Abstract
Cohort effects can be a major source of heterogeneity and play an important role in population dynamics. Silver-spoon effects, when environmental quality at birth improves future performance regardless of the adult environment, can induce strong lagged responses on population growth. Alternatively, the external predictive adaptive response (PAR) hypothesis predicts that organisms will adjust their developmental trajectory and physiology during early life in anticipation of expected adult conditions but has rarely been assessed in wild species. We used over 40 years of detailed individual monitoring of bighorn ewes (Ovis canadensis) to quantify long-term cohort effects on survival and reproduction. We then tested both the silver-spoon and the PAR hypotheses. Cohort effects involved a strong interaction between birth and current environments: reproduction and survival were lowest for ewes that were born and lived at high population densities. This interaction, however, does not support the PAR hypothesis because individuals with matching high-density birth and adult environments had reduced fitness. Instead, individuals born at high density had overall lower lifetime fitness suggesting a silver-spoon effect. Early-life conditions can induce long-term changes in fitness components, and their effects on cohort fitness vary according to adult environment.
Keywords: cohort effects, predictive adaptive response, silver-spoon, climate, density dependence, ungulate
1. Introduction
Individual differences in life-history traits play an important role in population processes [1,2], where sex and age are important structuring factors. Sex ratio and age structure affect population dynamics because survival typically varies between sexes [3], and reproduction and survival are often strongly dependent on age [4]. Cohort effects are another source of variation and occur when environmental conditions early in life generate average differences in future performance among individuals born in different years [5]. Cohort effects have been documented in many taxa including reptiles [6–8], birds [9,10], mammals [11,12] and humans [13] and can have a strong influence on population dynamics.
Early-life conditions can have delayed consequences on fitness components [9]. For example, conditions during ontogeny of zebra finches (Taeniopygia guttata) and great tits (Parus major) permanently affect clutch size [14]. In red squirrels (Tamiasciurus hudsonicus), high density at birth reduces adult survival [11]. These permanent influences of early-life conditions on life-history traits, independent of the adult environment, have been termed silver-spoon effects [15]. They increase heterogeneity because cohorts born under more favourable environmental conditions have higher lifetime performance. For example, cohorts of red-billed choughs (Pyrrhocorax pyrrhocorax) born in favourable years fledge more offspring over their lifetime than cohorts born in unfavourable years [10]. Hence, conditions during early life can have long-term consequences on population dynamics. Models have shown that in populations with overlapping generations, delayed performance effects can increase individual differences in fitness, potentially destabilizing population dynamics [16]. Cohort effects, however, can also be influenced by environmental conditions during adulthood. For example, a favourable adult environment could mask cohort differences induced by poor early conditions. This situation has recently been referred to as ‘beneficially saturated conditions’ [17] because very favourable adult conditions may enable maximum possible performance for all individual and mask differences between cohorts.
The external predictive adaptive response (PAR) hypothesis [18] suggests, however, that if conditions during early life anticipate those likely met as adults, the early environment may adaptively shape development and physiology to anticipate predicted adult conditions. Thus, individuals encountering matching environments when young and adult should have higher fitness. In meadow voles (Microtus pennsylvanicus), maternal exposure to different photoperiods influences the development of coat thickness of newborns [19], improving fitness by matching phenotype to birth season. In zebra finches, early-life exposure to heat stress increased survival of individuals who also experienced heat stress as adults [20]. Although support for this hypothesis has been found in humans [21] and in laboratory experiments [18,19], evidence for PAR in wild animals is weak [22–24]. In roe deer (Capreolus capreolus), fitness consequences of early-life environment fit the silver-spoon better than the PAR hypothesis [25]. Similarly, in red deer (Cervus elaphus), the effect of birth density on ageing is independent of density in adulthood [4]. The assumption that early-life environmental conditions reliably predict the future environment has been questioned [26]. For long-lived species, short-term environmental and ecological fluctuations may considerably reduce the predictive power of birth environment on future environment.
Interactions between environments during adulthood and early-life can also involve developmental constraints on future plasticity. Beckerman et al. [27] found such cohort variation in the plastic response to adult environment in soil mites (Sancassania berlesei). For example, rearing conditions interacted with density in adulthood so that the response of fecundity to adult density was stronger when rearing conditions had been favourable than when they had been unfavourable. Nussey et al. [28] also found that plasticity in offspring birth weight was constrained for red deer hinds born at high density. Hence, the consequences of early-life conditions on plasticity can be adaptive, as suggested by PAR, or non-adaptive as when individuals born in harsh conditions suffer reduced lifetime performance though silver-spoon effects.
Cohort effects are most commonly caused by factors affecting the entire population during early-life, such as environmental variation. Indeed, up to 30–50% of variation in individual performance can be explained by early-life environment in large mammals [12]. In Soay sheep (Ovis aries), the North Atlantic Oscillation (NAO) index during winter is linked to cohort variation in birth mass, birth date, twinning rate and age of primiparity [29]. In red deer, the amount of rainfall near parturition interacts with forage supplementation; high rainfall leads to higher body mass in the un-supplemented population [30]. In red squirrels, cohort effects on breeding success are linked to food abundance when pups are in the nest and to spring temperature in the year of birth [11]. Another source of cohort effects is density at birth. For example, high birth density delays primiparity in Soay sheep [29] and reduces body mass in red deer [31]. Cohort effects can also be caused by prenatal conditions. Soay sheep that experience high NAO values in utero delay primiparity [29]. With ongoing climate change, understanding how climate can cause cohort effects is crucial, because directional changes in early-life conditions may allow predictions of long-term population dynamics, which may differ from those observed under previous climate regimes.
Our study has three main objectives. First, we quantify the variance in survival and in weaning success of adult bighorn ewes (Ovis canadensis) explained by cohort and identify the environmental drivers of this variability. Second, we contrast the predictions from the silver-spoon and the PAR hypotheses. PAR predicts an interaction between early and current environmental conditions: individuals experiencing similar early and adult environments have higher fitness than those with dissimilar environment. The silver-spoon hypothesis, on the other hand, predicts long-term additive effects of early environment: individuals experiencing favourable conditions early in life will have superior fitness as adults. Third, we assess whether the variation observed at the cohort level arises from individual differences in fitness or from individual plasticity [32]. We used the long-term individual monitoring programme of bighorn sheep on Ram Mountain to identify the main environmental drivers of cohort effects and their lifelong consequences on survival and weaning success. Over 40 years, this population has experienced important changes in density (figure 1), weather and climate and shown substantial variation in reproduction and survival rates. We characterized early-life environment using density, a global climate index and local weather variables. We tested for the presence of PAR by determining whether animals with matching early-life and adult environments had higher fitness.
Figure 1.
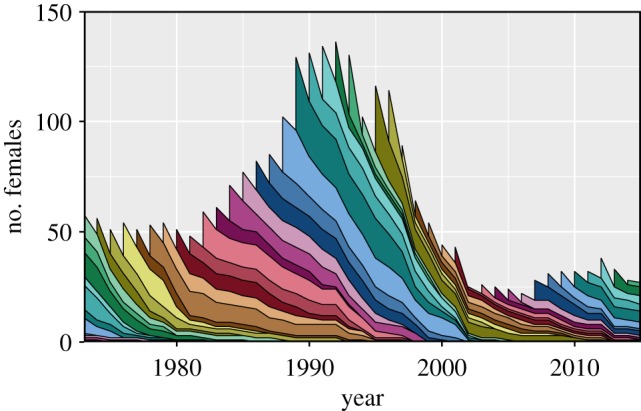
Population size and composition of female bighorn sheep at Ram Mountain between 1973 and 2014. Colour bands represent cohorts, with band height indicating the number of ewes. (Online version in colour.)
2. Method
(a). Study area and population
The study population is in Alberta, Canada (52° N, 115° W, elevation: 1080–2170 m). The study area covers about 38 km2 of alpine and subalpine habitat approximately 30 km east of the Rocky Mountains. The population has been closely monitored each year between late May and late September since 1972 [33]. Ewes are marked using visual collars. Lambs are marked with coloured ear tags, mostly within a few weeks of birth. Individuals were assigned to a cohort based on their year of birth (electronic supplementary material, table S4). Resighting probability is over 99% [34], so ewes are considered dead when not seen for a year. Because all females are marked and an exact census is made each year, we can precisely determine their survival rate. A female was considered to have weaned a lamb when the lamb survived past 15 September. She was considered unsuccessful if she either did not produce a lamb (i.e. was not seen with a lamb and was not lactating) or lost it before weaning. We restricted analyses to cohorts born from 1973 to 2005. Complete lifetime data on weaning success and survival up to 2014 are available for all individuals in these cohorts (N = 235), except for one female born in 2004 still alive in 2014 and 25 females from cohorts 1973 to 1977 which were experimentally removed [33]. We censored the last year of life of these experimentally removed ewes from analyses. Removing entirely individuals without complete life history or cohorts containing individuals with incomplete life history resulted in qualitatively identical results (results not shown); therefore, we present the results of analyses including truncated data to maximize sample size. Density was the number of females aged 2 years and older in June.
(b). Climatic and weather data
Data on precipitation (rainfall plus water equivalent of snowfall in mm) and average temperature (°C) were obtained from the Environment Canada meteorological station at Nordegg (52°30′ N, 116°03′ W, elevation: 1320 m). Local weather variables from when a cohort was in utero until its first winter were aggregated by seasons relevant to early development of bighorn sheep: winter of gestation (December–March before birth), spring (April–May during gestation), summer (June–15 September; mean birth date until approximate weaning date), autumn (mid-September to November) and first winter (December–March after birth). We calculated average daily temperature and total precipitation over each of these seasons. We used the annual mean of the Pacific Decadal Oscillation (PDO; Mantua et al. [35]) as a global climate index, characterized by shifts between warm and cool phases over decades. PDO values were obtained from http://research.jisao.washington.edu/pdo/PDO.latest and average by year.
(c). Statistical analyses
(i). Analysis of deviance
We tested for the effects of 12 early-life environment variables: total precipitation and mean temperature during winter and spring preceding birth (in utero), total precipitation and mean temperature during summer, autumn and winter after birth, annual mean PDO in the year of birth and density at birth. Variables were standardized (centred to 0 and divided by 1 standard deviation) prior to analyses. We tested the effects of standardized variables on ewe survival and probability of weaning a lamb with analysis of deviance (ANODEV) as advocated by Grosbois et al. [36], because it is more robust than likelihood ratio tests when the residual temporal variance in the focal model is high [37]. This approach also allows testing for annual variation while taking full advantage of individual-based data. The ANODEV approach is based on three hierarchical models: a constant null model with no early-life environment covariate (Mcst), a model including an early-life environment covariate of interest (Mco) and a fully time-dependent model where all possible deviance is captured by adding the year of birth as a discrete factor (Mt). We tested the linear effects of early-life environmental variables, their quadratic effects and their interactions with density by sequentially including them in the Mco model. When considering interactions between weather and density at birth, density was included in the null model (Mcst) to test for the added effect of the interaction only. To test for PAR, the Mco model included the additive effect of the environmental variable of interest both during early life, and in adulthood, as well as the interaction between the two. Further, the base model (Mcst) included the environmental variable of interest experienced as an adult and at birth without the interaction, to test the interaction between early-life and adult environments rather than just the addition of current environment. The ANODEV approach then compares the deviance of the three previously described models using the following formula:
where Dev and np are the deviance and the number of parameters of their respective models. Fcst/co/t follows a F-distribution with (np(Mco) − np(Mcst)) and (np(Mt) − np(Mco)) degrees of freedom. We also calculated the R2_Dev as described in Grosbois et al. [36] to measure effect size for early-life environment variables. This ANODEV approach was used to test the significance of the environmental covariate on both the probability to wean a lamb and survival.
(d). Probability of weaning a lamb
To test for cohort effects on the probability of weaning a lamb, we built generalized mixed effects models, including three maternal age classes (prime-aged = 2–7 years; old = 8–13; senescent = 14+) as a fixed effect [38]. Current year was added as a random effect to account for annual variability in survival and reproduction due to current environment. Ewe identity was included as a random effect to account for repeated individual responses. All models were fitted using the lme4 package (v. 1.1–10) [39] in R (v. 3.2.3) [40].
To evaluate if the interaction between current and early-life density on reproduction arose from variation between cohorts, between individuals or within-individual, we used the within-subject centring procedure of van de Pol and Wright [32]. This approach combines centring of explanatory variables within each subject with mixed effects models to partition ‘between’ from ‘within’ subject effects. We centred density within cohort, then within individual. In each case, we also applied likelihood ratio tests for variation in slope among subjects (random regression model) to determine if the response of weaning success to density varied between cohorts or between individuals. To test for the presence of selective disappearance, another mechanism that could explain variation in response to density, we added longevity as an explanatory variable [41]. A positive effect of longevity would suggest selective disappearance of individuals with low reproductive output.
(e). Survival
To test for cohort effects on annual survival probability, we first used mixed models to determine the amount of variance attributable to year of birth then tested each environmental covariate using ANODEV. Analyses of survival included ewes aged 2 years and older, to quantify only long-term effects and to use the same individuals included in the analysis of the probability of weaning a lamb. Given the limited variability in annual survival, we also looked at effects on longevity using Cox proportional hazard models. This approach also allowed us to include truncated information on 25 individuals which were experimentally removed [33]. Age was not included in these models because it was already accounted for by the baseline hazard function. When testing for PAR, current environment was included in the model as a time-dependent covariate along with the interaction with early environment. Similarly to the logistic models described previously, the deviance of these proportional hazard models was used according to the ANODEV approach to test for significant cohort effect. All models were fitted using the survival library (v. 2.39-4) in R [42]. In our approach, the many ways to quantify the environment result in a multitude of tests. Therefore, we corrected p-values for multiple testing using the Benjamini and Hochberg method [43] that controls the false discovery rate without reducing statistical power as drastically as the Bonferroni method. We used an α-level of 0.05.
3. Results
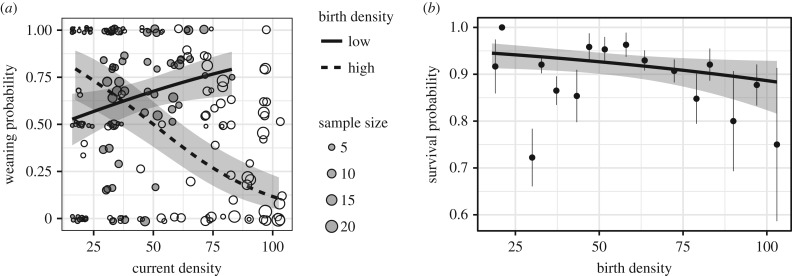
We analysed weaning success and survival of 227 ewes from 32 cohorts. Cohort identity explained 34.2% of variation in weaning success, compared to 64.0% for year and 1.4% for ewe identity. Very few effects were significant after correcting for multiple testing (electronic supplementary material, table S1), but a very strong effect of birth density on weaning success remained (p = 0.03; table 1). Birth density explained 32.1% of annual variation in weaning success, which was lower for cohorts born at higher density (slope = −0.525; CI = −0.721, −0.329). Adding the interaction between birth and current density (p < 0.001, table 1) increased the proportion of variance explained to 55.6% (electronic supplementary material, table S1). Contrary to the PAR hypothesis, however, harsh birth conditions did not increase fitness when adult conditions were also harsh (figure 2a). The weaning success of ewes born at low density increased slightly with the density they encountered as adults (slope of current density when born at lowest density = 0.682; CI = 0.372, 0.991). Ewes born at high density, however, were very sensitive to future environment and suffered a drastic reduction in weaning success when faced with high density as adults (slope of current density when born at lowest density = −1.385, CI = −1.076, −1.695). Models containing only birth or adult density reveal that birth density had a stronger effect (B = −0.54, p < 0.001) than adult density by itself (B = −0.25, p = 0.05).
Table 1.
Parameter estimates of the best model of weaning success for bighorn ewes at Ram Mountain, Canada (1975–2014). Prime-aged ewes (2–7) were used as reference.
| estimate | s.e. | Z-value | p-value | |
|---|---|---|---|---|
| intercept | 0.522 | 0.161 | 3.241 | 0.001 |
| age class (older) | −0.014 | 0.172 | −0.083 | 0.934 |
| age class (senescent) | −1.155 | 0.398 | −2.902 | 0.004 |
| current density | 0.009 | 0.141 | 0.062 | 0.950 |
| birth density | −0.268 | 0.121 | −2.211 | 0.027 |
| density interaction | −0.642 | 0.118 | −5.419 | <0.001 |
Figure 2.
(a) Weaning success of prime-aged bighorn ewes as a function of current and past density. For illustrative purposes, curves represent the 10th and 90th percentile of birth density. Points represent the observed data, grey for cohorts born above average density and clear for those born below average density. (b) Annual survival probability of prime-aged bighorn ewes as a function of birth density. Grey ribbons indicate 95% CI. Points represent mean (and s.e.) of observations binned according to birth density (bins of five individuals).
Cohort identity explained only 5.7% of variation in adult annual survival. Precipitation during the first winter as well as density explained some of the variation in adult survival between cohorts, but no early environment covariate remained significant after correcting for multiple testing (electronic supplementary material, table S2). The effect of early density on survival was weak and non-significant (B = −0.24, CI = −0.46, 0.0009; figure 2b), and it may simply be too small to be detected given our statistical power. Density at birth reduced longevity (hazard = 1.412, CI = 1.196–1.667), suggesting that cumulative small differences in yearly survival lead to shorter lifespan (electronic supplementary material, table S3). Density explained 27% of the variation in longevity between cohorts. Overall, the results showed no support for PAR.
A mixed model of weaning success as a response variable using within-subject centring revealed both within and between-subject effects of current density. When using within-subject centring by cohort, we found significant negative between-cohort effects of current density (β-between = −0.478, s.e. = 0.214, p = 0.025). On average, there was no within-cohort response to current density (β-within = −0.221, s.e. = 0.350, p = 0.530). However, we detected significant variation in the response to current density of different cohorts (Var = 1.795; χ2 = 33.897, d.f. = 2, p > 0.001, electronic supplementary material, figure S1a). Within-cohort response to current density, however, cannot distinguish between-individual effects from within-individual effects. We therefore also used within-subject centring by individual. Within-subject centring revealed significant between-individual effects of current density (β-between = −0.334, s.e. = 0.145, p = 0.021). On average, there was no within-individual response to current density (β-within = −0.078, s.e. = 0.151, p = 0.610). Unlike at the cohort level, we did not detect any significant variation in response of individuals to current density (χ2 = 0.056, d.f. = 2, p = 0.97, electronic supplementary material, figure S1b). Change within cohort and no change within individual imply a change in cohort composition through selective disappearance. Accordingly, we found a significant effect of longevity (β = 0.07, p < 0.001): individuals with low reproduction die at a younger age.
4. Discussion
Strong cohort effects are likely to cause important lag responses in population dynamics and increase individual differences in performance. Cohort explained a significant proportion of the variance in weaning success, which was only affected by density. None of the weather variables or the climate variable, PDO, significantly explained differences between cohorts in the probability of weaning a lamb. The effect of birth environment on reproduction included a strong interaction between adult and birth density. This interaction, however, was opposite in direction to the predictions of the PAR hypothesis. The probability of weaning a lamb was lowest for ewes that lived under matching high-density birth and adult environments. The effect of density on annual survival of ewes of different cohorts was weak and could only be detected through its cumulative effect on longevity. Traits with large fitness impact are expected to have lower variability due to canalization [44]. Canalization of adult survival is well documented in ungulates [45]. Additionally, selective early disappearance of unfit individual may reduce the detectability of long-term cohort effects. Overall, our results show that birth environment can strongly influence population dynamics over the lifetime of a cohort, but do not support the PAR hypothesis in this wild ungulate.
We did not find any strong effects of weather on either adult ewe survival or the probability of weaning a lamb. Spring and winter temperature and spring precipitation affect lamb survival [46]. Spring temperature also affects annual horn growth of rams [47]. Effects of weather, however, appear mostly short term. Spring temperature during the first year of life accounted for <1% of the variation in horn length of 3-year-old rams [47]. The long-term effects of spring temperature at birth on the probability of weaning a lamb were not significant after correction for multiple testing, suggesting that they are either non-existing or weak. Long-term effects of early-life conditions on fitness components may be primarily driven by other extrinsic factors such as density.
Long-term effects of early density on reproduction were highly significant. Delayed density-dependence has been documented in many ungulates [31,48,49] and plays an important role in their population dynamics. Populations showing lagged responses tend to be more variable over time [50]. Our results confirm that cohort effects can be an important mechanism by which density can have lagged effects [5,29]. Indeed, the effects of density at birth on reproduction were stronger than the effects of density in adulthood. As a consequence, delayed density dependence, driven by cohort effects, likely plays an important role in the regulation of this population. Although most studies test the effect of current density [51], given the importance of density at birth on reproduction and its marginal effect on survival, considering density at birth may be more informative. Our results underline the complexity of density effects on population growth. Indeed, the interaction between adult and birth density was highly significant in the reproduction models. Reproduction was highest for ewes that spent their entire life at low density. In addition, good conditions at birth (low density) seem to partly buffer individuals from adverse conditions later in life. Individuals born in harsh conditions (high density) benefited from no such protection and suffered reduced reproduction when faced with high density as adults. While a strict definition of silver-spoon effects implies a fixed advantage of favourable birth environment, several studies have found similar context-dependent silver-spoon effects [17,52]. Some have termed the protective effects that are more evident when adult conditions are harsh ‘beneficially saturated’ conditions [53]. Overall, our results support this protective silver-spoon effect rather than PAR [54]. Early nutrition is the main hypothesized cause of silver-spoon effects [55]. These differences impact later life due to the correlation in size from one year to the next or through the cost of increased growth. However, while high density lowers fitness, ewes born at high density appeared able to compensate for the poor start if environmental conditions improved [55]. Adult bighorn ewes show catch-up mass gain [56]. When density remains high, however, no compensation can occur and weaning success is reduced. The interaction between birth and adult environment may mask cohort effects if it is not specifically accounted for [11]. Surprisingly, when ewes were born at low density, density as an adult seemed to have a positive impact on weaning success. This unexpected result may be partly explained by the history of the population. Most of these ewes were monitored during the last 15 years of the study when density increased but remained low (figure 1). Their low weaning probability at very low density may indicate a component Allee effect [57], possibly caused in part by heavy cougar (Puma concolor) predation [58] in some recent years.
Individual plasticity appears unlikely to explain the negative effects of density on weaning success, because the within-individual effect of current density on reproduction was very small and non-significant. Further, there was no individual × environment interaction in response to current density, suggesting that all individuals had a similar response to current density and that this response was relatively weak compared to between-individual differences. These results strongly suggest a silver-spoon effect with a long-lasting impact of birth environment [54]. Apparently weak individual plasticity, however, may also be due to a lack of statistical power, because a large sample size is necessary to evaluate the variance of individual-specific slopes [59] and an adequate sample size is probably even larger for a logistic mixed model. Although we detected no individual plasticity in weaning success, we did find a significant between-individual effect as well as a within-cohort effect of current density. A change in the response of a cohort without a corresponding change in the response of its individual members is likely due to selective disappearance of weaker individuals [60], as supported by the positive association of reproduction and longevity.
The PAR hypothesis was not supported in this population, in agreement with another study testing for PAR in a wild ungulate population in France [25]. Instead, the response of ewes to birth environment supports a silver-spoon effect. In a recent meta-analysis, Uller et al. [22] found only weak support for PAR but their study did not include any mammals. Wells [26] argued that PAR is unlikely to evolve in long-lived species because the stochasticity of environmental variables makes the prediction of adult environment from maternal cues highly inaccurate. In our study, the correlation between environment in the year of birth and in adulthood was weak for all variables tested (mean = −0.04, s.d. = 0.03) except for density (0.28). These weak correlations make it unlikely that a PAR strategy would be adaptive, supporting Wells' suggestion that PAR may be a rare strategy in an unpredictable environment.
In conclusion, cohort effects in bighorn sheep ewes explained 5.7% and 34.2% of the variance in survival and reproduction, respectively. Effects of such magnitude will inevitably have important impacts on population growth. Given the longevity of bighorn sheep, the cohort effect could produce important lags in population dynamics. Our study suggests that changes in population growth are affected by complex interactions between past and present environments.
Supplementary Material
Acknowledgements
The authors are grateful to Anne Hubbs, Chiarastella Feder, Jack Hogg and Jon Jorgenson for their support of the Ram Mountain Research Program, to Mathieu Douhard and the reviewers for helpful comments on the manuscript and to all assistants and students who worked on this programme over decades.
Data accessibility
The data used for this study are available from the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.983kd [61].
Authors' contributions
F.P. and G.P. designed the study. G.P. conducted all analyses. All authors contributed to the writing and revision of the manuscript.
Competing interests
The authors declare no competing financial interests.
Funding
This work was funded by the Natural Sciences and Engineering Research Council of Canada, including Discovery Grants to F.P. and M.F.-B. and a scholarship to G.P., and the Alberta Conservation Association. F.P. holds the Canada Research Chair in Evolutionary Demography and Conservation.
References
- 1.Benton TG, Plaistow SJ, Coulson TN. 2006. Complex population dynamics and complex causation: devils, details and demography. Proc. R. Soc. B 273, 1173–1181. ( 10.1098/rspb.2006.3495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton TG. 2012. Individual variation and population dynamics: lessons from a simple system. Phil. Trans. R. Soc. B 367, 200–210. ( 10.1098/rstb.2011.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaillard JM, Festa-Bianchet M, Yoccoz NG. 1998. Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol. Evol. 13, 58–63. ( 10.1016/S0169-5347(97)01237-8) [DOI] [PubMed] [Google Scholar]
- 4.Nussey DH, Kruuk LE, Morris A, Clutton-Brock TH. 2007. Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr. Biol. 17, R1000–R1001. ( 10.1016/j.cub.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 5.Beckerman A, Benton TG, Ranta E, Kaitala V, Lundberg P. 2002. Population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 17, 263–269. ( 10.1016/S0169-5347(02)02469-2) [DOI] [Google Scholar]
- 6.Marquis O, Massot M, Le Galliard JF. 2008. Intergenerational effects of climate generate cohort variation in lizard reproductive performance. Ecology 89, 2575–2583. ( 10.1890/07-1211.1) [DOI] [PubMed] [Google Scholar]
- 7.Baron J-P, Le Galliard J-F, Tully T, Ferrière R. 2010. Cohort variation in offspring growth and survival: prenatal and postnatal factors in a late-maturing viviparous snake. J. Anim. Ecol. 79, 640–649. ( 10.1111/j.1365-2656.2010.01661.x) [DOI] [PubMed] [Google Scholar]
- 8.Le Galliard JF, Marquis O, Massot M. 2010. Cohort variation, climate effects and population dynamics in a short-lived lizard. J. Anim. Ecol. 79, 1296–1307. ( 10.1111/j.1365-2656.2010.01732.x) [DOI] [PubMed] [Google Scholar]
- 9.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 10.Reid JM, Bignal EM, Bignal S, McCracken DI, Monaghan P. 2003. Environmental variability, life-history covariation and cohort effects in the red-billed chough Pyrrhocorax pyrrhocorax. J. Anim. Ecol. 72, 36–46. ( 10.1046/j.1365-2656.2003.00673.x) [DOI] [Google Scholar]
- 11.Descamps S, Boutin S, Berteaux D, McAdam AG, Gaillard JM. 2008. Cohort effects in red squirrels: the influence of density, food abundance and temperature on future survival and reproductive success. J. Anim. Ecol. 77, 305–314. ( 10.1111/j.1365-2656.2007.01340.x) [DOI] [PubMed] [Google Scholar]
- 12.Hamel S, Gaillard J, Festa-Bianchet M, Côté S. 2009. Individual quality, early-life conditions, and reproductive success in contrasted populations of large herbivores. Ecology 90, 1981–1995. ( 10.1890/08-0596.1) [DOI] [PubMed] [Google Scholar]
- 13.Rickard IJ, Holopainen J, Helama S, Helle S, Russell AF, Lummaa V. 2010. Food availability at birth limited reproductive success in historical humans. Ecology 91, 3515–3525. ( 10.1890/10-0019.1) [DOI] [PubMed] [Google Scholar]
- 14.Haywood S, Perrins CM. 1992. Is clutch size in birds affected by environmental conditions during growth? Proc. R. Soc. Lond. B 249, 195–197. ( 10.1098/rspb.1992.0103) [DOI] [PubMed] [Google Scholar]
- 15.Grafen A. 1988. On the uses of data on lifetime reproductive success. In Reproductive success (ed. Clutton-Brock TH.), pp. 454–471. Chicago, IL: University of Chicago Press. [Google Scholar]
- 16.Lindström J, Kokko H. 2002. Cohort effects and population dynamics. Ecol. Lett. 5, 338–344. ( 10.1046/j.1461-0248.2002.00317.x) [DOI] [Google Scholar]
- 17.Krist M, Munclinger P. 2015. Context dependence of maternal effects: testing assumptions of optimal egg size, differential, and sex allocation models. Ecology 96, 2726–2736. ( 10.1890/14-2450.1) [DOI] [PubMed] [Google Scholar]
- 18.Gluckman PD, Hanson MA, Spencer HG. 2005. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533. ( 10.1016/j.tree.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 19.Lee TM, Zucker I. 1988. Vole infant development is influenced perinatally by maternal photoperiodic history. Am. J. Physiol. 255, R831–R838. [DOI] [PubMed] [Google Scholar]
- 20.Costantini D. 2013. Oxidative stress and hormetic responses in the early life of birds. In Adaptive and maladaptive aspects of developmental stress (eds Laviola G, Macrì S), pp. 257–273. New York, NY: Springer. [Google Scholar]
- 21.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. 1998. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177. ( 10.1016/S0140-6736(97)07244-9) [DOI] [PubMed] [Google Scholar]
- 22.Uller T, Nakagawa S, English S. 2013. Weak evidence for anticipatory parental effects in plants and animals. J. Evol. Biol. 26, 2161–2170. ( 10.1111/jeb.12212) [DOI] [PubMed] [Google Scholar]
- 23.Hayward AD, Lummaa V. 2013. Testing the evolutionary basis of the predictive adaptive response hypothesis in a preindustrial human population. Evol. Med. Public Health 2013, 106–117. ( 10.1093/emph/eot007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lea AJ, Altmann J, Alberts SC, Tung J. 2015. Developmental constraints in a wild primate. Am. Nat. 185, 809–821. ( 10.1086/681016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douhard M, Plard F, Gaillard J-M, Capron G, Delorme D, Klein F, Duncan P, Loe LE, Bonenfant C. 2014. Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. R. Soc. B 281, 20140276 ( 10.1098/rspb.2014.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells JC. 2007. Flaws in the theory of predictive adaptive responses. Trends Endocrinol. Metab. 18, 331–337. ( 10.1016/j.tem.2007.07.006) [DOI] [PubMed] [Google Scholar]
- 27.Beckerman AP, Benton TG, Lapsley CT, Koesters N. 2003. Talkin’ ‘bout my generation: environmental variability and cohort effects. Am. Nat. 162, 754–767. ( 10.1086/381056) [DOI] [PubMed] [Google Scholar]
- 28.Nussey DH, Clutton-Brock TH, Albon SD, Pemberton J, Kruuk LE. 2005. Constraints on plastic responses to climate variation in red deer. Biol. Lett. 1, 457–460. ( 10.1098/rsbl.2005.0352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forchhammer MC, Clutton-Brock TH, Lindström J, Albon SD. 2001. Climate and population density induce long-term cohort variation in a northern ungulate. J. Anim. Ecol. 70, 721–729. ( 10.1046/j.0021-8790.2001.00532.x) [DOI] [Google Scholar]
- 30.Rodriguez-Hidalgo P, Gortazar C, Tortosa FS, Rodriguez-Vigal C, Fierro Y, Vicente J. 2010. Effects of density, climate, and supplementary forage on body mass and pregnancy rates of female red deer in Spain. Oecologia 164, 389–398. ( 10.1007/s00442-010-1663-8) [DOI] [PubMed] [Google Scholar]
- 31.Mysterud A, Langvatn R, Yoccoz NG, Stenseth NC. 2002. Large-scale habitat variability, delayed density effects and red deer populations in Norway. J. Anim. Ecol. 71, 569–580. ( 10.1046/j.1365-2656.2002.00622.x) [DOI] [Google Scholar]
- 32.van de Pol M, Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim. Behav. 77, 753–758. ( 10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 33.Jorgenson JT, Festa-Bianchet M, Gaillard JM, Wishart WD. 1993. Harvesting bighorn ewes: consequences for population size and trophy ram production. J. Wildl. Manag. 57, 429–435. ( 10.2307/3809267) [DOI] [Google Scholar]
- 34.Jorgenson J, Festa-Bianchet M. 1997. Effects of age, sex, disease, and density on survival of bighorn sheep. Ecology 78, 1019–1032. ( 10.1890/0012-9658%281997%29078%5B1019%3AEOASDA%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 35.Mantua NJ, Hare SR, Zhang Y, Wallace JM, Francis RC. 1997. A Pacific interdecadal climate oscillation with impacts on salmon production. Bull. Am. Meteorol. Soc. 78, 1069–1079. ( 10.1175/1520-0477%281997%29078%3C;1069%3AAPICOW%3E;2.0.CO%3B2) [DOI] [Google Scholar]
- 36.Grosbois V, Gimenez O, Gaillard J-M, Pradel R, Barbraud C, Clobert J, Møller AP, Weimerskirch H. 2008. Assessing the impact of climate variation on survival in vertebrate populations. Biol. Rev. 83, 357–399. ( 10.1111/j.1469-185X.2008.00047.x) [DOI] [PubMed] [Google Scholar]
- 37.Skalski JR. 1996. Regression of abundance estimates from mark recapture surveys against environmental covariates. Can. J. Fish. Aquat. Sci. 53, 196–204. ( 10.1139/f95-169) [DOI] [Google Scholar]
- 38.Loison A, Festa-Bianchet M, Gaillard J-M, Jorgenson JT, Jullien J-M. 1999. Age-specific survival in five populations of ungulates: evidence of senescence. Ecology 80, 2539–2554. ( 10.1890/0012-9658%281999%29080%5B2539%3AASSIFP%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 39.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 40.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 41.van de Pol M, Verhulst S. 2006. Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 167, 766–773. ( 10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 42.Therneau T.2015. A package for survival analysis in S. version 2.38. See http://CRAN.R-project.org/package=survival .
- 43.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. 2001. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 125, 279–284. ( 10.2307/2346101) [DOI] [PubMed] [Google Scholar]
- 44.Stearns SC, Kawecki TJ. 1994. Fitness sensitivity and the canalization of life-history traits. Evolution 48, 1438 ( 10.2307/2410238) [DOI] [PubMed] [Google Scholar]
- 45.Gaillard J-M, Yoccoz NG. 2003. Temporal variation in survival of mammals: a case of environmental canalization? Ecology 84, 3294–3306. ( 10.1890/02-0409) [DOI] [Google Scholar]
- 46.Portier C, Festa-Bianchet M, Gaillard JM, Jorgenson JT, Yoccoz NG. 1998. Effects of density and weather on survival of bighorn sheep lambs (Ovis canadensis). J. Zool. 245, 271–278. ( 10.1111/j.1469-7998.1998.tb00101.x) [DOI] [Google Scholar]
- 47.Douhard M, Pigeon G, Festa-Bianchet M, Coltman DW, Guillemette S, Pelletier F. 2016. Environmental and evolutionary effects on horn growth of male bighorn sheep. Oikos. ( 10.1111/oik.03799) [DOI] [Google Scholar]
- 48.Festa-Bianchet M, Jorgenson J, Réale D. 2000. Early development, adult mass, and reproductive success in bighorn sheep. Behav. Ecol. 11, 633–639. ( 10.1093/beheco/11.6.633) [DOI] [Google Scholar]
- 49.Gaillard J, Loison A, Toiego C. 2003. Cohort effects and deer population dynamics. Ecoscience 10, 412–420. ( 10.1080/11956860.2003.11682789) [DOI] [Google Scholar]
- 50.Benton TG, Ranta E, Kaitala V, Beckerman AP. 2001. Maternal effects and the stability of population dynamics in noisy environments. J. Anim. Ecol. 70, 590–599. ( 10.1046/j.1365-2656.2001.00527.x) [DOI] [Google Scholar]
- 51.Gaillard J-M, Festa-Bianchet M, Yoccoz NG, Loison A, Toigo C. 2000. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 31, 367–393. ( 10.1146/annurev.ecolsys.31.1.367) [DOI] [Google Scholar]
- 52.Dziminski MA, Roberts JD. 2006. Fitness consequences of variable maternal provisioning in quacking frogs (Crinia georgiana). J. Evol. Biol. 19, 144–155. ( 10.1111/j.1420-9101.2005.00978.x) [DOI] [PubMed] [Google Scholar]
- 53.Engqvist L, Reinhold K. 2016. Adaptive trans-generational phenotypic plasticity and the lack of an experimental control in reciprocal match/mismatch experiments. Methods Ecol. Evol. 7, 1482–1488. ( 10.1111/2041-210x.12618) [DOI] [Google Scholar]
- 54.Monaghan P. 2008. Early growth conditions, phenotypic development and environmental change. Phil. Trans. R. Soc. B 363, 1635–1645. ( 10.1098/rstb.2007.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 56.Marcil-Ferland D, Festa-Bianchet M, Martin AM, Pelletier F. 2013. Despite catch-up, prolonged growth has detrimental fitness consequences in a long-lived vertebrate. Am. Nat. 182, 775–785. ( 10.1086/673534) [DOI] [PubMed] [Google Scholar]
- 57.Courchamp F, Clutton-Brock T, Grenfell B. 1999. Inverse density dependence and the Allee effect. Trends Ecol. Evol. 14, 405–410. ( 10.1016/S0169-5347(99)01683-3) [DOI] [PubMed] [Google Scholar]
- 58.Bourbeau-Lemieux A, Festa-Bianchet M, Gaillard J-M, Pelletier F. 2011. Predator-driven component Allee effects in a wild ungulate. Ecol. Lett. 14, 358–363. ( 10.1111/j.1461-0248.2011.01595.x) [DOI] [PubMed] [Google Scholar]
- 59.Martin JGA, Nussey DH, Wilson AJ, Réale D. 2011. Measuring individual differences in reaction norms in field and experimental studies: a power analysis of random regression models. Methods Ecol. Evol. 2, 362–374. ( 10.1111/j.2041-210X.2010.00084.x) [DOI] [Google Scholar]
- 60.Hayward AD, Wilson AJ, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB. 2013. Reproductive senescence in female Soay sheep: variation across traits and contributions of individual ageing and selective disappearance. Funct. Ecol. 27, 184–195. ( 10.1111/1365-2435.12029) [DOI] [Google Scholar]
- 61.Pigeon G, Festa-Bianchet M, Pelletier F. 2017. Data from: Long-term fitness consequences of early environment in a long-lived ungulate. Dryad Digital Repository. ( 10.5061/dryad.983kd) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pigeon G, Festa-Bianchet M, Pelletier F. 2017. Data from: Long-term fitness consequences of early environment in a long-lived ungulate. Dryad Digital Repository. ( 10.5061/dryad.983kd) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data used for this study are available from the Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.983kd [61].