Abstract
World experts of different disciplines, from molecular biology to macro-ecology, recognize the value of cave ecosystems as ideal ecological and evolutionary laboratories. Among other subterranean taxa, spiders stand out as intriguing model organisms for their ecological role of top predators, their unique adaptations to the hypogean medium and their sensitivity to anthropogenic disturbance. As the description of the first eyeless spider (Stalita taenaria), an array of papers on subterranean spider biology, ecology and evolution has been published, but a comprehensive review on these topics is still lacking. We provide a general overview of the spider families recorded in hypogean habitats worldwide, we review the different adaptations of hypogean spiders to subterranean life, and we summarize the information gathered so far about their origin, population structure, ecology and conservation status. Finally, we point out the limits of the knowledge we currently have regarding hypogean spiders, aiming to stimulate future research.
Keywords: Araneae, subterranean biology, evolution, hypogean habitat, adaptation
1. Background
Receiving poor energy inputs and being light-deficient, caves are generally considered extreme environments, characterized by low abundance and diversity of organisms. Because of their extraordinary adaptations, cave-dwelling animals offer unique study opportunities for pushing forward our current understanding of evolutionary and ecological processes [1,2]. Among other subterranean taxa, spiders are distinctive for their ecological role of top predators [3–5], and for the variety of functional adaptations [6], therefore representing undervalued models for the understanding the evolution of life in extreme habitats. Accordingly, subterranean spiders have served as models for physiological [7–9], ecological [4,5,10–12], ethological [13,14] and biogeographic studies [15–17], among others.
However, compared with other animal groups, the potential of spiders as model organisms is still under-expressed. It is possible that the paucity of studies is related to the difficulty of working in subterranean habitats and to the rarity of most subterranean species, which pose major impediments to data collection and analysis. In addition, the general lack of a state of the art on subterranean spider biology, ecology and evolution conceivably hinders advances in knowledge. In this review, we present a collection of information about spiders colonizing subterranean habitats—especially caves—and discuss their relevance in the understanding of cave life evolution and the ecology of subterranean animal communities.
(a). Terminology and acronyms
We use the term troglobiomorphism to distinguish the suite of adaptations, especially morphological, to subterranean life [18]. Despite the fact that ecological categorizations often oversimplify real cases and boundaries between categories can be vague, for the sake of this review, we use the traditional speleobiological nomenclature [19] to indicate ecological distributions of species:
(1) a troglobiont is strongly bound to hypogean habitats;
(2) a troglophile is able to maintain hypogean populations, but relies on epigean habitats for some biological functions;
(3) a trogloxene occurs only sporadically in a hypogean habitat and is unable to establish stable subterranean populations.
The acronym SSH refers to shallow subterranean habitats [20], i.e. the aphotic subterranean habitats close to the surface, harbouring species with troglobiomorphic traits, namely hypotelminorheic habitats, epikarst, lava tubes, deep leaf litter, soil strata and the milieu souterrain superficiel (MSS; see [21]).
We considered the terms ‘hypogean’ and ‘subterranean’ as synonyms.
2. Taxonomy and phylogeography
Spiders are a very diverse group of arthropods dating back to the Late Carboniferous [22], and comprising more than 46 500 described species [23]. They are considered one of the most successful groups of organisms in terms of evolutionary radiation [24] and ecological plasticity [25], as they have virtually colonized all terrestrial habitats, including subterranean ones.
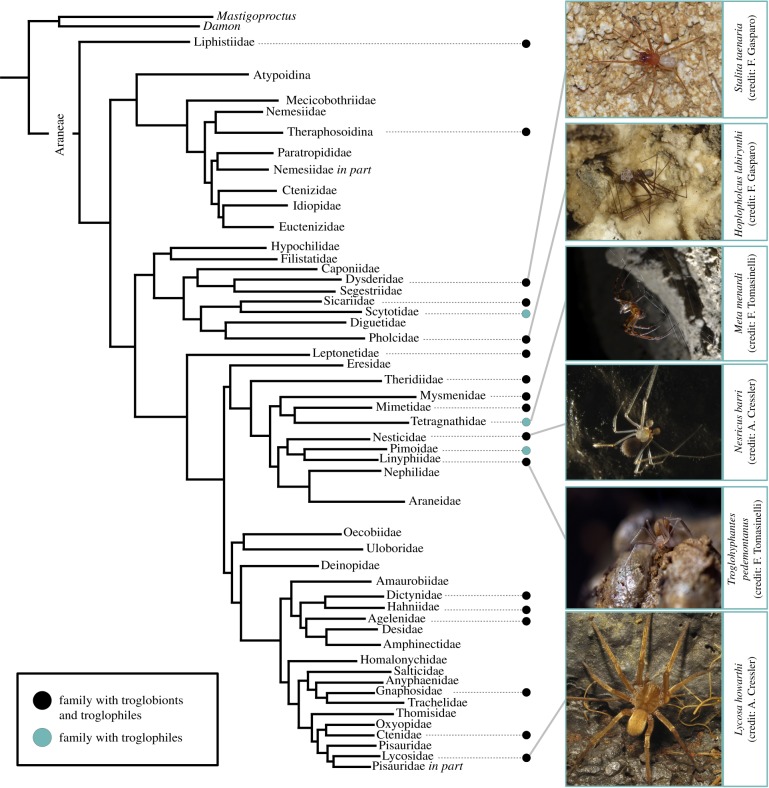
A number of spider taxa underwent remarkable diversification in subsurface habitats (figure 1), showing unique adaptations and exhibiting a wide variety of functional and morphological diversity [26,27]. The smallest spider ever described (Anapistula ataecina Cardoso & Scharff; approx. 0.4 mm [28]) as well as the largest one (Heteropoda maxima Jäger; legspan of approx. 30 cm [29]) provide two appropriate examples of the extraordinary morphological diversity of subterranean spiders.
Figure 1.
Appearance of troglobiomorphic features during the evolution of spiders. Tree topology according to [24]—not all spider families are included. (Online version in colour.)
The description of the first troglobiont spider dates back to 1847, when Schiödte (1812–1884) described Stalita taenaria (Dysderidae) from the Postojna cave in Slovenia. S. taenaria shows remarkable troglobiomorphic traits, namely the absence of eyes—‘oculi nulli’—and a pronounced cuticular depigmentation—‘abdomine niveo’ [30].
The discovery of a spider showing extraordinary adaptations to the subterranean environment, paved the way for future generations of scientists to disclose the huge biological diversity hidden in subterranean habitats around the world.
According to available estimates [26,27], around 1000 troglobiomorphic spiders have been classified as troglobionts. In addition, a countless number of species have been reported to be associated with caves. During our bibliographic survey, we recorded spiders showing subterranean adaptations in at least 48 families (table 1)—out of the 113 currently listed [23].
Table 1.
List of the spider families including troglobiont/troglophile species.
| family | distribution | notes |
|---|---|---|
| ORTOGNATHA | ||
| Barychelidae | Cuba, Dominican Republic | troglobionts in Troglothele and Trichopelma |
| Cyrtaucheniidae | Guinea | Acontius stercoricola is the only troglobiont in the family |
| Dipluridae Hexatelidae | Central and South America, Philippines, Australia New Zealand | troglophiles and troglobionts in Euargus, Harmonicon, Masteria and Troglodiplura Hexathele cavernicola is the only troglobiont in the family |
| Liphistiidae | Malaysia, Thailand | troglobionts in Liphistius |
| Microstigmatidae | Ecuador | Spelocteniza ashmolei is the only troglobiont in the family |
| Theraphosidae | Mexico, Brazil | troglobionts in Tmesiphantes and Hemirrhagus |
| LABIDOGNATHA | ||
| Agelenidae | Europe, USA, China | various troglobiomorphic genera (e.g. Coelotes, Histopona, Draconarius) |
| Amaurobiidae | Europe | some troglophiles in Amaurobius |
| Anapidae | South Africa, Korea | troglobionts in Crozetulus and Conculus |
| Austrochilidae | Tasmania | Hickmania troglodytes is the only troglophile in the family |
| Ctenidae | Dominican Republic, Java, Cuba, Australia, South Africa | troglobionts in Ciba and Janusia |
| Cybaeidae | Holartic | troglobionts in Cybaeus and Cybaeozyga |
| Cycloctenidae | Australia, Tasmania | troglobionts in Cycloctenus and Toxopsiella |
| Dyctinidae | Europe, USA, Mexico, Japan | troglobionts in Cicurina |
| Dysderidae | Mediterranean basin, Canary Islands | various degree of adaptation in several genera |
| Gnaphosidae Grandungulidae | Cuba, southeast Asia Australia, New Zealand | troglobionts in Herpyllus, Hongkongia and Micythus cave-dwelling species in various genera |
| Hahniidae | Europe | troglobionts in Hanhia, Iberina |
| Holarchaeidae | Tasmania and New Zealand | cave-dwelling species in Holarchaea |
| Leptonetidae | Holartic, Mexico, South America | numerous troglobionts and troglophiles |
| Linyphiidae | Holartic, Africa, Australia, Central America | numerous troglobionts and troglophiles |
| Liocranidae | Europe, North Africa, Canary Islands | cave-dwelling species in Brachyanillus, Liocranum and Agraecina |
| Lycosidae | Hawaii, French Polinesia | three troglobitic species in Adelocosa, Lycosa and Nukuhiva |
| Mimetidae | Sri Lanka | Mimetus strinatii is the only troglobiont in the family |
| Mysmenidae | Mexico, Tasmania, China (?) | troglobionts in Maymena and Trogloneta |
| Nesticidae | Holartic, Brazil, Africa, Oceania | numerous troglobionts and troglophiles |
| Ochyroceratidae | North and South America, Antilles, Asia, Africa | cave-dwelling species in several genera |
| Oonopidae | Mexico, Cuba, China, Ecuador, India, Iran | troglobionts in several genera (e.g. Dysderoides, Gamasomorpha, Wanops) |
| Orsolobidae | New Zealand | Anopsolobus subterraneus is the only troglobiont in the family |
| Pholcidae | Northern and Southern Hemispheres | numerous troglobiomorphic species |
| Phyxelididae | South Africa | cave-associated species and one troglobiont (Phyxelida makapanensis) |
| Pimoidae | Holartic | troglophiles especially in Pimoa |
| Prodidomidae | Galápagos Islands, Brazil | troglobiont in Lygromma |
| Scytotidae | Philippines | cave-associated species in Scytodes |
| Sicariidae Sparassidae | Africa, South America Asia, East Africa | cave-associated species and few troglobionts in Loxosceles troglobiomorphic species in Sinopoda and Berlandia |
| Stiphidiidae | Australia, Tasmania | troglobionts in several genera (e.g. Baiami, Stiphidion, Tartarus) |
| Symphytognathidae | Australia, Europe (Portugal) | troglobionts in Anapistula |
| Synotaxidae | Tasmania | species with troglobiomorphic traits in Tupua |
| Telemidae | North-Central America, Europe, Asia, South Africa | troglobionts in several genera (e.g. Apneumonella, Telema, Telemofila, Usofila) |
| Tetrablemmidae | Mexico, China, Japan, Sumatra | numerous troglophiles |
| Tetragnathidae | Holartic, Tasmania | troglophiles in Meta, Metellina, Okileucauge and Orsinome |
| Theridiidae | USA, Oceania, Europe, Galápagos Islands | troglophiles and troglobionts in various genera |
| Theridiosomatidae | Venezuela, Sri Lanka | cave-dwelling species, especially in Plato |
| Trochanteridae | Oceania | troglobionts in Desognanops and Olin |
| Trogloraptoridae | caves in Oregon (northwest USA) | monospecific family (Trogloraptor marchingtoni, troglophile) |
| Zoropsidae | Oregon (USA) | troglobionts in Phanotea and Tengella |
The appearance of troglobiomorphic features occurred multiple times during the 380 Myr of spider evolution, in families scattered all over the spider tree of life (figure 1). As well as most hypogean taxa [31], troglobiomorphic spiders represent filtered subsamples of regional epigean species pools [4]. However, there are genera and families without epigean relatives, like Trogloraptoridae [32].
In terms of foraging guilds (sensu [31]), a functional disharmony is often observed, with entire guilds being scarcely represented, if not absent, in caves—e.g. sensing web weavers, ambush hunters. Aside from the notable exception of the Iberian Peninsula [4], there are no extensive compilations about functional groups in subterranean spiders, making it difficult to draw any general pattern.
Subterranean spiders are distributed in all continents but Antarctica (table 1). As for other taxonomic groups [33], the core of the diversity is found in temperate regions—especially in the Northern Hemisphere. In the tropics, around 40 species of eyeless spiders are currently known, having been reported from caves in Central and South America [34–39], Caribbean [40], Hawaii [41], Galápagos [42], Asia and Oceania [43–46]. In this regard, cave fauna in the tropics is probably understudied [47], and it is likely that the number of tropical troglobiont spiders will grow in the near future [40].
3. Subterranean adaptations
Species under the same selective pressure evolve convergent adaptive traits. Being subjected to strong environmental filters, hypogean habitats are a remarkable example of this phenomenon [1]. The primary limiting factors for subterranean species are the lack of solar radiation and the scarcity of food resources—but see [48] for an in-depth discussion. The process of adaptation implies the evolution of well-defined and often convergent biological traits [49]. Indeed, troglobiomorphic spiders share adaptive features found in other groups of cave animals, supposedly due to the strong and similar selective pressures acting underground [1].
We here discuss the details on these adaptations, grouping them according to morphology, physiology and behaviour.
(a). Morphology
Morphological adaptations are directly related to progressive or regressive evolution. They include reduction or loss of cuticular pigments, eye regression or loss, thinning of integuments, leg elongation and heavy spination [38,39,50–59].
Data referring to the European species of Troglohyphantes (Linyphiidae) suggest that pigmentation is the first character undergoing regression in the process of subterranean adaptation [51], whereas the loss of pigment around the eyes and the progressive eye reduction represent a second step in the adaptation process [51,59]. Tentatively, this is possibly related to the minor role played in spiders by visual perception and the consequent negligible development of eye apparatus in most epigean species, a feature that could represent a pre-adaptation to life in darkness. Conversely, evidence suggests that the presence of pigments in epigean populations is selectively maintained [60]. Exceptions include spider families relying on sight for prey capture [41,53], such as Lycosidae, which in fact include only three troglobionts [46], or Salticidae, which lack troglobionts.
Compared with their epigean relatives, cave species often develop longer legs [51,53–55]. However, caution should be exercised in generalizing this pattern, as leg elongation is apparently a morphological feature depending on habitat size [20] and may not occur in spiders inhabiting small interstices—see Ecology.
An interesting related aspect refers to intraspecific variation in troglobiomorphic traits, such as eye reduction and depigmentation. Intraspecific polymorphism was documented in Neoleptoneta (Leptonetidae) [61], Cicurina (Dictynidae) [62], Troglohyphantes [63] and Porrhomma (Linyphiidae) [58,59]. Variability in troglobiomorphic traits was documented both at a regional and local scale, but a reasonable explanation for these patterns has never been provided. For example, [61] observed a range of variation between the populations of two different species of Tayshaneta (Leptonetidae) found in central Texas along a latitudinal gradient, ‘from darkly pigmented, large-eyed individuals to lightly pigmented, reduced-eyed forms to depigmented, blind individuals’. At a local scale, individuals of Kryptonesticus eremita (Simon) (Nesticidae) found in caves show variability in pigmentation patterns within a single population depending on the distance from the entrance [64].
(b). Physiology
Physiological adaptations pertain to reduction of the metabolic rate, higher resistance to starvation, alteration in circadian rhythm, reduction in fecundity, delayed maturity, slower development and the tendency to lay a smaller number of eggs, though larger [50–53,65]. However, quantitative studies aiming to shed light on these adaptations are scarce and, as far as we are aware, no studies accounted for phylogenetic effects, making difficult to generalize results across families.
Observations conducted on different species kept in captivity pointed towards a delayed maturation of juveniles and longer lifespan [51,54,65]. A reduced number of eggs/cocoons (less than 10) was also documented for several troglobionts [28,50,65–67]. For comparison, a number of eggs/eggsacs one order of magnitude higher was documented for troglophile species able to disperse outside the cave habitat [68,69]. Deeleman-Reinhnold [51] provided a comprehensive analysis on this topic focusing on the genus Troglohyphantes, comparing size and number of eggs/eggsacs in 20 regressed and non-regressed species. She observed fewer eggs in regressed species (mean = 12.3 mm versus mean = 16.8 mm in non-regressed species) of slightly larger size (mean = 0.36 mm versus mean = 0.41 mm in non-regressed species).
Experimental and field data suggest that hypogean spiders have fine-tuned their physiological tolerance to the constant and narrow temperature and relative humidity ranges of their habitats over time [70]. With the thinning of the integuments, subterranean organisms are more prone to desiccation, and thus are preferentially associated with humid microhabitats [3]. Experiments on adults and spiderlings of Lycosa howarthi Gertsch (Lycosidae) provided evidence of a pronounced sensitivity to saturation deficit [7,8].
Moreover, Novak et al. [9] tested lower lethal temperatures in various troglophiles and troglobionts, including spiders. They concluded that while troglophiles retained their ability to withstand temperature variations, most troglobionts lost their thermoregulatory mechanisms due to regressive evolution.
Although these pieces of evidence are compelling, in lack of correction for phylogeny the figures remain crude. Specifically, it remains unresolved whether differences in thermal tolerance are adaptive or are due to phylogenetic relatedness [71].
(c). Behaviour
Little is known about the behaviour of subterranean species, as in most cases the rarity of species often precludes anything but sporadic observations. Documented observations in spiders—mostly anecdotal [27]—primarily refer to reproductive behaviour [13,66,68,72]. Complex courtships were documented in troglophiles [13,68], whereas maternal care was observed in troglobiont Nesticidae [66,73]. Sociality in cave spiders was never documented [14]. Non-territorial ‘subsociality’, in the form of free movements of the spiders inside and between interconnected webs, was tentatively proposed for Goeldia zyngierae Almeida-Silva, Brescovit & Dias (Titanoecidae), a troglophile inhabiting Brazilian caves [68].
4. Origin: subterranean evolution
Available phylogenetic studies suggest that troglobiomorphy has evolved several times independently [56,61]. The origin of troglobionts is generally explained by two major theories. The theory of active colonization [74] or adaptive shift hypothesis [7], puts emphasis on the process of active colonization of the hypogean domain, being the species driven by the opportunity to occupy new, unexploited ecological niches. This theory generally refers to hypogean speciation in tropical areas [42,75].
The theory of relicts and refuges, or climate relict hypothesis [76,77], puts emphasis on long-term climatic changes, such as glaciation cycles, indicating them as the main factors prompting the colonization of the subterranean habitat, meanwhile causing the obliteration of surface-dwelling populations [78]. To date, the climate relict hypothesis has been mostly used to explain the radiation of troglobiont spiders in temperate regions [16,17,59,70,79]. However, it is worth noticing that the two theories are not incompatible, and it is likely that in some cases they both played a role in hypogean speciation.
Whichever the causes and mechanisms, the processes are similar to primary successions over time. At first, generalist pioneer species begin the colonization, being then gradually replaced by specialized and competitive elements. Subterranean habitats are characterized by a constant flux of invaders and migrants [1], with the colonization process starting from transitional zones in the vicinity of the surface [57], including the SSHs [20]. In the first phases, organisms with adaptive traits suitable for subterranean life—exaptations—are generally favoured. For example, eye reduction, depigmentation and highly developed chemoreceptors in moss-, soil- and litter-dwelling spider species [16,51,58,61,80] generally promote the colonization of subterranean habitats.
When the process of colonization advances, spiders move deeper in the subterranean domain, facing environmental changes that act as ecological filters. The attainment of mechanisms that may be more efficient in the regulation of hydric balance and metabolism, combined with the ability to carry out the entire life cycle in darkness, implies a complete adaptation to the subterranean conditions. At this point, troglophiles undergo further selective pressures, fine-tuning their adaptive traits and determining true subterranean specialization.
Segregation mechanisms hindering gene flow between epigean and hypogean populations—allopatry or parapatry—seem to play a major role in the speciation process [81]. Arnedo et al. [56] documented a compelling case of coexistence of sister-species pairs of Dysdera (Dysderidae) in lava tubes in the Canary Islands. They hypothesized that trophic segregation is the cause of the high level of sympatry between these species, supporting the only documented case of sympatric speciation within the terrestrial hypogean environment. Conversely, sympatric speciation was observed in freshwater subterranean ecosystems in Mexican cave fish [82,83] and Australian hypogean diving beetles [84,85].
5. Dispersal and genetic structuring
Troglobionts often exhibit lower physiological tolerance, which hampers their dispersal ability via non-subterranean habitats. Moreover, caves and other subterranean habitats are generally concentrated in certain geological areas—e.g. karst—and are often isolated from each other by non-suitable habitats. These considerations paved the way for the use of caves as ideal systems in which to study isolation and population structuring.
Many authors have attempted to address these issues, using crickets, beetles, crustaceans and fish [81,86]. In spiders, studies on troglophiles have often uncovered moderate gene flows between populations and relatively shallow population structures [17,69,87]—but see [88]—suggesting the existence of extra-cave dispersal [89].
When considering troglobionts a different picture emerges. Studies uncovered pronounced genetic structuring and low gene flow—if any—between cave populations, as found with Nesticidae [15,79,87,90], Linyphiidae [17] and Telemidae [91]. Although it is difficult to separate speciation from population structuring [92], the lack of gene flow supports the ‘caves as islands’ scenario sensu [90], in which dispersal is virtually absent.
However, Paquin & Hedin [62] found shared haplotypes in isolated cave populations of Cicurina, which can be interpreted either as ancestral molecular polymorphism or as ongoing gene flow across species boundaries. Similar results were obtained with Neoleptoneta [61].
In this respect, it is worth remembering that subterranean habitats are often connected through the networks of cracks, voids and other SSHs, which may enhance the dispersal of subterranean species [20,21].
6. Population structure
Generalizations on population size of subterranean species are often trivial, as it is likely to depend on the carrying capacity of the single cave system. Furthermore, caves are connected with rock fissures and other habitats inaccessible to men [20,21], which often precludes correct population estimations. However, in some circumstances, protocols for monitoring spider populations in caves have been successfully applied [89,93].
As a rule of thumb, larger populations are expected in prey-rich tropical caves rather than in temperate oligotrophic caves. Population structures in caves are usually skewed towards juveniles, with values up to two-thirds juveniles in Tetragnathidae [10,11], or even more in Dyctinidae [94]. Female-biased sex ratio was also documented in numerous species. In Troglohyphantes, adult females outnumber adult males with ratios from 2.5 : 1 to 10 : 1, or more [12,50]. In Cicurina spp., the estimated ratio of adult females/males is around 10 : 1 [94], and in S. taenaria 4 : 1 [95]. Ratios for troglophile species align these results, as in Meta ssp. (Tetragnathidae) (from 3 : 1 to 5 : 1; [5,12]), Kryptonesticus eremita (3 : 1; [95]) and Pimoa graphitica Mammola et al. (Pimoidae) (3 : 1; [69]).
When considering subadult spiders, some evidence suggests that sexes are actually not skewed towards females [51,54]. The longer lifespan of females and the high mortality rates of males after the last moult may explain these patterns. In the case of Anapistula ataecina (Symphytognathidae), parthenogenesis was also proposed to explain strongly biased sex ratios [28], although it remains to be proven.
7. Ecology
(a). Habitat and microhabitat
Troglobiomorphic spiders are mostly reported from subterranean habitats accessible to man, such as caves and artificial sites—mines, bunkers and cellars. Moreover, other habitats such as lava tubes [3,41,56], the MSS [21] animal burrows and other dark, moist SSHs [51] (figure 2) proved to be suitable for hosting communities of subterranean spiders.
Figure 2.
Breadth of habitats colonized by subterranean spiders across the world. (a) Natural caves; (b) deep strata of mountain screes and boulder fields; (c) mines and quarries; (d) milieu souterrain superficiel (MSS); (e) man-made military fortifications; (f) cellars; (g) shallow subterranean habitats (SSH) such as deep soil and litter strata. By courtesy of: (a,c): Mauro Paschetta; (b) Federico Pianciola; (d) Jacopo Orlandini; (e,f,g) Dino Mammola. (Online version in colour.)
The spatial distribution of spiders within caves—in terms of linear distance from the entrance—appears to be related to the degree of subterranean adaptation. The less adapted species are preferentially found close to the entrance. Orb-weaver spiders are associated with cave walls and roofs of the twilight zone [96], characterizing the so-called ‘entrance spiders’ [80]. Conversely, space- and sheet-weavers and ground hunters generally colonize cave floors, especially rocky debris [97]. The presence of spiders in millimetric and centimetric interstices—MSS and other SSHs—deserves some further comments, because it can be limited by the habitat size. According to available data, such a habitat seems preferentially colonized by spiders spinning small webs or by active ground dwellers. Conversely, orb-weavers prefer wider interstices—i.e. caves [21,96,97]. In fact, large orb-weavers have also been documented in SSHs characterized by large voids, such as boulder fields [59], offering enough three-dimensional space.
(b). Predation and diet
Being top predators, spiders are preferentially found in prey-rich areas, where they maximize food intake [5,11,12,98]. Many subterranean spiders also evolved unusual foraging behaviours and feeding habits, in order to comply with the general low prey availability. In resource-deprived environments, space and sheet webs are favoured, given their high efficiency in catching prey. On the other hand, orb webs targeting flying insects are rare, and are preferentially found in the twilight zone, where the availability of flying prey is usually higher.
For the same reason, many cave lineages widen their diets and lose trophic specialization [31]. Detritivores, small-sized predators and external animals blundering underground are among the most frequent prey captured by cave spiders. Occasional captures of vertebrates by subterranean spiders have also been reported, with prey, such as fish [99], amphibians and reptiles [100].
Meta menardi well exemplifies the diet of a subterranean spider, as demonstrated by numerous studies. Arthropods comprise the bulk of the diet, but unusual prey such as Gastropoda and even conspecifics of smaller size are also consumed [5,11,12,101].
On the other hand, exceptions have been observed in Dysdera spp., which are regarded as specialist woodlouse hunters [56]. In this respect, Cardoso [4] hypothesized that Dysdera may switch from specialist to generalist during the transition from the epigean to the hypogean habitat.
(c). Cave communities and interspecific competition
The richness of spider species in caves is orders of magnitude lower than in epigean ecosystems, each cave having a limited capacity to host a considerable number of species [4]. The association of troglobiont and troglophile spiders is common, as different species usually exploit distinct ecological niches. Coexistence of multiple troglobionts in the same cave is less frequent [4,40,56,97], and often mediated by niche partitioning.
There is a consistency, across studies, in stating that the linear distance from the cave entrance is the environmental gradient over which the niche diversification is generally achieved. Whether the spatial distribution of spider species is driven by competition alone, or by its combination with other driving forces—e.g. trophic availability, microclimatic conditions—coexisting species tend to occupy distinct areas of the cave, thus reducing niche overlaps [5,12,98,102]. Other documented mechanisms enhancing niche partitioning include the trophic niche differentiation [10], spatial segregation [103], temporal niche shifts [10] and conditional differentiation [11].
8. Conservation
Hypogean fauna is highly susceptible to disturbance [1], and spiders are not an exception. In fact, recently numerous authors stressed the importance of protecting their habitats [4,67,91,93,104].
There are several intrinsic factors related to the biology of troglobionts that determine a higher vulnerability in hypogean species compared with their superficial counterparts:
(1) the small range of distribution of most species implies higher vulnerability. Eight species of subterranean spiders assessed according to the IUCN criteria [105], have often fulfilled the criteria of the threatened species;
(2) low dispersal implies a reduced ability to shift distribution in face of habitat disruption. Moreover, the genetic pool in a single subterranean population is often reduced;
(3) the physiological specialization of most troglobionts implies a higher risk of extinction, especially in case of climatic alterations—e.g. global warming [70];
(4) the reduced carrying capacity of most subterranean systems implies the occurrence of simplified ecological communities in the cave ecosystem, which leads to a higher risk of disruption of the trophic web. Moreover, most spiders are directly susceptible to variations in trophic availability, which in turn, depend upon events outside the cave [106];
(5) the population structure and reproductive peculiarity of most subterranean spider species, expose them to additional disturbance. For example, observations on Hickmania troglodytes (Higgins & Petterd) (Austrochilidae) indicated that the population abundance and mean body size in tourist caves are noticeably displaced in comparison to natural caves [107].
It is clear that the conservation of subterranean spiders is often complicated by the lack of information about their distribution and auto-ecology [66]. As a direct consequence, assessing species diversity and population abundances may be difficult, and thus determining the status of conservation of the different species.
Furthermore, the current species hypothesis regarding speciose taxa are often far for being solid [62,67,88,92,94], which is a crucial factor hindering the conservation of cryptic and restricted species [108].
Given the lack of detailed information, general measures of conservations should consider:
(1) minimizing the impacts on rare species—e.g. avoiding collecting using indiscriminate methods such as massive use of pitfall traps.
(2) minimizing the alterations of local microclimate;
(3) promoting IUCN assessment of troglobiont species [109];
(4) listing troglobiont spiders as legally protected species, in order to increase the tutelage of their habitats.
Although self-evident, it should be plainly stated that increasing the knowledge about cave spiders will in turn increase the awareness of the cave natural heritage—both in cavers and visitors of touristic caves—which will have positive feedback on their tutelage.
9. Conclusion
(1) Worldwide, approximately 1000 subterranean spiders across 48 families have been described so far (table 1). However, recent descriptions of new species, or even entire new families [32], in regions where the cave fauna is relatively well known, show that in the hypogean habitats there are still a lot of surprises in store. By some estimates, only one-third of the spider species have been described [110], and it is expected that a significant portion of this estimated diversity pertains to the hypogean ecosystems—especially in the tropics [40].
(2) The process of adaptation to the subterranean domain in spiders is only partially understood and our knowledge mostly relies on sporadic observations. Comparative studies supported by modern investigation tools—and accounting for phylogenetic effects—represent stimulating endeavours that would allow further light to be shed on this topic.
(3) In the last 20 years, the use of a molecular approach to study subterranean spiders has proven to be a promising field of research, aiming to disclose biogeographic patterns of species diversification [16,17,79], delimit species boundaries [91,92] and unravel the role of dispersal in highly isolated populations [15,90].
(4) When compared with surface ecosystems, subterranean environments exhibit lower biodiversity and simplified trophic webs, harbouring specialized biocoenoses of considerable scientific interest. In this context, the role of spiders as top-predators represents a stimulating feature to revisit classical ecological concepts such as species interactions and niche dynamics. In recent years, subterranean spiders have also proved to be effective models for macro-ecological studies at the regional scale [4], e.g. acting as potential bioindicator of the effects of global warming on subterranean communities [70].
(5) Caves are fragile ecosystems and their fauna is susceptible to disturbance and environmental alterations. While the conservation status of many caves is acceptable, it would be useful to list troglobiont spiders as legally protected species and assess their conservation status according to the latest IUCN criteria.
Acknowledgements
We are grateful to all photographers who kindly provided photos of subterranean spiders and their habitats. We thank Carles Ribera, Martina Pavlek and Pedro Cardoso for helping us to improve the quality of the manuscript. A special thank goes to Alexandra Jones for proofreading our English.
Authors' contributions
S.M. conceived the idea, led the writing, prepared figures; M.I. revised the manuscript, prepared figures. Both authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This review has no funding.
References
- 1.Culver DC, Pipan T. 2009. The biology of caves and other subterranean habitats. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Poulson TL, White WB. 1969. The cave environment. Science 165, 971–981. ( 10.1126/science.165.3897.971) [DOI] [PubMed] [Google Scholar]
- 3.Howarth FG. 1983. Ecology of cave arthropods. Annu. Rev. Entomol. 28, 365–389. ( 10.1146/annurev.en.28.010183.002053) [DOI] [Google Scholar]
- 4.Cardoso P. 2012. Diversity and community assembly patterns of epigean vs. troglobiont spiders in the Iberian Peninsula. Int. J. Speleol. 41, 83–94. ( 10.5038/1827-806X.41.1.9) [DOI] [Google Scholar]
- 5.Mammola S, Piano E, Isaia M. 2016. Step back! Niche dynamics in cave-dwelling predators. Acta Oecol. 75, 35–42. ( 10.1016/j.actao.2016.06.011) [DOI] [Google Scholar]
- 6.Cardoso P, Pekar S, Jocqueé R, Coddington JA. 2011. Global patterns of guild composition and functional diversity of spiders. PLoS ONE 6, e21710 ( 10.1371/journal.pone.0021710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howarth FG. 1980. The zoogeography of specialized cave animals: a bioclimatic model. Evolution 34, 394–406. ( 10.2307/2407402) [DOI] [PubMed] [Google Scholar]
- 8.Hadley NF, Ahearn GA, Howarth FG. 1981. Water and metabolic relations of cave-adapted and epigean lycosid spiders in Hawaii. J. Arachnol. 9, 215–222. [Google Scholar]
- 9.Novak T, Šajna N, Antolinc E, Lipovšek S, Devetak D, Janžekovič F. 2014. Cold tolerance in terrestrial invertebrates inhabiting subterranean habitats. Int. J. Speleol. 43, 265–272. ( 10.5038/1827-806X.43.3.3) [DOI] [Google Scholar]
- 10.Novak T, Tkvac T, Kuntner M, Arnett EA, Delakorda SL, Perc M, Janžekovič F. 2010. Niche partitioning in orbweaving spiders Meta menardi and Metellina merianae (Tetragnathidae). Acta Oecol. 36, 522–529. ( 10.1016/j.actao.2010.07.005) [DOI] [Google Scholar]
- 11.Mammola S, Isaia M. 2014. Niche differentiation in Meta bourneti and M. menardi (Araneae, Tetragnathidae) with notes on the life history. Int. J. Speleol. 43, 343–353. ( 10.5038/1827-806X.43.3.11) [DOI] [Google Scholar]
- 12.Mammola S, Isaia M. 2016. The ecological niche of a specialized subterranean spider. Invertebr. Biol. 135, 20–30. ( 10.1111/ivb.12113) [DOI] [Google Scholar]
- 13.Doran NE, Richardson AMM, Swain R. 2001. The reproductive behaviour of the Tasmanian cave spider Hickmania troglodytes (Araneae, Austrochilidae). J. Zool. 253, 405–418. ( 10.1017/S0952836901000371) [DOI] [Google Scholar]
- 14.Yap LM, Norma-Rashid Y, Liu F, Liu J, Li D. 2011. Comparative biology of cave-dwelling spitting spiders (Araneae: Scytodidae): parental care, cooperative prey-capture, cannibalism, natal dispersal and reproductive behaviour. Raffles Bull. Zool. 59, 269–284. [Google Scholar]
- 15.Hedin MC. 1997. Molecular phylogenetics at the population/species interface in cave spiders of the southern Appalachians (Araneae: Nesticidae: Nesticus). Mol. Biol. Evol. 14, 309–324. ( 10.1093/oxfordjournals.molbev.a025766) [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Li S, Wang R, Parquin P. 2008. Phylogeographic analysis of Pimoidae (Arachnida: Araneae) inferred from mitochondrial cytochrome c oxidase subunit I and nuclear 28S rRNA gene regions. J. Zool. Syst. Evol. Res. 46, 96–104. ( 10.1111/j.1439-0469.2007.00441.x) [DOI] [Google Scholar]
- 17.Mammola S, Isaia M, Arnedo MA. 2015. Alpine endemic spiders shed light on the origin and evolution of subterranean species. PeerJ 3, e1384. ( 10.7717/peerj.1384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christiansen KA. 1962. Proposition pour la classification des animaux cavernicoles. Spelunca 2, 75. [Google Scholar]
- 19.Sket B. 2008. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 42, 1549–1563. ( 10.1080/00222930801995762) [DOI] [Google Scholar]
- 20.Culver DC, Pipan T. 2014. Shallow subterranean habitats: ecology, evolution, and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 21.Mammola S, Giachino PM, Piano E, Jones A, Barberis M, Badino G, Isaia M. 2016. Ecology and sampling techniques of an understudied subterranean habitat: the Milieu Souterrain Superficiel (MSS). Sci. Nat. 103, 88 ( 10.1007/s00114-016-1413-9) [DOI] [PubMed] [Google Scholar]
- 22.Selden PA, Shcherbakov DE, Dunlop JA, Eskov KY. 2014. Arachnids from the Carboniferous of Russia and Ukraine, and the Permian of Kazakhstan. Paläontol. Z. 88, 297–307. ( 10.1007/s12542-013-0198-9) [DOI] [Google Scholar]
- 23.World Spider Catalog. 2017. World spider catalog. Version 17.5. Bern, Switzerland: Natural History Museum. (http://wsc.nmbe.ch) (accessed on 12 January 2017)
- 24.Garrison NL, et al. 2016. Spider phylogenomics: untangling the spider tree of life. PeerJ 4, e1719 ( 10.7717/peerj.1719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbull AL. 1973. Ecology of the true spiders (Araneomorphae). Annu. Rev. Entomol. 18, 305–348. ( 10.1146/annurev.en.18.010173.001513) [DOI] [Google Scholar]
- 26.Ribera C. 2004. Arachnida: Araneae (Spiders). In Encyclopaedia of caves and karst sciences (ed. Gunn J.), pp. 71–73. Fitzroy Dearborn: Taylor and Francis. [Google Scholar]
- 27.Reddell JR. 2005. Spiders and related groups. In Encyclopedia of caves, 2nd edn (eds Culver DC, White WB), pp. 786–797. Amsterdam, The, Netherlands: Elsvier. [Google Scholar]
- 28.Cardoso P, Scharff N. 2009. First record of the spider family Symphytognathidae in Europe and description of Anapistula ataecina sp. n. (Araneae). Zootaxa 2246, 45–57. [Google Scholar]
- 29.Jäger P. 2001. A new species of Heteropoda (Araneae: Sparassidae: Heteropodinae) from Laos—the largest huntsman spider? Zoosystema 23, 461–465. [Google Scholar]
- 30.Schiödte JC. 1847. Forelöbig Beretning om Untersögelser om den underjordiske Fauna i Hulerme i Krain og Istrien. Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhandlinger 1847, 75–81. [Google Scholar]
- 31.Gibert J, Dehrveng L. 2002. Subterranean ecosystems: a truncated functional biodiversity. Bioscience 52, 473–481. ( 10.1641/0006-3568(2002)052%5B0473:SEATFB%5D2.0.CO;2) [DOI] [Google Scholar]
- 32.Griswold CE, Audisio T, Ledford JM. 2012. An extraordinary new family of spiders from caves in the Pacific Northwest (Araneae, Trogloraptoridae, new family). ZooKeys 215, 77–102. ( 10.3897/zookeys.215.3547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culver DC, Deharveng L, Bedos A, Lewis JJ, Madden M, Reddell JR, Sket B, Trontelj P, White D. 2006. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 29, 120–128. ( 10.1111/j.2005.0906-7590.04435.x) [DOI] [Google Scholar]
- 34.Trajano E, Bichuette ME. 2009. Diversity of Brazilian subterranean invertebrates, with a list of troglomorphic taxa. Subt. Biol. 7, 1–16. [Google Scholar]
- 35.Machado EO, Ferreira RL, Brescovit AD. 2011. A new troglomorphic Metagonia Simon 1893 (Araneae, Pholcidae) from Brazil. Zootaxa 3135, 59–62. [Google Scholar]
- 36.Bertani R, Bichuette ME, Pedroso DR. 2013. Tmesiphantes hypogeus sp. nov. (Araneae, Theraphosidae), the first troglobitic tarantula from Brazil. Anais da Academia Brasileira de Ciências. 85, 235–243. ( 10.1590/S0001-37652013005000007) [DOI] [PubMed] [Google Scholar]
- 37.Candia-Ramirez DT, Valdez-Mondragon A. 2014. A new troglobitic species of the spider genus Tengella Dahl (Araneae, Tengellidae) from Chiapas, Mexico. Zootaxa 3764, 377 ( 10.11646/zootaxa.3764.3.7) [DOI] [PubMed] [Google Scholar]
- 38.Marroquín JIM. 2014. Taxonomic revision of Hemirrhagus Simon, 1903 (Araneae: Theraphosidae, Theraphosinae), with description of five new species from Mexico. Zool. J. Linn. Soc. 170, 634–689. ( 10.1111/zoj.12112) [DOI] [Google Scholar]
- 39.Pedroso DR, Baptista RLC. 2014. A new troglomorphic species of Harmonicon (Araneae, Mygalomorphae, Dipluridae) from Pará, Brazil, with notes on the genus. ZooKeys 389, 77–88. ( 10.3897/zookeys.389.6693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bloom T, Binford GA, Esposito L, Garcia GA, Peterson I, Nishida A, Loubet-Senear K, Agnarsson I. 2014. Discovery of two new species of eyeless spiders within a single Hispaniola cave. J. Arachnol. 42, 148–154. ( 10.1636/K13-84.1) [DOI] [Google Scholar]
- 41.Gertsch WJ. 1973. The cavernicolous fauna of the Hawaiian lava tubes. 3. Areaneae (Spiders). Pac. Insects 16, 415–426. [Google Scholar]
- 42.Peck SB, Finston T. 1993. Galapagos islands troglobites: the questions of tropical troglobites, parapatric distributions with eyed-sister-species, and their origin by parapatric speciation. Mém. Biospéol. 20, 19–37. [Google Scholar]
- 43.Jäger P. 2012. Asian species of the genera Anahita (Karsch 1879), Ctenus (Walckenaer 1805) and Amauropelma (Raven, Stumkat & Gray 2001) (Arachnida: Araneae: Ctenidae). Zootaxa 3429, 1–63. [Google Scholar]
- 44.Jäger P. 2012. Revision of the genus Sinopoda Jäger, 1999 in Laos with discovery of the first eyeless huntsman spider species (Sparassidae: Heteropodinae). Zootaxa 3415, 37–57. ( 10.11646/33) [DOI] [Google Scholar]
- 45.Miller JA, Rahmadi C. 2012. A troglomorphic spider from Java (Araneae, Ctenidae, Amauropelma). ZooKeys 163, 11 ( 10.3897/zookeys.163.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Framenau VW, Lehtinen PT. 2014. Nukuhiva Berland, 1935 is a troglobitic wolf spider (Araneae: Lycosidae), not a nursery-web spider (Pisauridae). Zootaxa 4028, 129–135. ( 10.11646/zootaxa.4028.1.6) [DOI] [PubMed] [Google Scholar]
- 47.Trajano E, Gallão JE, Bichuette ME. 2016. Spots of high diversity of troglobites in Brazil: the challenge of measuring subterranean diversity. Biodivers. Conserv. 25, 1805–1828. ( 10.1007/s10531-016-1151-5) [DOI] [Google Scholar]
- 48.Culver DC, Pipan T. 2016. Shifting paradigms of the evolution of cave life. Acta Carsol. 44, 415–425. ( 10.3986/ac.v44i3.1688) [DOI] [Google Scholar]
- 49.Protas M, Jeffery WR. 2012. Evolution and development in cave animals: from fish to crustaceans. Wiley Interdiscip. Rev. Dev. Biol. 1, 823–845. ( 10.1002/wdev.61) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fage L. 1931. Biospeologica LV; Araneae; précédée d'un essai sur l'Evolution souterraine et son déterminisme. Arch. Zool. Exp. Gen. 5, 99–291. [Google Scholar]
- 51.Deeleman-Reinhold CL. 1978. Revision of the cave-dwelling and related spiders of the genus Troglohyphantes Joseph (Linyphiidae), with special reference to the Jugoslav species. Op. Acad. Scient. et art. Slovenica 23, 1–221. [Google Scholar]
- 52.Deeleman-Reinhold CL, Deeleman PR. 1980. Remarks on troglobitism in spiders. In Proc. 8th Int. Congress Arachnol., Vienna, Austria (ed. H Egermann), pp. 433–438.
- 53.Gertsch WJ. 1992. Distribution patterns and speciation in North American cave spiders with a list of the troglobites and revision of the cicurinas of the subgenus Cicurella. Texas Mem. Mus. Speleol. Monogr. 3, 75–122. [Google Scholar]
- 54.Cokendolpher JC. 2004. Cicurina spiders from caves in Bexar County, Texas (Araneae: Dictynidae). Texas Mem. Mus. Speleol. Monogr. 6, 13–58. [Google Scholar]
- 55.Miller JA. 2005. Cave adaptation in the spider genus Anthrobia (Araneae, Linyphiidae, Erigoninae). Zool. Scripta. 34, 565–592. ( 10.1111/j.1463-6409.2005.00206.x) [DOI] [Google Scholar]
- 56.Arnedo MA, Oromí P, Múrria C, Macías-Hernández N, Ribera C. 2007. The dark side of an island radiation: systematics and evolution of troglobitic spiders of the genus Dysdera Latreille (Araneae : Dysderidae) in the Canary Islands. Invert. Syst. 21, 623 ( 10.1071/IS07015) [DOI] [Google Scholar]
- 57.Růžička V. 1999. The first steps in subterranean evolution of spiders (Araneae) in Central Europe. J. Nat. Hist. 33, 255–265. ( 10.1080/002229399300407) [DOI] [Google Scholar]
- 58.Růžička V, Laška V, Mikula J, Tuf IH. 2011. Morphological adaptations of Porrhomma spiders (Araneae: Linyphiidae) inhabiting soil. J. Arachnol. 39, 355–357. ( 10.1636/JOACP10-66.1) [DOI] [Google Scholar]
- 59.Růžička V, Šmilauer P, Mlejnek R. 2013. Colonization of subterranean habitats by spiders in Central Europe. Int. J. Speleol. 42, 133–140. ( 10.5038/1827-806X.42.2.5) [DOI] [Google Scholar]
- 60.Oxford GS, Gillespie RG. 1998. Evolution and ecology of spider coloration. Annu. Rev. Entomol. 43, 619–643. ( 10.1146/annurev.ento.43.1.619) [DOI] [PubMed] [Google Scholar]
- 61.Ledford J, Paquin P, Cokendolpher J, Campbell J, Griswold C. 2011. Systematics of the spider genus Neoleptoneta Brignoli, 1972 (Araneae: Leptonetidae) with a discussion of the morphology and relationships for the North American Leptonetidae. Invert. Syst. 25, 334–388. ( 10.1071/IS11014) [DOI] [Google Scholar]
- 62.Paquin P, Hedin M. 2004. The power and perils of ‘molecular taxonomy’: a case study of eyeless and endangered Cicurina (Araneae: Dictynidae) from Texas caves. Mol. Ecol. 13, 3239–3255. ( 10.1111/j.1365-294X.2004.02296.x) [DOI] [PubMed] [Google Scholar]
- 63.Isaia M, Mammola M, Mazzuca P, Arnedo MA, Pantini P. 2017. Advances in the systematics of the spider genus Troglohyphantes (Araneae, Linyphiidae). Syst. Biodivers. 15, 307–326 ( 10.1080/14772000.2016.1254304) [DOI] [Google Scholar]
- 64.Dresco E, Huber M. 1967. Étude des variations oculaires chez Nesticus eremita Simon (Araneae, Nesticidae). Arch. Zool. Exp. Gen. 108, 3–31. [Google Scholar]
- 65.Juberthie C. 1985. Cycle vital de Telema tenella dans la Grotte-Laboratoire de Moulis et strategies de reproduction chez les Araignees cavernicoles. Mém. Biospéol. 12, 77–89. [Google Scholar]
- 66.Carver LM, Perlaky P, Cressler A, Zigler KS. 2016. Reproductive seasonality in Nesticus (Araneae: Nesticidae) cave spiders. PLoS ONE 11, e0156751 ( 10.1371/journal.pone.0156751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ledford J, Paquin P, Cokendolpher J, Campbell J, Griswold C. 2012. Systematics, conservation and morphology of the spider genus Tayshaneta (Araneae, Leptonetidae) in Central Texas Caves. Zookeys 167, 1–102. ( 10.3897/zookeys.167.1833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Almeida-Silva LM, Brescovit AD, Dias SC. 2009. A new species of Goeldia (Araneae: Titanoecidae) with notes on its natural history. Zoologia (Curitiba) 26, 363–368. ( 10.1590/S1984-46702009000200021) [DOI] [Google Scholar]
- 69.Mammola S, Hormiga G, Arnedo MA, Isaia M. 2016. Unexpected diversity in the relictual European spiders of the genus Pimoa (Araneae, Pimoidae). Inv. Syst. 30, 566–587. [Google Scholar]
- 70.Mammola S, Goodacre SL, Isaia M. 2017. Climate change may drive cave spiders to extinction. Ecography 40, 1–10. ( 10.1111/ecog.02974) [DOI] [Google Scholar]
- 71.Rohlf FJ. 2006. A comment on phylogenetic correction. Evolution 60, 1509–1515. ( 10.1554/05-550.1) [DOI] [PubMed] [Google Scholar]
- 72.Ledford JM. 2004. A revision of the spider genus Calileptoneta Platnick (Araneae, Leptonetidae), with notes on morphology, natural history and biogeography. J. Arachnol. 32, 231–269. ( 10.1636/H02-41) [DOI] [Google Scholar]
- 73.Gertsch WJ. 1984. The spider family Nesticidae (Araneae) in North America, Central America, and the West Indies. Texas Memorial Mus. Bull. 31, 1–91. [Google Scholar]
- 74.Rouch R, Danielpol D. 1987. L'origine de la faune aquatique souterraine, entre le paradigme du refuge et le modèle de la colonisation active. Stygologia 3, 345–372. [Google Scholar]
- 75.Howarth FG. 1987. The evolution of non-relictual tropical troglobites. Int. J. Speleol. 16, 1–16. ( 10.5038/1827-806X.16.1.1) [DOI] [Google Scholar]
- 76.Holsinger JR. 1988. Troglobites: the evolution of cave-dwelling organisms. Am. Sci. 76, 147–153. [Google Scholar]
- 77.Botosaneanu L, Holsinger J. 1991. Some aspects concerning colonization of the subterranean realm—especially subterranean waters: a response to Rouch and Danielopol, 1987. Stygologia 6, 11–39. [Google Scholar]
- 78.Culver DC, Pipan T. 2010. Climate, abiotic factors, and the evolution of subterranean life. Acta Carsol. 39, 577–586. ( 10.3986/ac.v39i3.85) [DOI] [Google Scholar]
- 79.Zhang Y, Li S. 2013. Ancient lineage, young troglobites: recent colonization of caves by Nesticella spiders. BMC Evol. Biol. 13, 183 ( 10.1186/1471-2148-13-183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deeleman-Reinhold CL. 1981. Remarks on origin and distribution of troglobitic spiders. In Proc. 8th Int. Congress Speleol, Bowling Green, KY (ed. Barry F. Beck), pp. 305–308.
- 81.Juan C, Guzik MT, Jaume D, Cooper SJ. 2010. Evolution in caves: Darwin's ‘wrecks of ancient life’ in the molecular era. Mol. Ecol. 19, 3865–3880. ( 10.1111/j.1365-294X.2010.04759.x) [DOI] [PubMed] [Google Scholar]
- 82.Wilkens H, Hüppop K. 1986. Sympatric speciation in cave fishes? J. Zool. Syst. Evol. Res. 24, 223–230. ( 10.1111/j.1439-0469.1986.tb00630.x) [DOI] [Google Scholar]
- 83.Strecker U, Hausdorf B, Wilkens H. 2012. Parallel speciation in Astyanax cave fish (Teleostei) in Northern Mexico. Mol. Phylogenet. Evol. 62, 62–70. ( 10.1016/j.ympev.2011.09.005) [DOI] [PubMed] [Google Scholar]
- 84.Cooper SJB, Hinze S, Leys R, Watts CHS, Humphreys WF. 2002. Islands under the desert: molecular systematics and evolutionary origins of stygobitic water beetles (Coleoptera: Dytiscidae) from central Western Australia. Invert. Syst. 16, 589–598. ( 10.1071/IT01039) [DOI] [Google Scholar]
- 85.Leys R, Watts C, Cooper SJB, Humphreys WF. 2003. Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57, 2819–2834. ( 10.1111/j.0014-3820.2003.tb01523.x) [DOI] [PubMed] [Google Scholar]
- 86.Bohonak AJ. 1999. Dispersal, gene flow and population structure. Q. Rev. Biol. 74, 21–45. ( 10.1086/392950) [DOI] [PubMed] [Google Scholar]
- 87.Cesaroni D, Allegrucci G, Caccone A, Sbordoni MC, De Matthaeis E, Di Rao M, Sbordoni V. 1981. Genetic variability and divergence between populations and species of Nesticus cave spiders. Genetica 56, 81–92. ( 10.1007/BF00055410) [DOI] [PubMed] [Google Scholar]
- 88.Yao Z, Zheng G, Fu J, Li S. 2016. High endemism at cave entrances: a case study of spiders of the genus Uthina. Sci. Rep. 6, 35757 ( 10.1038/srep35757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smithers P. 2005. The early life history and dispersal of the cave spider Meta menardi (Latreille, 1804) (Araneae: Tetragnathidae). Arachnology 13, 213–216. [Google Scholar]
- 90.Snowman CV, Zigler KS, Hedin M. 2010. Caves as islands: mitochondrial phylogeography of the cave-obligate spider species Nesticus barri (araneae: Nesticidae). J. Arachnol. 38, 49–56. ( 10.1636/A09-057.1) [DOI] [Google Scholar]
- 91.Zhang Y, Li S. 2014. A spider species complex revealed high cryptic diversity in South China caves. Mol. Phylogenet. Evol. 79, 353–358. ( 10.1016/j.ympev.2014.05.017) [DOI] [PubMed] [Google Scholar]
- 92.Hedin MC. 2015. High-stakes species delimitation in eyeless cave spiders (Cicurina, Dictynidae, Araneae) from central Texas. Mol. Ecol. 24, 346–361. ( 10.1111/mec.13036) [DOI] [PubMed] [Google Scholar]
- 93.Carter J, Fowles A, Angele C. 2010. Monitoring the population of the linyphid spider Porrhomma rosenhaueri (L. Koch, 1872) (Araneae: Linyphiidae) in Lesser Garth Cave, Cardiff, UK. J. Cave Karst Sci. 37, 3–8. [Google Scholar]
- 94.Paquin P, Dupérré N. 2009. A first step towards the revision of Cicurina: redescription of type specimens of 60 troglobitic species of the subgenus Cicurella (Araneae: Dictynidae), and a first visual assessment of their distribution. Zootaxa 2002, 1–67. [Google Scholar]
- 95.Gasparo F, Thaler K. 2000. I ragni cavernicoli del Venezia Giulia (Italia nord-orientale) (Arachnida, Araneae). Atti Mem. Comm. Grotte ‘E. Boegan’. 37, 17–55. [Google Scholar]
- 96.Moseley M. 2009. Size matters: scalar phenomena and a proposal for an ecological definition of ‘cave’. J. Cave Karst Stud. 35, 89–94. [Google Scholar]
- 97.Isaia M, Paschetta M, Lana E, Pantini P, Schonhofer AL, Christian E, Badino G. 2011. Subterranean arachnids of the Western Italian Alps (Arachnida: Araneae, Opiliones, Palpigradi, Pseudoscorpiones). Torino, Italy: Museo di Scienze Naturali. [Google Scholar]
- 98.Poulson TL. 1977. A tale of two spiders. Cave Res. Found. Annual Report, 245–248.
- 99.Horstkottet J, Riidiger RR, Plath M, Jäger P. 2010. Predation by three species of spiders on a cave fish in a Mexican sulphur cave. Arachnology 15, 55–58. ( 10.13156/arac.2010.15.2.55) [DOI] [Google Scholar]
- 100.Rasalan JB, Barrion-Dupo ALA, Bicaldo PRD, Sotto MP. 2015. Spider assemblages of Puting Bato cave and surrounding karst forest environs, with additional notes on the cave-dwelling nature of Phlogiellus kwebaburdeos. Mus. Publ. Nat. Hist. 4, 18–25. [Google Scholar]
- 101.Smithers P. 2005a. The diet of the cave spider Meta menardi (Latreille 1804) (Araneae, Tetragnathidae). J. Arachnol. 33, 243–246. ( 10.1636/CT-05-2.1) [DOI] [Google Scholar]
- 102.Resende LPA, Bichuette ME. 2016. Sharing the space: coexistence among terrestrial predators in Neotropical caves. J. Nat. Hist. 50, 2107–2128. ( 10.1080/00222933.2016.1193641) [DOI] [Google Scholar]
- 103.Bourne JD. 1976. Notes préliminaires sur la distribution spatiale du Meta menardi, Triphosa dubitata, Triphosa sabaudiata, Nelima aurantiaca et Culex pipiens au sain d'un écosystéme cavernicole (Grotte de Scierce: Mte. Savoie). Int. J. Speleol. 8, 253–267. ( 10.5038/1827-806X.8.3.2) [DOI] [Google Scholar]
- 104.Lin Y, Li S. 2010. Long-legged cave spiders (Araneae, Telemidae) from Yunnan-Guizhou Plateau, southwestern China. Zootaxa 2445, 1–34. [Google Scholar]
- 105.IUCN. 2015. The IUCN Red List of Threatened Species. Version 2015-4. (http://iucnredlist.org) (accessed on 19 November 2016).
- 106.Trajano E. 2000. Cave faunas in the Atlantic tropical rain forest: composition, ecology, and conservation. Biotropica 32, 882–893. ( 10.1111/j.1744-7429.2000.tb00626.x) [DOI] [Google Scholar]
- 107.Doran NE, Kiernan K, Swain R, Richardson AMM. 1999. Hickmania troglodytes, the Tasmanian cave spider, and its potential role in cave management. J. Insect. Conserv. 3, 257–262. ( 10.1023/A:1009677531343) [DOI] [Google Scholar]
- 108.Proudlove G, Wood PJ. 2003. The blind leading the blind: cryptic subterranean species and DNA taxonomy. Trends Ecol. Evol. 18, 272–273. ( 10.1016/S0169-5347(03)00095-8) [DOI] [Google Scholar]
- 109.Cardoso P, Borges PAV, Triantis KA, Ferrández MA, Martín JL. 2011. Adapting the IUCN red listing criteria for invertebrates. Biol. Conserv. 144, 2432–2440. ( 10.1016/j.biocon.2011.06.020) [DOI] [Google Scholar]
- 110.Agnarsson I, Coddington JA, Kuntner M. 2013. Systematics—progress in the study of spider diversity and evolution. In Spider research in the 21st century: trends and perspectives (ed. Penney D.), pp. 58–111. Manchester, UK: Siri Scientific Press. [Google Scholar]