastic population dynamics may lead to incorrect projections of population growth and extinction risk [11,19,49].
Although large floater-to-breeder ratios have long been suggested to indicate stable breeding populations [50,51], under which circumstances the pool of floaters may compensate for the detrimental impacts of stochasticity on the breeding portion of the population remains unclear. Recent studies suggest that non-breeders, such as floaters, are most probably to buffer sudden breeder losses when environmental changes cause reductions in the number of breeders but do not simultaneously influence floater fates in a significant way [11,12,49]. However, a number of key questions remain unsolved: how large should the pool of floaters be to cope with the detrimental impacts of stochasticity? Likewise, how should floater survival vary to compensate for breeder losses under different scenarios of stochasticity? In addition, because a high and abrupt mortality of breeding adults may provoke an increasing recruitment of young floaters with low fitness prospects [11], to what extent and under which conditions is the short-term buffer effect of floaters able to lead to the long-term survival of populations? Adequate responses to these questions will only arise from detailed assessments of the size and demography of floaters and breeders under different stochastic scenarios and in a range of species with diverse life histories.
The buffer effect of floaters is expected to be particularly relevant in scenarios of severe habitat loss and fragmentation, where species often survive as small local breeding populations subjected to detrimental and synergic impacts of stochasticity and the Allee effect [48]. As widespread phenomena and major drivers of eco-evolutionary dynamics [9], habitat loss and fragmentation may provide researchers with a suitable general framework to investigate whether and how a suitable surplus of non-breeding individuals, such as floaters, may ameliorate the resilience and stability of small populations. In addition, a better understanding of the factors that influence the occurrence of floaters in fragmented and degraded habitats may also provide new insights into our knowledge of the mechanisms underlying how small populations are able to persist in spite of human-induced perturbations. Our study illustrates some of these issues, such as the importance of assessing floater occurrence and the persistence of small populations in fragmented and degraded habitats, which we develop more thoroughly in the following lines.
Because the connectivity of populations did not have a severe influence on floater occurrence, an increase in isolation is unlikely to prevent floaters from reaching distant local patches in our study system. Instead, the fact that floaters were less likely to occur in small habitat patches suggests that the Allee effect in fragmented habitats may be associated with a high mortality of floaters in response to habitat fragmentation. Indeed, recent simulation studies suggest that the Allee effect in breeding populations may be explained by a high mortality of floaters and, therefore, by a reduction in the potential for the renovation of populations [11,14]. Alternatively, floating woodpeckers may actively avoid small patches through habitat selection. Bayne & Hobson [21] found a reduction in the occurrence of floaters under fragmentation conditions in the ovenbird, probably because of permanent dispersal from small fragments in search of new territories (i.e. habitat selection) rather than fragmentation increasing mortality [52]. Regardless of the mechanism (floater mortality or habitat selection), we suggest that the Allee effect in fragmented habitats may be associated with overlooked reductions in the numbers of floaters able to replace the lost breeders.
The Allee effect may also arise by a disruption in social information as patch size, and thus population size, decreases. In fact, because conspecific attraction is a widespread behavioural mechanism during habitat selection [53], small patches with no or small numbers of conspecifics may have a reduced potential to attract settlers. In an elegant experiment on the migratory least flycatcher (Empidonax minimus), Fletcher [54] demonstrated that a reduction in conspecific attraction may explain the patch size effect in fragmented landscapes. Our results on the preference of floating woodpeckers for highly populated patches provide, for the first time in a resident species, empirical support for the conspecific attraction hypothesis in fragmented landscapes. Because the effect of population size on floater occurrence remained in the models after controlling for patch size and the density of large oaks, we believe that the attraction to highly populated areas is not due to other confounding factors associated with habitat quality (e.g. the use of private information through the assessment of habitat structure). Nonetheless, the exclusion of this possibility would require experiments beyond the scope of this study.
In line with evolutionary game theory models (e.g. [17]), floating woodpeckers occupied high-quality habitat patches (i.e. large patches with high density of large oaks), suggesting that they may prefer to increase their fitness by waiting for a vacancy in a good-quality site instead of settling in a low-quality patch. On the contrary, the fact that at the population level floating woodpeckers apparently rejected low-quality sites is against classical ecological theory, which advocates for competitive displacements of low-quality birds from good breeding sites to low-quality ones. These results match those by Gołąb et al. [20] showing that (i) experimental induction of habitat degradation yielded substantial reductions in the number of floaters in the damselfly Calopteryx splendens and (ii) floating damselflies were more likely to take over high-quality territories than low-quality ones. Likewise, Walters & García [55] found that floating was a suitable strategy to acquire a high-quality territory in the cooperatively breeding red-cockaded woodpecker (Picoides borealis).
The strength of floaters for buffering the extinction risk of populations through the replacement of the lost breeders may be limited if the floating population is composed of low-quality individuals with poor fitness prospects. Recent studies, however, indicate that floating may not lead to particularly low fitness prospects. For example, although floaters exhibit relatively high mortality in the red-cockaded woodpecker [37], floating and natal helping had similar fitness prospects in terms of numbers of fledglings over the breeding lifetime [55], suggesting that floating did not lead to reductions in long-term fecundity compared with delaying dispersal. Moreover, floaters exhibited higher fitness prospects than both solitary (unpaired) territorial males and unrelated helpers that dispersed out of their natal territory, which were attached to low-quality territories [55]. In fragmented landscapes, territorial ovenbirds dispersed out of small fragments due to reduced mating and nesting success, whereas floating ovenbirds were likely to acquire a good territory when a vacancy occurred in highly populated contiguous forests [21,52]. This suggests that becoming a floater may be a better strategy than staying unpaired or mating in low-quality territories in fragmented landscapes. The presence of unpaired territorial males in small patches and the occurrence of floaters in larger ones in our study system ([23], this study) resemble the situation described for the ovenbirds. However, under which situations floating is an individual strategy that is translated into a substantial buffering of the extinction risk at the population level remains unclear.
Acknowledgements
We are grateful to the numerous volunteers who collected data, especially to M. Robles (Chano) for his logistic support and fieldwork effort since the beginning of this study. Two anonymous referees and the editors provided useful comments that improved an earlier version of the article.
Data accessibility
Data used in the analyses have been deposited in the Dryad Digital Repository, http://dx.doi.org/10.5061/dryad.f0450 [56].
Authors' contributions
H.R. developed the research questions, designed the study, collected the data, analysed the data and wrote the paper. C.C. was involved in data collection, analysing data and approval of the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
C.C. was financed by the Spanish Ministry of Education (FPU grant), and H.R. by Junta de Castilla y León, University of León, Ángeles Alvariño programme (Xunta de Galicia) and Plan I2C (Xunta de Galicia). The Spanish Ministry of Education and Science (REN 2002-03587/GLO) and Xunta de Galicia (INCITE08PXIB103259PR) partially financed this research. We especially thank M. Robles (Chano) for his financial sponsorship, particularly during the numerous years with no external funding.
References
- 1.Pettersson B. 1985. Extinction of an isolated population of the middle spotted woodpecker Dendrocopos medius (L.) in Sweden and its relation to general theories on extinction. Biol. Conserv. 32, 335–353. ( 10.1016/0006-3207(85)90022-9) [DOI] [Google Scholar]
- 2.Lande R. 1993. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 142, 911–927. ( 10.1086/285580) [DOI] [PubMed] [Google Scholar]
- 3.Pimm SL, Diamond J, Reed TM, Russell GJ, Verner J. 1993. Times to extinction for small populations of large birds. Proc. Natl Acad. Sci. USA 90, 10 871–10 875. ( 10.1073/pnas.90.22.10871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanski I, Moilanen A, Pakkala T, Kuussaari M. 1996. The quantitative incidence function model and persistence of an endangered butterfly metapopulation. Conserv. Biol. 10, 578–590. ( 10.1046/j.1523-1739.1996.10020578.x) [DOI] [Google Scholar]
- 5.Robles H, Ciudad C. 2012. Influence of habitat quality, population size, patch size, and connectivity on patch-occupancy dynamics of the middle spotted woodpecker. Conserv. Biol. 26, 284–293. ( 10.1111/j.1523-1739.2011.01816.x) [DOI] [PubMed] [Google Scholar]
- 6.Lens L, Van Dongen S, Norris K, Githiru M, Matthysen E. 2002. Avian persistence in fragmented rainforest. Science 298, 1236–1238. ( 10.1126/science.1075664) [DOI] [PubMed] [Google Scholar]
- 7.Groom MJ, Meffe GK, Carroll CR. 2006. Principles of conservation biology. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 8.Lindenmayer DB, Fischer J. 2006. Habitat fragmentation and landscape change. An ecological and conservation synthesis. Washington, DC: Island Press. [Google Scholar]
- 9.Legrand D, Cote J, Fronhofer EA, Holt RD, Ronce O, Schtickzelle N, Travis JMJ, Clobert J. 2017. Eco-evolutionary dynamics in fragmented landscapes. Ecography 40, 9–25. ( 10.1111/ecog.02537) [DOI] [Google Scholar]
- 10.Allee WC. 1931. Animal aggregations. Q. Rev. Biol. 2, 367–398. ( 10.1086/394281) [DOI] [Google Scholar]
- 11.Penteriani V, Ferrer M, Delgado MM. 2011. Floater strategies and dynamics in birds, and their importance in conservation biology: towards an understanding of nonbreeders in avian populations. Anim. Conserv. 14, 233–241. ( 10.1111/j.1469-1795.2010.00433.x) [DOI] [Google Scholar]
- 12.Penteriani V, Otalora F, Ferrer M. 2005. Floater survival affects population persistence. The role of prey availability and environmental stochasticity. Oikos 108, 523–534. ( 10.1111/j.0030-1299.2005.13514.x) [DOI] [Google Scholar]
- 13.Penteriani V, Otalora F, Ferrer M. 2006. Floater dynamics can explain positive patterns of density-dependent fecundity in animal populations. Am. Nat. 168, 697–703. [DOI] [PubMed] [Google Scholar]
- 14.Penteriani V, Otalora F, Ferrer M. 2008. Floater mortality within settlement areas can explain the Allee effect in breeding populations. Ecol. Modell. 213, 98–104. ( 10.1016/j.ecolmodel.2007.11.009) [DOI] [Google Scholar]
- 15.Moffat CB. 1903. The spring rivalry of birds: some views on the limit to multiplication. Irish. Nat. 12, 152–166. [Google Scholar]
- 16.Brown JL. 1969. Territorial behavior and population regulation in birds: a review and re-evaluation. Wilson Bull. 81, 293–329. [Google Scholar]
- 17.Kokko H, Sutherland WJ. 1998. Optimal floating and queuing strategies: consequences for density dependence and habitat loss. Am. Nat. 152, 354–366. ( 10.1086/286174) [DOI] [PubMed] [Google Scholar]
- 18.Walters JR, Crowder LB, Priddy JA. 2002. Population viability analysis for red-cockaded woodpeckers using an individual-based model. Ecol. Appl. 12, 249–260. ( 10.1890/1051-0761(2002)012%5B0249:PVAFRC%5D2.0.CO;2) [DOI] [Google Scholar]
- 19.Grimm V, Revilla E, Groeneveld J, Kramer-Schadt S, Schwager M, Tews J, Wichmann M, Jeltsch F. 2005. Importance of buffer mechanisms for population viability analysis. Conserv. Biol. 19, 578–580. ( 10.1111/j.1523-1739.2005.000163.x) [DOI] [Google Scholar]
- 20.Gołąb MJ, Sniegula S, Drobniak SM, Zajac T, Serrano-Meneses MA. 2013. Where do floaters settle? An experimental approach in odonates. Anim. Behav. 86, 1069–1075. ( 10.1016/j.anbehav.2013.09.013) [DOI] [Google Scholar]
- 21.Bayne EM, Hobson KA. 2001. Effects of habitat fragmentation on pairing success of ovenbirds: the importance of male age and floater behavior. Auk 118, 380–388. ( 10.1642/0004-8038(2001)118%5B0380:EOHFOP%5D2.0.CO;2) [DOI] [Google Scholar]
- 22.Pettersson B. 1985. Relative importance of habitat area, isolation and quality for the occurrence of middle spotted woodpecker Dendrocopos medius (L.) in Sweden. Holarct. Ecol. 8, 53–58. ( 10.1111/j.1600-0587.1985.tb01152.x) [DOI] [Google Scholar]
- 23.Robles H, Ciudad C, Vera R, Olea PP, Matthysen E. 2008. Demographic responses of middle spotted woodpeckers (Dendrocopos medius) to habitat fragmentation. Auk 125, 131–139. ( 10.1525/auk.2008.125.1.131) [DOI] [Google Scholar]
- 24.Ciudad C, Robles H, Matthysen E. 2009. Postfledging habitat selection of juvenile middle spotted woodpeckers: a multi-scale approach. Ecography 32, 676–682. ( 10.1111/j.1600-0587.2009.05806.x) [DOI] [Google Scholar]
- 25.Robles H, Olea PP. 2003. Distribución y abundancia del Pico Mediano (Dendrocopos medius) en una población meridional de la Cordillera Cantábrica. Ardeola 50, 275–280. [Google Scholar]
- 26.Robles H, Ciudad C, Vera R, Baglione V. 2007. No effect of habitat fragmentation on post-fledging, first-year and adult survival in the middle spotted woodpecker. Ecography 30, 685–694. ( 10.1111/j.2007.0906-7590.05179.x) [DOI] [Google Scholar]
- 27.Root RB. 1973. Organization of a plant–arthropod association in simple and diverse habitats: the fauna of collards. Ecol. Monogr. 45, 95–120. ( 10.2307/1942161) [DOI] [Google Scholar]
- 28.Lampila P, Mönkkönen M, Desrochers A. 2005. Demographic responses by birds to forest fragmentation. Conserv. Biol. 19, 1537–1546. ( 10.1111/j.1523-1739.2005.00201.x) [DOI] [Google Scholar]
- 29.Stamps JA. 1988. Conspecific attraction and aggregation in territorial species. Am. Nat. 131, 329–347. ( 10.1086/284793) [DOI] [Google Scholar]
- 30.Pasinelli G. 2003. Dendrocopos medius middle spotted woodpecker. BWP Update 5, 49–99. [Google Scholar]
- 31.Pasinelli G, Hegelbach J, Reyer HU. 2001. Spacing behavior of the middle spotted woodpeckers in Central Europe. J. Wildlife Manage. 65, 432–441. ( 10.2307/3803095) [DOI] [Google Scholar]
- 32.Michalek KG, Winkler H. 2001. Parental care and parentage in monogamous great spotted woodpeckers (Picoides major) and middle spotted woodpeckers (P. medius). Behaviour 138, 1259–1285. ( 10.1163/15685390152822210) [DOI] [Google Scholar]
- 33.Onrubia A, Robles H, Salas M, González-Quirós P, Olea PP. 2004. Pico Mediano, Dendrocopos medius. In Libro rojo de las aves de España (eds Madroño A, González C, Atienza JC), pp. 304–307. Madrid, Spain: Dirección General para la Biodiversidad-SEO/BirdLife. [Google Scholar]
- 34.Camprodón J, Campión D, Martínez-Vidal R, Onrubia A, Robles H, Romero JL, Senosiain A. 2007. Estatus, selección del hábitat y conservación de los pícidos ibéricos. In Conservación de la biodiversidad, fauna vertebrada y gestión forestal (eds Camprodón J, Plana E), pp. 391–434. Barcelona, Spain: Centro Tecnológico Forestal de Cataluña-Universitat de Barcelona. [Google Scholar]
- 35.Robles H, Ciudad C, Vera R, Olea PP, Purroy FJ, Matthysen E. 2007. Sylvopastoral management and conservation of the middle spotted woodpecker at the south-western edge of its distribution range. Forest Ecol. Manage. 242, 343–352. ( 10.1016/j.foreco.2007.01.052) [DOI] [Google Scholar]
- 36.Onrubia A, Robles H, Salas M, González-Quirós P, Olea PP. 2003. Pico Mediano, Dendrocopos medius. In Atlas de las aves reproductoras de España (eds Martí R, Moral JC), pp. 358–359. Madrid, Spain: Dirección General para la Biodiversidad-SEO/BirdLife. [Google Scholar]
- 37.Zeilig SL, Walters JR. 2014. Population models for social species: lessons learned from models of Red-cockaded Woodpeckers (Picoides borealis). Ecol. Appl. 24, 2144–2154. ( 10.1890/13-1275.1) [DOI] [PubMed] [Google Scholar]
- 38.Rohner C. 1996. The numerical response of great horned owls to the snowshoe hare cycle: consequences of non-territorial ‘floaters’ on demography. J. Anim. Ecol. 65, 359–370. ( 10.2307/5882) [DOI] [Google Scholar]
- 39.Hanski I. 1994. A practical model of metapopulation dynamics. J. Anim. Ecol. 63, 151–162. ( 10.2307/5591) [DOI] [Google Scholar]
- 40.Moilanen A, Hanski I. 2001. On the use of connectivity measures in spatial ecology. Oikos 95, 147–151. ( 10.1034/j.1600-0706.2001.950116.x) [DOI] [Google Scholar]
- 41.Pasinelli G. 2000. Oaks Quercus sp. and only oaks? Relations between habitat structure and home range size of the middle spotted woodpecker Dendrocopos medius. Biol. Conserv. 93, 227–235. ( 10.1016/S0006-3207(99)00137-8) [DOI] [Google Scholar]
- 42.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 43.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information-theoretic approach. Berlin, Germany: Springer. [Google Scholar]
- 44.Bartón K.2014. MuMIn: multi-model inference. R package version 1.12-2. See http://cran.r-project.org/web/packages/MuMIn/index.html .
- 45.Johnson JB, Omland KS. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108. ( 10.1016/j.tree.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 47.R Development Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 48.Dennis B, Assas L, Elaydi S, Kwessi E, Livadiotis G. 2016. Allee effects and resilience in stochastic populations. Theor. Ecol. 9, 323–335. [Google Scholar]
- 49.Lee AM, Reid JM, Beissinger SR. 2017. Modelling effects of nonbreeders on population growth estimates. J. Anim. Ecol. 86, 75–87. ( 10.1111/1365-2656.12592) [DOI] [PubMed] [Google Scholar]
- 50.Hunt WG. 1998. Raptor floaters at Moffat's equilibrium. Oikos 82, 191–197. ( 10.2307/3546929) [DOI] [Google Scholar]
- 51.Newton I. 1998. Population limitation in birds. London, UK: Academic Press. [Google Scholar]
- 52.Bayne EM, Hobson KA. 2002. Apparent survival of male ovenbirds in fragmented and forested boreal landscapes. Ecology 83, 1307–1316. ( 10.1890/0012-9658(2002)083%5B1307:ASOMOI%5D2.0.CO;2) [DOI] [Google Scholar]
- 53.Stamps JA. 2001. Habitat selection by dispersers: integrating proximate and ultimate approaches. In Dispersal (eds Clobert J, Danchin E, Dhondt AA, Nichols JD), pp. 230–242. New York, NY: Oxford University Press. [Google Scholar]
- 54.Fletcher RJ. 2009. Does conspecific attraction explain the patch-size effect? An experimental test. Oikos 118, 1139–1147. ( 10.1111/j.1600-0706.2009.17342.x) [DOI] [Google Scholar]
- 55.Walters JR, García V. 2016. Red-cockaded woodpeckers: alternative pathways to breeding success. In Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior (eds Koenig WD, Dickinson JL), pp. 58–76. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 56.Robles H, Ciudad C. 2017. Data from: Floaters may buffer the extinction risk of small populations: an empirical assessment. Dryad Digital Repository. ( 10.5061/dryad.f0450) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Robles H, Ciudad C. 2017. Data from: Floaters may buffer the extinction risk of small populations: an empirical assessment. Dryad Digital Repository. ( 10.5061/dryad.f0450) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data used in the analyses have been deposited in the Dryad Digital Repository, http://dx.doi.org/10.5061/dryad.f0450 [56].