Abstract
Articular cartilage is a physiologically non-self-renewing avascular tissue with a singular cell type, the chondrocyte, which functions as the load-bearing surface of the arthrodial joint. Injury to cartilage often progresses spatiotemporally from the articular surface to the subchondral bone, leading to development of degenerative joint diseases such as osteoarthritis (OA). Although lacking intrinsic reparative ability, articular cartilage has been shown to contain a population of stem cells or progenitor cells, similar to those found in many other adult tissues, that are thought to be involved in the maintenance of tissue homeostasis. These so-called cartilage-derived stem/progenitor cells (CSPCs) have been observed in human, equine and bovine articular cartilage, and have been identified, isolated and characterized on the basis of expression of stem-cell-related surface markers, clonogenicity and multilineage differentiation ability. However, the origin and functions of CSPCs are incompletely understood. We review here the current status of CSPC research and discuss the possible origin of these cells, what role they might have in cartilage repair, and their therapeutic potential in OA.
Introduction
In 1743, the anatomist William Hunter noted: “…from Hippocrates down to the present age, we shall find, that an ulcerated cartilage is universally allowed to be a troublesome disease… and that, when destroyed, it is never recovered.”1 Articular cartilage is matrix-rich, hypocellular and vasculature-free, a structure that enables it to function as a lubricating and load-bearing surface in the joints. Injury to cartilage often progresses spatiotemporally from the articular surface to the subchondral bone, leading to the development of degenerative joint diseases such as osteoarthritis (OA). OA is characterized by progressive loss of articular cartilage, subchondral bone sclerosis, osteophyte formation and synovial inflammation; clinical symptoms include activity limitation and pain.2 OA is the most common cause of mobility loss, severely affects quality of life, work productivity and cost of health care, and is the most prevalent form of musculoskeletal disease worldwide.3–5
Because of their ability to form multiple tissue types, stem cells are a promising candidate cell type for tissue regeneration, particularly for the repair of degenerated tissues, including articular cartilage. Although lacking intrinsic reparative ability, articular cartilage has been shown to contain a population of cells with progenitor-like qualities,6–11 similar to populations of stem cells found in many other tissues.12 These cartilage-derived stem/progenitor cells (CSPCs) have been observed in human, equine and bovine articular cartilage, and have been identified, isolated and characterized on the basis of their capacity for self-renewal, expression of stem-cell-related surface markers, and ability to differentiate along multiple lineages. However, the origin and functions of these CSPCs are incompletely understood. In this Review, we focus on the current status of CSPC research and address the following questions. Firstly, what is the origin of CSPCs? Secondly, what role might CSPCs play in cartilage repair? Finally, what is the therapeutic potential of CSPCs in OA?
Cartilage stem/progenitor cells
Stem cells in a nonreparative tissue
Stem cells are characterized principally by their ability to self-renew and differentiate along multiple lineages. Embryonic stem cells, derived from the inner cell mass of blastocysts, are termed pluripotent because of their ability to progress along the endodermal, mesodermal, and ectodermal lineages.13 On the other hand, adult stem cells, such as mesenchymal stem cells (MSCs) and haematopoietic stem cells (HSCs), are usually referred to as multipotent, because of their more limited lineage differentiation abilities.14,15 Advances in the reprogramming of adult somatic cells have generated induced pluripotent stem cells (iPSCs), which resemble embryonic stem cells in terms of their unlimited replicative activity, pluripotency and germ-line integrative ability.16
During development, it is generally assumed that the potency of stem cells becomes more restricted, and that some stem cells can exist in certain tissues as quiescent progenitor cells.17 Numerous investigations have shown that these tissue-specific stem cells are present, and that they are probably involved in the maintenance of tissue homeostasis, in many tissues.12,18,19 For example, red blood cells, with an average life span of ~100–120 days, need to be replenished from lineage-committed stem cells. Regarding cartilage, certain adult stem cell populations, such as bone-marrow-derived MSCs, are thought to have chondrogenic potential.14,15 Thus, marrow-stimulation techniques such as microfracture have been clinically developed whereby penetration of the subchondral bone enables the bone marrow to seep into the cartilage defect area to form reparative cartilage, which usually consists of a structurally inferior fibrous tissue.20 The existence of an endogenous population of progenitor or stem cells in articular cartilage that function as reparative cells, however, was considered questionable because articular cartilage is unable to heal effectively after injury.13 The lack of a perichondrium and vasculature in articular cartilage also probably contribute to the nonreparative nature of this tissue, but neither transplantation of perichondrium nor connection of bone marrow with microfracture has been found to contribute to efficient articular cartilage repair.21–24 Nonetheless, recent studies have identified, isolated and characterized a population of stem or progenitor cells from articular cartilage.6–11
Characterization of CSPCs
Although stem cells are probably present in most tissues, their isolation and verification are not easy tasks. In the case of HSCs, a well-defined combination of cell-surface antigens is required for cell tracing to identify the phenotype and function of different stem cell subpopulations.25 For stem or progenitor cells in cartilage tissue, a definitive biomarker is lacking, but a number of clues exist regarding their characteristics.
Chondrocytes, long considered the only cell type within articular cartilage, have been shown to quickly lose their ability to produce a cartilaginous extracellular matrix (ECM) after in vitro expansion,26 a phenomenon referred to as chondrocyte dedifferentiation. However, in 2003, Barbero et al.27 observed the presence of colony-forming cells in dedifferentiated adult human articular chondrocytes in culture; these cells had chondrogenic, osteogenic and adipogenic differentiation potential. The following year Alsalameh et al.28 reported the presence of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage, based on the co-expression of the cell surface markers CD105 (endoglin) and CD166 (activated leukocyte cell adhesion molecule), and that these cells were comparable to bone-marrow-derived MSCs in terms of their capacity to differentiate into adipocytes or osteocytes. Several independent research groups have since reported observations of stem or progenitor cells from human articular cartilage tissue.8,29–33
These cells were characterized according to properties of adult stem cells, such as self-renewal capacity, multilineage differentiation potential and the presence of a repertoire of MSC-related surface markers, similar to the identification of bone-marrow-derived MSCs (Figure 1). The self-renewal capacity of the cells was verified on the basis of the colony-forming unit (CFU) test7,32 and the generation of side populations (as identified by Hoechst dye exclusion on flow cytometry).6,30 The Archer group also reported longer telomere length and higher telomerase activity in the clonally derived cells, compared with chondrocytes isolated from the full depth of the articular cartilage, after many population-doublings,6 further corroborating their self-renewal ability. Multilineage potency was established via osteogenic, chondrogenic and adipogenic differentiation in vitro.28 Furthermore, as a potential cell source for cartilage repair, articular cartilage progenitor cells were comparable to bone-marrow-derived stromal cells in an equine model.34 Phenotypic analysis detected the expression of various stem-cell-related surface markers, individually and in combination, including CD9,8 CD29 (integrin β-1),32 CD44,32 CD49e (integrin α-5),6 CD54 (intercellular adhesion molecule 1),8 CD73 (5′-nucleotidase),32 CD90 (Thy-1 membrane glycoprotein),32 CD105,28,32 CD166,28,35 Notch 1 (neurogenic locus notch homologue protein 1),29,36 and STRO-1,30 among others. Another characteristic stem cell behavior, migratory ability, was observed by two different groups: Koellings et al.32 detected migratory chondrogenic progenitor cells in degenerated cartilage sites in late-stage OA, and Seol and colleagues reported that chondrogenic progenitor cells emerged and migrated to the site of injury caused by blunt impact31 or mild enzymatic insult to the ECM37 in healthy cartilage explants. Taken together, these observations suggest that CSPCs exist in adult articular cartilage tissue and might have functional roles similar to other tissue-specific stem cells.
Figure 1.
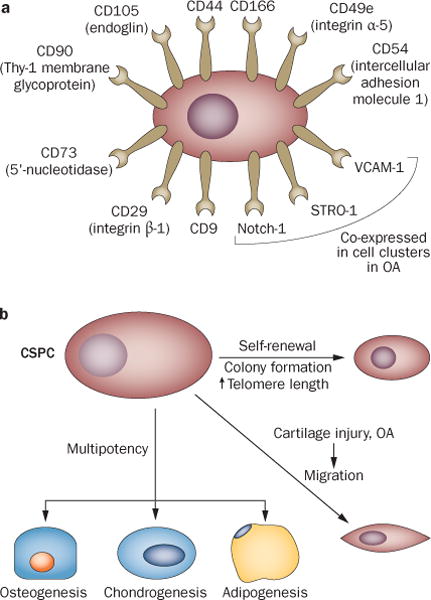
Identification and stem-cell-like properties of CSPCs. CSPCs have been observed in human, equine54 and bovine11 articular cartilage. Identification, isolation and characterization of CSPCs have been based on a | expression of stem-cell-related surface markers (individually and in combination, under various conditions) and b | properties such as clonogenicity and multipotency.6,7,8 Migratory activity of CSPCs has also been found in response to injury31 and in late-stage OA cartilage.32 Abbreviations: CSPC, cartilage stem/progenitor cell; OA, osteoarthritis.
Origin of CSPCs
CSCs and CPCs in developing cartilage
The three types of cartilaginous tissue in human body—hyaline cartilage, fibrocartilage and elastic cartilage—are distinguishable by the unique molecular composition and organization of their ECM; articular cartilage is the hyaline cartilage that covers the end of diarthrodial joints. Developmentally, most hyaline cartilage exists as a transitional stage tissue during embryonic skeletogenesis (Box 1).
Box 1. Cartilage formation during embryonic skeletogenesis.
Embryonic limb development begins with proliferation and condensation of limb mesenchymal cells, followed by their chondrogenic differentiation and production of a cartilaginous ECM to form the cartilage anlage, the model of the future long bone.74 Partitioning of the cartilage anlage forms a noncartilaginous region known as the interzone, where a process of cavitation later results in the formation of the future joint cavity. The cartilage model is then replaced by bone from the center to the distal ends via the process of endochondral ossification.75 As the interzone emerges, flattened cells appear on either side.76,77 These flattened cells become articular chondrocytes that reside at the surface of diarthrodial joints and give rise to permanent hyaline cartilage;78,79 postnatal reorganization forms the zonal structure that characterizes mature cartilage.80 CPCs were first reported by Dowthwaite et al.,46 who identified them as a subpopulation of superficial zone cells for appositional growth of bovine articular cartilage that have enhanced affinity to fibronectin and expressed high level of Notch-1.
Abbreviations: CPC, cartilage progenitor cell; ECM, extracellular matrix; Notch-1, neurogenic locus notch homologue protein 1.
The developmental origin of human CSPCs has not been well-defined because of a lack of stable markers for tracing their lineage, but some important clues are nevertheless available. Quintin et al.7 reported the isolation of cells capable of multilineage differentiation from human fetal femurs at weeks 14–16 of embryogenesis, and Wu et al.38 used microdissection to identify different subpopulations of cartilage-forming cells in human embryonic limb buds. In the latter study, two cell sub-populations present at weeks 5–6 were of particular interest: CD166low/−CD73−CD146+ cells, with the capacity to undergo multilineage differentiation, including chondro genesis; and CD166low/−CD73+CD146low/−LIN−CD44low cells, which were able to undergo only chondrogenesis.38 These findings suggest that at least two different sub populations of chondrogenic cells coexist in developing cartilage—a population of multipotent cartilage stem cells (CSCs) and a population of oligopotent chondrogenic cartilage progenitor cells (CPCs).
However, the definitive identification of CSCs and CPCs in human adult articular cartilage has remained elusive. This difficulty could be due not only to the lack of a well-defined marker to enable specific purification of these cells, but also to the change in cellular phenotype upon isolation and monolayer expansion of both normal39 and OA40,41 chondrocytes. In vitro culture has been shown to alter stem cell phenotype, and stem cell surface markers, such as Notch-1 and STRO-1, are not well maintained in monolayer cultures.30,39,42,43 Thus, although CSPCs in human cartilage are reported by different research groups,8,27,28 as yet no single cell-specific surface marker is available to directly identify CSCs or CPCs, or distinguish them from each other, either in vitro or in vivo.33
Mature articular cartilage has a zonal architecture (Box 2), with different zones characterized by variations in matrix biochemical composition44 and cell distribution.45 Cells with phenotypic and functional properties similar to mesenchymal stem cell or progenitor cell populations, such as those derived from bone marrow, have been detected mostly in the superficial zone of mature articular cartilage (Figure 2a).28,29,46,47 These cells were characterized by the expression of surface markers, including CD105,28,48,49 vascular cell adhesion protein 1 (VCAM-1, also known as CD106),26,30 CD166,8,28,48 Notch 1,9,30,36 STRO-19,30,43 and smooth-muscle actin.50 For example, Lotz et al. reported a high percentage (>45%) of cells positive for Notch-1, STRO-1 and VCAM-1 throughout normal cartilage, mostly in the superficial zone.30,51
Box 2. Zonal structure of mature articular cartilage.
The superficial zone (tangential layer) consists of two to three layers of small, flattened chondrocytes arranged parallel to the surface that produce high quantities of a specific proteoglycan lubricant (proteoglycan 4, also known as lubricin). In the middle or transitional zone, chondrocytes are spherical; in the deep or radial zone, chondrocytes are enlarged and form columns perpendicular to the joint surface. In the middle and deep zones, the cartilage ECM is composed mainly of type II collagen, aggrecan and hyaluronan. Finally, in the calcified zone, chondrocytes are hypertrophic and embedded in a calcified ECM connected to the subchondral bone, separated from the uncalcified zone by a histologically distinct structure, the tidemark.
Abbreviation: ECM, extracellular matrix.
Figure 2.
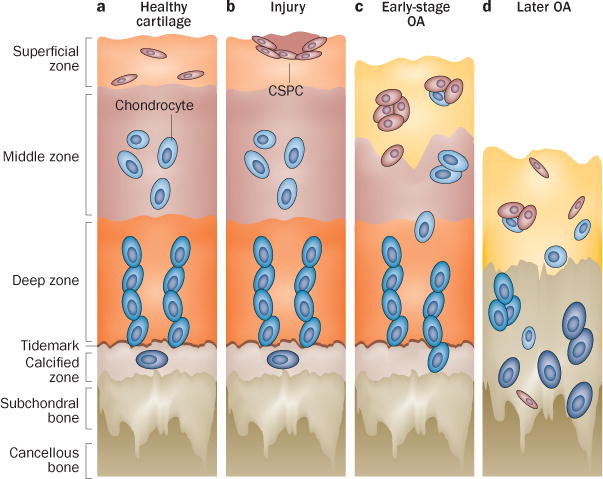
CSPC distribution in normal articular cartilage and during OA pathogenesis. Schematic of healthy and OA cartilage illustrating the zonal architecture and distribution of chondrocytes and CSPCs. a | In normal adult cartilage, Notch-1-expressing CSPCs probably reside in the superficial zone.45 b | In response to injury, spindle-shaped CSPCs appear and migrate to the injury site.31 c | In early OA, characterized by loss of the superficial zone and changes to the internal structure of articular cartilage, cells express surface markers such as CD105 and CD166,28 and cell clusters emerge, containing cells positive for stem-cell-related markers such as Notch-1, STRO-1 and VCAM-1.30,58 d | In late-stage OA, characterized by continued ECM loss and chondrocyte hypertrophy, CSPCs seem to migrate throughout the articular cartilage.32 Abbreviations: CSPC, cartilage stem/progenitor cell; ECM, extracellular matrix; Notch-1, neurogenic locus notch homologue protein 1; OA, osteoarthritis; VCAM-1, vascular cell adhesion protein 1.
Function of CSPCs in cartilage repair
In view of the ‘stem-like’ or ‘progenitor-like’ properties of CSPCs, it has been proposed that these cells probably have a role in cartilage injury and repair;31,32 however, a major caveat is the distinctive inability of hyaline cartilage to self-repair. In many other tissues, tissue-specific stem cells have been shown to contribute to tissue renewal and homeostasis. In injured muscle fibre, for example, satellite cells contribute to both myofibre repair and satellite cell repopulation.12 Although direct tracing of CSPCs has not been possible without a definitive and consistent biomarker, a number of findings strongly suggest that CSPCs respond to tissue damage, as would be expected for a chondrogenic stem-cell or progenitor-cell population.
Given the lack of a universally accepted set of bio-markers, most research groups have generally used the term ‘CPCs’ to define chondrogenic progenitor cells that respond to injury and migrate;31,37,52,53 in one particular study, migratory cells from OA cartilage were termed ‘CSPCs’.32 As discussed earlier, the distinction between CSCs and CPCs in adult cartilage remains unclear; we have thus adopted the terminology of the original studies in this Review.
CPCs in response to injury
The emergence of CPCs in a healthy cartilage explant in response to a blunt mechanical impact or scratch injury was reported by Seol et al.,31 who observed the appearance, within the ECM surrounding the injury site after 7–14 days, of a migratory cell population that showed characteristics of chondrogenic progenitor cells. Furthermore, isolated CPCs seem to respond to the biological stimuli and changes that accompany joint injury, such as ECM degradation, blood infiltration and synovitis. For example, mild enzymatic insult to the cartilage ECM promoted CPCs migration in cultured articular cartilage explants.37 The soluble factors released after injury are thought to initiate such migration, a hypothesis supported by the similar response of CPCs to dead-cell debris and to medium conditioned by injured cartilage.31 For example, high mobility group protein B1 (HMGB1) released by OA synoviocytes has been implicated as a biochemical mediator of inflammation, acting in cooperation with IL-1β.31,54 Other soluble factors could also enhance the chemotactic activity of chondrocytes, such as insulin-like growth factor 1 (IGF-1) in serum55 and platelet-derived growth factor (PDGF)55,56 in synovial fluid57 or post-traumatic bleeding.58,59 Thus, migration of CPCs is likely to be regulated by multiple tissue-injury-related signals. Interestingly, exposure to low-intensity pulsed ultrasound reportedly promotes migration of CPCs in cartilage explants,52 suggesting that both chemical and physical factors could affect CPC migration. A cell physiology study also suggested that purinergic signalling might be involved in regulating calcium oscillations in migrating CPCs and the subsequent chondrogenic differentiation of these cells.53
The functional role of the migratory cells is not clearly understood. In the study by Seol et al.,31 migratory cells harvested from the cartilage injury area produced more side populations identified by flow cytometry, and expressed higher levels of IL-6 and lower levels of cartilage ECM genes, such as collagen type II and aggrecan, compared with normal chondrocytes. The migratory cells are thus proliferative and exhibit a phenotype different from chondrocytes. Interestingly, these migratory cells also showed a high level of expression of the PRG4 gene, which encodes lubricin (proteoglycan 4), compared with bone-marrow-derived MSCs from the same donor.31 Lubricin is an important cartilage superficial zone protein responsible for joint lubrication and protection of the articular cartilage surface in the joint cavity. The functions of lubricin in the articular joint also include preventing synovial cell overgrowth and chondrocyte apoptosis.60–62 Inflammation reduces lubricin levels in both cartilage and synovium.62 In vitro and animal experiments showed that injecting lubricin into an injured joint could ‘repaint’ the joint cavity, including resurfacing of the cartilage tissue,63 thus delaying joint degeneration.64 Because PRG4 expression was higher in migratory cells than bone-marrow-derived MSCs, these migratory CSPCs probably contribute to the biological resurfacing of cartilage after injury (Figure 2b).
CSPCs in osteoarthritis
Cell proliferation and stem cell surface markers are increased in OA tissue, evidenced by the finding that the proportion of CD105+CD166+ cells in OA cartilage was ~8%, compared with ~4% in normal cartilage,28 suggesting the possible involvement of these cells in OA pathogenesis.
Other clues indicate that CSPCs are involved in all stages of OA. In early OA, cell clusters were found in and near the fissures and clefts of the articular cartilage; within these clusters, both anabolic and catabolic markers were detected12,51 and most cells expressed Notch-1, STRO-1 and VCAM-1.30,51 In proximity to the cell clusters, positive staining for these three stem cell markers was also found in the middle zone of OA cartilage.30,51 These findings suggest that CSPCs might be involved in ECM remodelling in early OA (Figure 2c), but more studies are needed to confirm this.
In late OA, Koelling et al.32 reported a migratory population of chondrogenic progenitor cells in degenerated cartilage sites. These cells were located in areas of tissue repair and could have been local cells or cells that had migrated from neighbouring bone tissue, or both, as the cartilage tidemark was broken and neovascularization underneath the cartilage tissue was evident.32 This disruption of the cartilage ECM in late OA probably alters the mode and extent of communication between the articular cartilage and the subchondral bone,65 synovium66 and other neighbouring tissues;67 perhaps these migratory cells could act as a messenger-cell population that shuttles between tissues (Figure 2d). The functional roles of CSPCs in the progression of OA are not yet understood, and studies on how these migratory cells interact with the subchondral bone and synovium, and whether they are involved in OA associated pathologies such as osteophyte formation,68 are clearly needed.
CSPCs in osteoarthritis therapy
Available evidence strongly suggests that the phenotype of CSPCs in OA correlates with the degree of ECM degeneration in the articular cartilage, but the function of these cells remains unclear and is likely to be different at different stages of OA. In early OA, which is marked by loss of the superficial zone and structural changes in the internal matrix despite an increase in cell number and the formation of cell clusters, the cells remain embedded within the ECM (Figure 3a).12,51 In later OA, the matrix undergoes further loss of organization, and the cells seem to be able to migrate freely (Figure 3b).32 Thus, any therapeutic strategies that target CSPCs must take into consideration the stage-specific nature of the cells.
Figure 3.
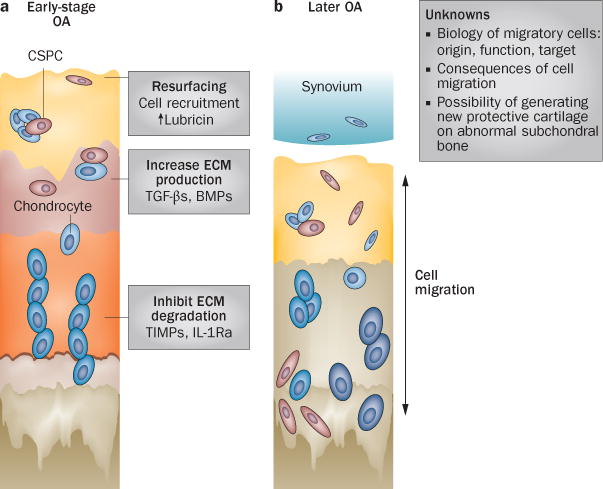
Schematic of degenerative disease progression in OA and possible CSPC-based therapies. a | Potential therapeutic strategies involving CSPCs in early OA could enhance the capacity of these cells for joint resurfacing, ECM production and chondroprotection. b | ECM loss and structural changes in late-stage OA cartilage enable cell passage, but several challenges and questions regarding the role of migratory CSPCs remain to be addressed. Abbreviations: BMP, bone morphogenic protein; CSPC, cartilage stem/progenitor cell; ECM, extracellular matrix; IL-Ra, IL-1 receptor antagonist; OA, osteoarthritis; TGF-β, transforming growth factor β; TIMP, tissue inhibitor of metalloproteinases.
In early OA, the main goal of therapeutic strategies is to preserve tissue structure and function. Given the known characteristics of CSPCs, CSPC-based strategies could aim to enhance, for instance, joint resurfacing, ECM production or chondroprotection. Firstly, targeting CSPCs to enhance joint resurfacing would recruit cells producing high amounts of lubricin to generate a new surface layer, thus replacing the degenerated tissue, as discussed above. Secondly, targeting CSPCs to enhance ECM production would promote tissue homeostasis. Although few studies on the metabolic regulation of CSPCs are available, signalling molecules such as transforming growth factor β and bone morphogenic proteins are clearly effective in enhancing chondrocyte re-differentiation and ECM production, as shown in studies on isolated CSPCs in vitro.32 Thirdly, targeting CSPCs to enhance the intrinsic chondroprotective ability of these cells through inhibition of matrix-degrading enzymes would induce, for example, the production of tissue inhibitors of metalloproteinases (TIMPs),69 inhibitors of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS),70 or anti-inflammatory cytokines such as IL-1 receptor antagonist (IL-Ra).71 Progress in one or more of these directions will help to modulate ECM turnover and delay cartilage degeneration in early OA.
In later stages of OA, the loss of cartilage ECM and the accompanying tissue structural changes result in the formation of channels from the synovium to the subchondral bone, enabling cell passage between these tissues (Figure 3B). This scenario necessitates an in-depth analysis of the biology of the migratory cells—their origins, functions and migratory tracks, and the consequences of their migration. Although the migratory ability of CSPCs is known, the ability of synovial cells, such as synovial fibroblasts, to migrate into the cartilage ECM is unknown. If uncontrolled cell migration is a consequence of or accelerates tissue disruption in later-stage OA, blocking cell migration might delay degeneration. On the other hand, if the travelling cells function in tissue rebuilding, cell migration should be promoted. As end-stage OA is characterized by cartilage loss and thickening of the subchondral bone, adapting the altered tissue structure to facilitate joint movement is of critical importance, in addition to pain management. Interestingly, in some clinical cases, a very thin, protective, cartilage-like layer can be found on the surface of the abnormal subchondral bone;72 this layer was thought to result from chondrocyte aggregation with accompanying lubricin production, suggesting endogenous joint-resurfacing activity.73 Although the mechanisms responsible for this neocartilage formation in the joint environment of such late-stage OA are unknown, the involvement of CSPCs is highly probable and could be a target for OA therapy.
Conclusions
Growing evidence suggests that CSPCs are present in human articular cartilage. Although the lack of a specific cell marker prevents in vivo tracing, these cells are characterized by a self-renewal ability, differentiation multipotency and migration. In cartilage tissue, these cells actively respond to injury and OA, having different phenotypes in the early and late stages of OA. Understanding the function of CSPCs in vivo at different stages of disease is critical to the design of CSPC-based OA therapies.
Key points.
-
■
Human articular cartilage contains a population of stem or progenitor cells that can be isolated and characterized in vitro on the basis of having self-renewal, multilineage differentiation and migratory abilities
-
■
During human embryonic development, cartilage stem cells and cartilage progenitor cells represent distinct subpopulations; however, no single, specific cell marker can trace or distinguish these cell populations in vivo
-
■
Upon injury to healthy cartilage, cartilage stem/progenitor cells (CSPCs) emerge and migrate to the injury site where they are thought to participate in tissue reparative activities
-
■
CSPCs exhibit different phenotypes in the early and late stages of osteoarthritis (OA), specifically with respect to cell migratory ability
-
■
Changes in the distribution of CSPCs during OA progression suggest that these cells might be responsible for communication between the articular cartilage, subchondral bone and other joint tissues
-
■
CSPCs are a candidate therapeutic target for OA, potentially involving strategies to enhance joint resurfacing, extracellular matrix production or chondroprotection
Review criteria.
We searched the PubMed database for full-text English-language articles, with a focus on papers published since 2000. Initial searches were broad, using the terms “cartilage progenitor cells”, “chondrogenesis”, “cartilage stem cells”, “chondrocytes”, “cartilage cell clusters” and “osteoarthritis”. The retrieved papers were evaluated and appropriate articles were selected according to relevant scientific and clinical data.
Acknowledgments
The authors acknowledge research support from the Commonwealth of Pennsylvania Department of Health (SAP 4100050913), the US Department of Defense (W81XWH-10-1-0850, W81XWH-08-2-0032 and W81XWH-13-2-0052) and the NIH (1U18TR000532), and editorial assistance from N. Baker.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Both authors contributed substantially to each stage of the preparation of this manuscript for submission.
References
- 1.Hunter W. Of the structure and diseases of articulating cartilages. Phil Trans Royal Soc. 1743;470:514–521. [Google Scholar]
- 2.National Collaborating Centre for Chronic Conditions (UK) National Clinical Guideline for Care and Management in Adults. Royal College of Physicians of London; 2008. [PubMed] [Google Scholar]
- 3.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1–9. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 4.WHO. The World Health Report 2002: reducing risks, promoting healthy life. WHO; 2002. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2010–2012. MMWR Morb Mortal Wkly Rep. 2013;62:869–873. [PMC free article] [PubMed] [Google Scholar]
- 6.Williams R, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE. 2010;5:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quintin A, et al. Plasticity of fetal cartilaginous cells. Cell Transplant. 2010;19:1349–1357. doi: 10.3727/096368910X506854. [DOI] [PubMed] [Google Scholar]
- 8.Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6:R422–R432. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsson C, Lindahl A. Articular cartilage stem cell signalling. Arthritis Res Ther. 2009;11:121. doi: 10.1186/ar2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007;358:99–103. doi: 10.1016/j.bbrc.2007.04.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes AJ, Tudor D, Nowell MA, Caterson B, Hughes CE. Chondroitin sulfate sulfation motifs as putative biomarkers for isolation of articular cartilage progenitor cells. J Histochem Cytochem. 2008;56:125–138. doi: 10.1369/jhc.7A7320.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toh WS, et al. Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007;25:950–960. doi: 10.1634/stemcells.2006-0326. [DOI] [PubMed] [Google Scholar]
- 14.Chen FH, Rousche KT, Tuan RS. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 15.Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diekman BO, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:19172–19177. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 18.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 20.Jiang YZ, Zhang SF, Qi YY, Wang LL, Ouyang HW. Cell transplantation for articular cartilage defects: principles of past, present and future practice. Cell Transplant. 2010;20:593–607. doi: 10.3727/096368910X532738. [DOI] [PubMed] [Google Scholar]
- 21.Bouwmeester PS, Kuijer R, Homminga GN, Bulstra SK, Geesink RG. A retrospective analysis of two independent prospective cartilage repair studies: autogenous perichondrial grafting versus subchondral drilling 10 years post-surgery. J Orthop Res. 2002;20:267–273. doi: 10.1016/S0736-0266(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 22.Bouwmeester SJ, Beckers JM, Kuijer R, Van der Linden AJ, Bulstra SK. Long-term results of rib perichondrial grafts for repair of cartilage defects in the human knee. Int Orthop. 1997;21:313–317. doi: 10.1007/s002640050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knutsen G, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 24.Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40:325–331. doi: 10.1177/0363546511425651. [DOI] [PubMed] [Google Scholar]
- 25.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel M, et al. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10:62–70. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 27.Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315–1325. doi: 10.1002/art.10950. [DOI] [PubMed] [Google Scholar]
- 28.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 29.Hiraoka K, Grogan S, Olee T, Lotz M. Mesenchymal progenitor cells in adult human articular cartilage. Biorheology. 2006;43:447–454. [PubMed] [Google Scholar]
- 30.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seol D, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64:3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koelling S, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Tong T, Heng B, Ouyang H. Cartilage injuries: role of implantation of human stem/progenitor cells. In: Hayat MA, editor. Stem Cells and Cancer Stem Cells. Vol. 3. Springer; 2012. pp. 327–333. [Google Scholar]
- 34.McCarthy HE, Bara JJ, Brakspear K, Singhrao SK, Archer CW. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J. 2012;192:345–351. doi: 10.1016/j.tvjl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Ozbey O, Sahin Z, Acar N, Ustunel I. Distribution of CD105 and CD166 positive cells in the proximal epiphysis of developing rat humerus. Histol Histopathol. 2010;25:1437–1445. doi: 10.14670/HH-25.1437. [DOI] [PubMed] [Google Scholar]
- 36.Ustunel I, et al. The immunohistochemical localization of notch receptors and ligands in human articular cartilage, chondroprogenitor culture and ultrastructural characteristics of these progenitor cells. Acta Histochem. 2008;110:397–407. doi: 10.1016/j.acthis.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Seol D, et al. Effect of short-term enzymatic treatment on cell migration and cartilage regeneration: in vitro organ culture of bovine articular cartilage. Tissue Eng Part A. 2014;20:1087–1014. doi: 10.1089/ten.tea.2013.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, et al. Human developmental chondrogenesis as a basis for engineering chondrocytes from pluripotent stem cells. Stem Cell Reports. 2013;1:575–589. doi: 10.1016/j.stemcr.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz-Romero J, et al. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202:731–742. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes AM, et al. Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage. PLoS ONE. 2013;8:e62994. doi: 10.1371/journal.pone.0062994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dehne T, Karlsson C, Ringe J, Sittinger M, Lindahl A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res Ther. 2009;11:R133. doi: 10.1186/ar2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HJ, Choi BH, Min BH, Park SR. Changes in surface markers of human mesenchymal stem cells during the chondrogenic differentiation and dedifferentiation processes in vitro. Arthritis Rheum. 2009;60:2325–32. doi: 10.1002/art.24786. [DOI] [PubMed] [Google Scholar]
- 43.Otsuki S, et al. Tissue neogenesis and STRO-1 expression in immature and mature articular cartilage. J Orthop Res. 2010;28:96–102. doi: 10.1002/jor.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poole AR, et al. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;391(Suppl):S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 45.Grogan SP, et al. Zone-specific gene expression patterns in articular cartilage. Arthritis Rheum. 2013;65:418–428. doi: 10.1002/art.37760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dowthwaite GP, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 47.Henson FM, Bowe EA, Davies ME. Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage. 2005;13:537–544. doi: 10.1016/j.joca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Pretzel D, et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther. 2011;13:R64. doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernstein P, Sperling I, Corbeil D, Hempel U, Fickert S. Progenitor cells from cartilage—no osteoarthritis-grade-specific differences in stem cell marker expression. Biotechnol Prog. 2013;29:206–212. doi: 10.1002/btpr.1668. [DOI] [PubMed] [Google Scholar]
- 50.Hung SC, Kuo PY, Chang CF, Chen TH, Ho LL. α-smooth muscle actin expression and structure integrity in chondrogenesis of human mesenchymal stem cells. Cell Tissue Res. 2006;324:457–466. doi: 10.1007/s00441-006-0156-x. [DOI] [PubMed] [Google Scholar]
- 51.Lotz MK, et al. Cartilage cell clusters. Arthritis Rheum. 2010;62:2206–2218. doi: 10.1002/art.27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang KW, et al. Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound Med Biol. 2014;40:1177–1186. doi: 10.1016/j.ultrasmedbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matta C, et al. Purinergic signalling is required for calcium oscillations in migratory chondrogenic progenitor cells. Pflügers Arch. doi: 10.1007/s00424-014-1529-8. http://dx.doi.org/10.1007/s00424-014-1529-8. [DOI] [PubMed]
- 54.Garcia-Arnandis I, Guillen MI, Castejon MA, Gomar F, Alcaraz MJ. Haem oxygenase-1 down-regulates high mobility group box 1 and matrix metalloproteinases in osteoarthritic synoviocytes. Rheumatology (Oxford) 2010;49:854–861. doi: 10.1093/rheumatology/kep463. [DOI] [PubMed] [Google Scholar]
- 55.Joos H, Wildner A, Hogrefe C, Reichel H, Brenner RE. Interleukin-1β and tumor necrosis factor α inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res Ther. 2013;15:R119. doi: 10.1186/ar4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008;26:1407–1412. doi: 10.1002/jor.20668. [DOI] [PubMed] [Google Scholar]
- 57.Beekhuizen M, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis Cartilage. 2013;21:918–922. doi: 10.1016/j.joca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Ohnishi H, et al. Evidence for “response to injury” hypothesis. Life Sci. 1982;31:2595–2602. doi: 10.1016/0024-3205(82)90734-2. [DOI] [PubMed] [Google Scholar]
- 59.Pierce GF, et al. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995;96:1336–1350. doi: 10.1172/JCI118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waller KA, et al. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci USA. 2013;110:5852–5857. doi: 10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhee DK, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746–1755. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 63.Gleghorn JP, Jones AR, Flannery CR, Bonassar LJ. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res. 2009;27:771–777. doi: 10.1002/jor.20798. [DOI] [PubMed] [Google Scholar]
- 64.Flannery CR, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 65.Prasadam I, et al. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 2010;62:1349–1360. doi: 10.1002/art.27397. [DOI] [PubMed] [Google Scholar]
- 66.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bastiaansen-Jenniskens YM, et al. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin F2α. Arthritis Rheum. 2013;65:2070–2080. doi: 10.1002/art.37996. [DOI] [PubMed] [Google Scholar]
- 68.Blom AB, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Kafienah W, Al-Fayez F, Hollander AP, Barker MD. Inhibition of cartilage degradation: a combined tissue engineering and gene therapy approach. Arthritis Rheum. 2003;48:709–718. doi: 10.1002/art.10842. [DOI] [PubMed] [Google Scholar]
- 70.Chen P, et al. The amelioration of cartilage degeneration by ADAMTS-5 inhibitor delivered in a hyaluronic acid hydrogel. Biomaterials. 2014;35:2827–2836. doi: 10.1016/j.biomaterials.2013.12.076. [DOI] [PubMed] [Google Scholar]
- 71.Chen B, Qin J, Wang H, Magdalou J, Chen L. Effects of adenovirus-mediated bFGF, IL-1Ra and IGF-1 gene transfer on human osteoarthritic chondrocytes and osteoarthritis in rabbits. Exp Mol Med. 2010;42:684–695. doi: 10.3858/emm.2010.42.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Callaghan JJ, editor. The Adult Knee. Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 73.Zhang D, Johnson LJ, Hsu HP, Spector M. Cartilaginous deposits in subchondral bone in regions of exposed bone in osteoarthritis of the human knee: histomorphometric study of PRG4 distribution in osteoarthritic cartilage. J Orthop Res. 2007;25:873–883. doi: 10.1002/jor.20344. [DOI] [PubMed] [Google Scholar]
- 74.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 75.Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today. 2009;87:351–371. doi: 10.1002/bdrc.20167. [DOI] [PubMed] [Google Scholar]
- 76.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 77.Shum L, Coleman CM, Hatakeyama Y, Tuan RS. Morphogenesis and dysmorphogenesis of the appendicular skeleton. Birth Defects Res C Embryo Today. 2003;69:102–122. doi: 10.1002/bdrc.10012. [DOI] [PubMed] [Google Scholar]
- 78.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–155. doi: 10.1002/bdrc.10015. [DOI] [PubMed] [Google Scholar]
- 79.Pitsillides AA, Beier F. Cartilage biology in osteoarthritis—lessons from developmental biology. Nat Rev Rheumatol. 2011;7:654–663. doi: 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- 80.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–413. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]


