Abstract
Tissue engineered constructs have the potential to function as in vitro pre-clinical models of normal tissue function and disease pathogenesis for drug screening and toxicity assessment. Effective high throughput assays demand minimal systems with clearly defined performance parameters. These systems must accurately model the structure and function of the human organs and their physiological response to different stimuli. Musculoskeletal tissues present unique challenges in this respect, as they are load-bearing, matrix-rich tissues whose functionality is intimately connected to the extracellular matrix and its organization. Of particular clinical importance is the osteochondral junction, the target tissue affected in degenerative joint diseases, such as osteoarthritis (OA), which consists of hyaline articular cartilage in close interaction with subchondral bone. In this review, we present an overview of currently available in vitro three-dimensional systems for bone and cartilage tissue engineering that mimic native physiology, and the utility and limitations of these systems. Specifically, we address the need to combine bone, cartilage and other tissues to form an interactive microphysiological system (MPS) to fully capture the biological complexity and mechanical functions of the osteochondral junction of the articular joint. The potential applications of three-dimensional MPSs for musculoskeletal biology and medicine are highlighted.
Keywords: Tissue engineering, osteoarthritis, drug screening, biomaterial scaffold, bone, cartilage
Introduction. The osteochondral complex
The osteochondral complex of the articular joint is a highly organized structure formed by hyaline cartilage and subchondral bone, joined at the osteochondral junction1,2 (Figure 1). In cartilage, the uppermost superficial zone is characterized by squamous chondrocytes with collagen fibrils aligning parallel to the articular surface. In the middle/intermediate zone, rounded chondrocytes as well as collagen fibrils are less organized relative to the surface. In the deep zone, vertical columns of chondrocytes and fibrils are organized perpendicular to the articular surface. The highest concentration of proteoglycans is found in the deep zone. A wavy tidemark of basophilic matrix highlights the boundary between the deep and calcified zones (Figure 2). Collagen fibrils lengthen from the deep zone to calcified cartilage passing through the tidemark.3 Mechanically, this region transfers the forces through vertically oriented collagen fibrils.4 The hypertrophic chondrocytes in this zone are larger in size and more dispersed.5 Overall, the calcified zone marks the transition from soft cartilage to stiff subchondral bone and is important for attaching the non-calcified cartilage to bone. The subchondral bone is interdigitated with calcified cartilage except that the fibrils do not extend from the calcified zone to the bone. Immediately below the cartilage, cortical bone exhibits low porosity and vascularity, while the subchondral trabecular bone contains randomly oriented trabeculae. This physical linkage between cartilage and bone is a critical component affected in the pathogenesis of degenerative diseases such as OA.6
Figure 1.
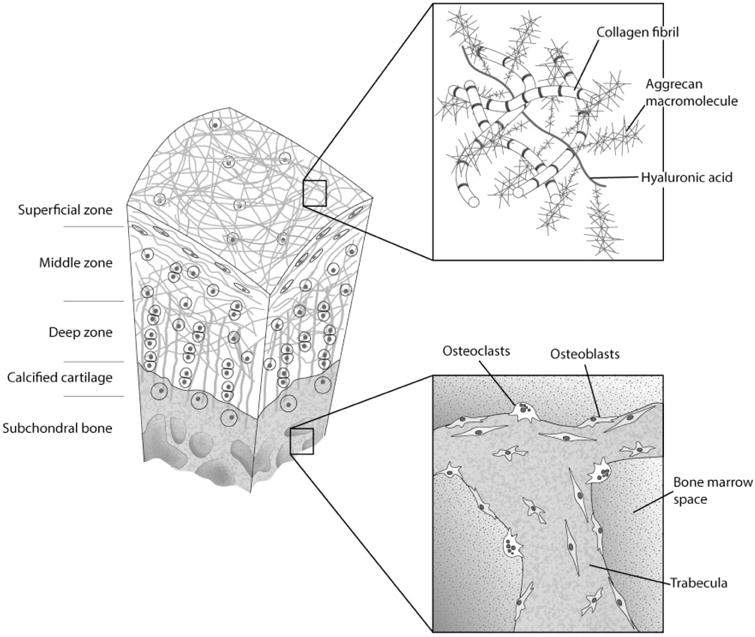
Structure of the osteochondral complex (modified from Thompson et al.7)
Figure 2.
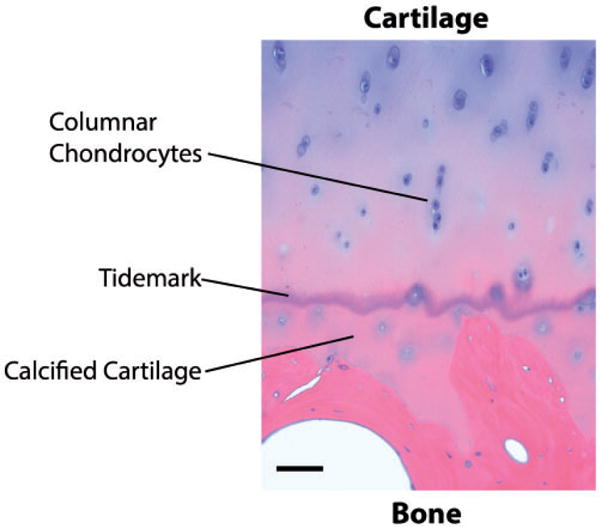
Hematoxylin and eosin (H&E) staining of a section of native osteochondral tissue in which tidemark, calcified cartilage and columnar chondrocytes are highlighted as prominent features in the osteochondral junction. Bar = 100 μm. (A color version of this figure is available in the online journal.)
In general, bone presents a more heterogeneous cell population than cartilage,8 comprising osteocytes, osteoblasts, and osteoclasts that directly remodel the tissue. Furthermore, the trabecular pores host the bone marrow, rich in hematopoietic (HSCs) and mesenchymal stem cells (MSCs), and act as an adipose depot.6 Bone is vascularized as well as innervated, and others cells such as neurons and endothelial cells are also present and may play a relevant role in bone biology. In fact, it is generally considered that bone vascularization itself is one of the reasons for the active self-repair capacity of bone.
Historically, cartilage and bone have been among the first targets of tissue engineering technologies.9,10 The low cellular densities and the prominent presence of the extracellular matrix (ECM) have led researchers to experiment on a variety of structures and scaffolds that could host cells and mimic the properties of native tissue. The cells of choice have most often been bone marrow-derived MSCs, and more recently adipose-derived MSCs.11–13 Both cell types are relatively easy to access and can differentiate following established protocols into bone and cartilage. All of these factors have contributed to the active, ongoing efforts focused on the development of musculoskeletal tissue engineering and to a relatively fast track from in vitro to in vivo animal testing in current cartilage and bone research practice.14 However, when moving toward a microphysiological system (MPS) approach, the prominence of the ECM in cartilage and bone tissues has come to represent a major obstacle as it poses greater challenges to the degree of miniaturization, hence of throughput, that is achievable.15,16
Osteoarthritis: a degenerative joint disease of cartilage and bone
Osteoarthritis (OA) is a chronic, degenerative disease of the articular joint that involves cartilage, synovium, ligaments, bone, meniscus, tendon, and peri-articular muscle.17 Cartilage destruction is one of the common characteristics of OA progression and results in malfunction of the affected joint. Normal articular cartilage is comprised of large amounts of ECM, produced and maintained by chondrocytes, the sole cell type in the cartilage. During disease progression, net loss of cartilage matrix results from an imbalance between cartilage matrix degradation and synthesis by chondrocytes in the cartilage.18–20 Currently, there is no effective therapy for the treatment of OA except for palliative measures to relieve the symptoms of the diseases until the joints need to be replaced by surgery. Typical pharmacological management includes the administration of non-steroidal anti-inflammatory drugs (NSAIDs), specific inhibitors of cyclooxygenase-2, and intra-articular corticosteroid injection.21 However, the underlying structural damage of the joint is not restored by these treatments.
Both biomechanics and biochemistry play an important role in OA. Irregularities or perturbations in the joint structure caused by genetic or environmental factors create abnormal forces within the joint that are highly correlated with the development of OA.22,23 More severe stress such as those found in chronic overuse or joint trauma also contribute to the etiology of OA,24–26 enhanced by genetic or environmental factors.27,28 From epidemiological and animal studies, hallmarks of post-traumatic cartilage damage that result in OA include cell death/apoptosis, matrix degradation, alteration of chondrocyte phenotype characterized by higher proliferative rate, and expression of markers characteristic for hypertrophy including Runx2 and collagen type X.29,30
This characterization of OA etiology emphasizes the chondral component of the disease. However, whether OA begins in the cartilage or the bone and whether subchondral bone or articular cartilage is the best target for disease modifying OA drug (DMOAD) development, are subjects of debate. Supporters of the “bone side” of the debate maintain that, as the “substrate” for articular cartilage, subchondral bone plays a supporting role in cartilage health, and that any perturbations to its structure and composition are amplified as pathological conditions and transferred from bone to cartilage. For example, osteophyte formation and changes in subchondral bones are seen to appear before measurable changes in articular cartilage thickness and related joint space narrowing.31 Another post-traumatic OA study also linked skeletal changes associated with OA and alterations in articular cartilage.32 Similarly, in the Hartley guinea pig model of OA, altered mechanical properties of subchondral bone precede the onset of cartilage degradation.33 In the rat anterior cruciate ligament transection models of OA, increased subchondral bone resorption is associated with early development of cartilage lesions.34 Further evidence for the “bone first” theory include findings suggesting that subchondral bone dysplasia leads to OA.35 For example, abnormal anatomies of either the femoral head or the acetabulum that interfere with rotation of the femoral head leads to OA that is treatable by periacetabular osteotomy.36,37 Other studies suggest that healthy subchondral bone is essential for healthy cartilage. They also report that chronic overuse and joint trauma lead to bone bruising, resulting in changed subchondral bone biomechanical properties that negatively impact the cartilage above. Furthermore, growing evidence indicates that, in vivo, cartilage receives nutrients, cytokines, and hormones through the osteochondral junction, characterized by calcified cartilage and a basophilic tidemark, and vice versa.38–40 Other studies conclude that changes in subchondral bone gene expression characterize OA,41 and that inhibitors of bone resorption suppress later cartilage symptoms of OA.42
Proponents of the “cartilage first theory” argue that while early changes to cartilage during OA are clearly coupled to bone alterations via mechanical and soluble factors, changes to the bone seem to be secondary to alterations in articular cartilage.43 Supporting evidence suggests that OA changes to cartilage alter the mechanical environment of the bone cells and induce them, in turn, to modulate tissue structure. Several studies report that thickening of calcified cartilage along with tidemark advancement contributes to thinning of articular cartilage.44 This leads to increased mechanical stress in the matrix of the deep zone of cartilage and contributes to OA cartilage deterioration.45
The evidence for both bone and/or cartilage etiologies, taken together, thus suggests that OA should be considered a disease of the osteochondral tissue, if not the entire joint that includes the synovial lining – mediator of endocrine signaling, metabolic homeostasis and immune responses between the articular cartilage and the rest of the body – and the blood vessels and vasculature within the osseous environment where vessels may go right up to the cartilage.46,47 This etiological origin implies that in vivo and in vitro studies of OA must include at least a functioning osteochondral unit, since interactions between both bone and cartilage are central to disease progression, and that bone and cartilage can no longer be considered separately in the study of OA.
We believe that this consideration is not simply an academic point of view. OA is the most common form of arthritis, a musculoskeletal disease with severe societal burden that affects an estimated 12–15 million people in the USA alone. Abnormal mechanical forces are both a cause and a result of OA, leading to pain, joint impairment, and decreased mobility. Thus, there is an urgent need for pre-surgical, medical therapies to prevent or slow the progression of OA.
In Vitro models of cartilage and bone
The starting point for these microphysiological models is the preparation of the target tissue in vitro. Although cartilage and bone formation are intimately linked during embryogenesis in endochondral ossification and at the osteochondral junction, the increasingly reductionistic studies of the past century have often entailed studying the development and maintenance of these tissues in isolation.
Chondral tissue
Micromass high density culture of embryonic mesenchymal cells
The development of in vitro systems for cartilage tissue began in the context of embryology and developmental biology. In vitro techniques for the study of chondrogenic differentiation of embryonic limb bud mesenchymal cells from chick or mouse have been available for more than half a century. Early methods required high-density confluent monolayer cultures of the cells.48 The micromass culture method developed by Solursh and colleagues49 represented a convenient system for the observations and analysis of the differentiation processes and phenomena analogous to those exhibited by the limb cartilage anlagen in vivo. In these cultures, isolated embryonic limb bud mesenchymal cells are plated at 20×106 cells/mL in 10–20 mL droplets. These cells undergo condensation that gives rise to cell aggregates that differentiate into cartilage nodules,50,51 paralleling cartilage formation during embryonic limb development in vivo.52–55 For this reason, the micromass limb bud mesenchyme culture system (Figure 3) gained great popularity for the analysis of the mechanisms and regulation of cellular condensation and differentiation,56–60 maturation of the cartilage anlagen and, upon treatment with the thyroid hormone triiodothyronine (T3), cartilage hypertrophy61,62 and calcification.63 For example, this system was used in establishing the importance of ECM and cadherin-mediated cell–cell adhesion in cellular condensation,64,65 TGF-β and BMP signaling in chondrogenic differentiation,66 the action of BMPs and WNTs in joint formation,67 and the activity of natriuretic peptides in chondrocyte hypertrophy.68 The micromass system is very flexible and has even been used as a platform for drug testing.69
Figure 3.
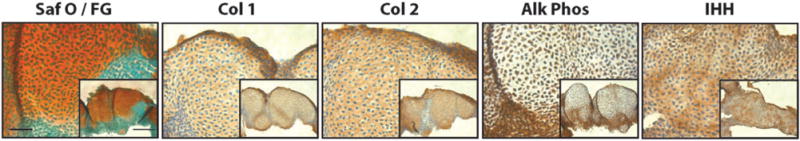
Limb bud mesenchymal micromass. Embryonic chick limb bud micromass cultured for 21 days and stained with Safranin O/Fast green (Saf O/FG), and immunostained for collagen type I (Col 1), collagen type II (Col 2), alkaline phosphatase (Alk Phos), and Indian hedgehog (IHH). Bar =50 μm, Inset Bar 100 μm. (A color version of this figure is available in the online journal.)
Cell pellet: high density culture of MSCs
Nearly five decades ago, Friedenstein et al.70 described a population of non-HSCs isolated from human bone marrow with the in vitro ability to adhere, proliferate, and differentiate into chondrocytes, osteoblasts, and adipocytes. The ease with which these MSCs undergo skeletogenic differentiation prompted scientists and physicians alike to employ them for musculoskeletal engineering. Although MSCs are able to show signs of differentiation in 2D culture stimulated by TGFβ-superfamily members,71,72 much more robust chondrogenesis is observed when the cells are induced to condense within high density droplets or after pelleting by mild (300×g) centrifugation (Figure 4), like the limb bud mesenchyme described earlier.73,74 This reflects the importance and utility of the cell-aggregation technique to induce adult MSC chondrogenesis in vitro, imitating embryonic prechondrogenic aggregates in vivo. Pellet cultures, i.e. high density cell aggregates of about 250,000 cells,75 represent another 3-dimensional (3D) model especially used in cartilage engineering.
Figure 4.
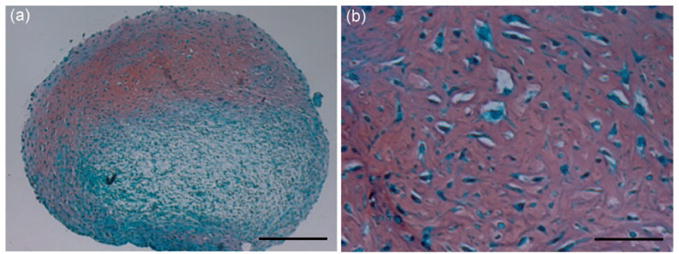
MSC pellet culture. Human bone marrow-derived MSCs were pelleted and cultured in TGF-β1 containing chondrogenic medium for 21 days. Samples were processed for histology and stained with Safranin O/Fast green. Red staining indicates matrix sulfated glycosaminoglycan deposition. Bar =250 μm in a and 50 μm in b. (A color version of this figure is available in the online journal.)
Studies using pellet cultures of human MSCs have been used to verify that chondrogenic mechanisms for adult stem cells are similar to those of embryonic cells, but not identical.76–78 Stimulation of MSC recruitment to articular cartilage defects by microfracture or implantation of stem cells in the context of high-density pellets resulted in short-term hyaline cartilage formation, followed by a slow but certain conversion to fibrocartilage or calcified cartilage and eventual degeneration within the joint.79–81
Biomaterial scaffolds: three-dimensional culture of MSCs
The failure of current cartilage repair surgical procedures is generally considered to be a consequence of aberrant cell differentiation resulting in poor matrix production and inferior tissue mechanical properties, or in matrix calcification.82 A large number of biomaterials have been tested for the maintenance and support of human MSC chondrogenesis in vivo.83,84 These biomaterials include hydrogels, sponges and fibrils, comprised of natural and synthetic materials to form biomimetic hierarchical structures for the support of MSC chondrogenic differentiation (Figure 5). Hydrogels were among the first materials to be employed because of the ease of use, high hydration (conferring chondro-supportive mechanical properties), and potentially chondro-inductive, bioactive epitopes, depending on the polymer used.85 The confluence of these properties has proven to be supportive of chondrogenesis in many contexts, including in vitro differentiation, biomechanical characterization of cartilage-like tissue, and repair of articular cartilage defects.86
Figure 5.
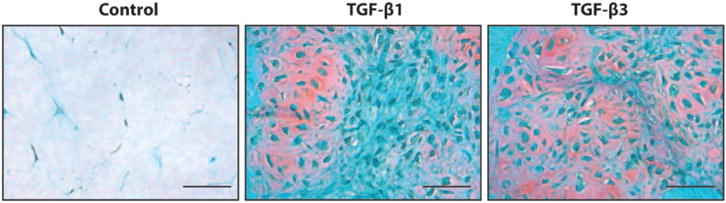
MSCs chondrogenic differentiation in 3D. Human bone marrow derived MSCs were seeded within 1 mg/mL collagen type I gels and cultured for 21 days with TGF-β1 or TGF-β3 containing chondrogenic medium or control medium. Samples were processed for histology and stained with Safranin O/Fast green staining. Red staining indicates matrix sulfated glycosaminoglycan deposition. Bar =50 μm. (A color version of this figure is available in the online journal.)
Encapsulation within inert hydrogels such as agarose or polyethylene glycol (PEG) itself induces a spherical cytoskeletal arrangement conducive to the chondrogenic phenotype.87,88 In such cultures, chondrocytes maintain their phenotype, while MSCs show enhanced expression of the chondrogenic transcription factor, Sox9, and collagen type II, aggrecan and other ECM molecules, producing a more functional, mechanically resilient matrix.89,90 Biomimetics has also inspired the use of micro- and nano-fibers resembling the collagen fibrils so prominent in native cartilage matrix.91–94 The addition of appropriate growth factors (TGFβ1, TGFβ3, BMP6 and IGF)95,96 and appropriate molecular environments by using gelatin, collagen type II, hyaluronan, or devitalized cartilage matrix serves to induce greater and more mature matrix production indicated by the expression on collagen types IX and XI, proteoglycan link protein, hyaluronan and other minor proteoglycans in almost all scaffold types tested.97,98 The addition of uniaxial, unconfined compression or cyclic hydrostatic pressure increases chondrogenesis and serves as another critical stimulus to enhance the production and remodeling of a robust ECM.99–101 Hydrogel combinatorial approaches have produced the most promising neo-cartilage matrix while also reducing the production of hypertrophic markers such as Runx2, collagen type X, and matrix metalloproteinase 13 (MMP13).102 The change in chondrocyte phenotype responsible for this change in matrix and ultimate failure of the constructs in vitro and in vivo remains a challenge yet to be overcome.
In general, typical hydrogel based cartilage scaffolds can attain compressive moduli of 0.05–0.25 MPa or aggregate moduli ranging between 0.02 and 0.12 MPa.103,104 These compare poorly to the properties of native cartilage: 1–2 MPa compressive and up to 0.85 MPa aggregate moduli. Given that the articular surface in the human knee can experience peak loads of 10–12 MPa,105 it is no surprise that these constructs fail in vivo in long-term studies. A significant, mostly unmet need is to develop scaffolds with sufficient initial mechanical properties to permit cell survival and tissue development in the challenging joint environment. The most successful scaffolds of this type are solid three-dimensional porous matrices106,107 or woven microfibers.108,109 The composition and porosity of the solid matrices is created through the use of particulate leaching, phase separation, or microsphere compaction and formed through molding or 3D printing techniques. The addition of certain natural (e.g. chitosan, bioceramics) and synthetic (e.g. polyvinyl alcohol (PVA)) components easily provides the mechanical strength to match that of native cartilage. However, the strength of these scaffolds often completely shields incorporated cells from mechanical forces, which have been shown to be critical to cartilage and bone cell and tissue development and homeostasis.110,111
Osseous tissue
Micromass: high density culture of osteoblastic cells
The simplest system for studying osteogenesis is a micromass of cultured osteoblastic cells in osteogenic medium, often comprised of 10% serum-containing basal medium, supplemented with 10 nM β-glycerol phosphate, 50 μg/mL ascorbate, and 10 μM dexamethasone.112 1,25-dihydroxy vitamin D3 is also included in some cultures. Gene expression, including alkaline phosphatase (ALK), bone sialoprotein (BSP), collagen type I, osteonectin (ON), osteopontin (OPN), and osteocalcin (OC), indicates an advanced degree of osteogenic differentiation.113 Calcium deposition has also been observed in these systems, although these cultures do not produce the complex structure characteristic or cortical and trabecular bone.114
Biomaterial scaffold: three-dimensional cultures of human MSCs for osteogenesis
As generally the engineering of bone has been aimed at direct clinical applicability, particular stress has been put on materials and structures that could be both osteoinductive and osteoconductive.115 Furthermore, considering the need for cell infiltration and vascularization after implant, a great deal of attention has been put towards generating structure that could couple mechanical strengths and significant porosity, a major challenge in itself. Consequently, a host of different scaffolding approaches can be found in the literature for bone tissue engineering, exploring different architectures and material properties.116,117 Some of the most commonly used approaches are summarized below to illustrate the diversity of the bone biomaterials field.
Calcium phosphate
Inspired by the ground-breaking observation that de-vitalized bone matrix could stimulate osteogenesis of encapsulated cells,118 many investigators have focused on developing scaffolds with material properties similar to bone, including bone powder or calcium phosphate in its various forms (hydroxyapatite (HA), β-tri-calcium phosphate (β-TCP) and/or biphasic calcium phosphate (BCP))119,120 as well as ceramics and cements.121–123 The mechanical properties of bone vary between cancellous and cortical bone. For example, Young’s modulus of cortical bone is between 15 and 20 GPa and that of cancellous bone is between 0.1 and 2 GPa, and compressive strength varies between 100 and 200 MPa for cortical bone, and between 2 and 20 MPa for cancellous bone.124,125 As a consequence, the scaffolds for bone engineering must have substantial mechanical strength. Scaffolds comprised of calcium phosphate may have compressive strength equivalent to cancellous bone (approximately 10 MPa). A recent study showed that a combination of macro-porosity (250–350 μm) and micro-porosity (2–8 μm) even resulted in lamellar and woven bone formation.126,127
Synthetic/natural polymers
Other commonly used synthetic polymers for 3D scaffolds in bone tissue engineering include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(ε -caprolactone) (PCL), and poly(lactic-co-glycolide) (PLGA), PVA, and poly(propylene fumarate) (PPF).128,129 Among natural polymers, collagen type I and chitosan are the most commonly used. In contrast to cartilage engineering where these polymers are often used to form micro- or nanofibers,130 in bone engineering, rigid 3D sponges are common.131,132 The pores within the sponge are formed by various techniques, including molding, thermally induced phase separation, particle leaching, micro-particle sintering, among others. Optimal bone formation is obtained with interconnected porosity of 200–300 μm. The addition of hydroxyapatite crystals within all of these scaffolds dramatically increases osteogenesis by cells within the scaffold.133–138 While synthetic polymers such as PPF have a high compressive strength comparable to cortical bone, the degradation products of many polymers result in physiologically challenging microenvironments, such as low pH or poorly cleared monomers, that often induce an undesirable inflammatory response in the host and potential cell death.
In Vitro modeling of the osteochondral complex
The osteochondral junction provides a mechanical transition from the cartilage to bone, and a biochemical barrier that is severely disrupted in OA. More recently, the osteochondral junctions is being viewed as a channel for communication between the subchondral bone and overlying cartilage, as in situ studies (including immunohistochemical and biochemical analyses) have provided evidence that cytokines, such as HGF, may travel between the subchondral bone and cartilage.139,140 Hence, an engineered osteochondral construct that properly incorporates articular cartilage, osteochondral interface (calcified cartilage), and the subchondral bone as an interactive micro-tissue unit should be of significant utility in elucidating the pathogenesis of degenerative joint diseases as well as assessing the efficacy of potential therapeutics against the disease by recapitulating the complex signaling occurring across the junction. In developing such a system, a number of factors should be taken into account.
Challenges to the development of an osteochondral MPS
There are several hurdles that must be overcome in developing an in vitro osteochondral micro-tissue. The first is the disparate conditions in which these two tissues develop and exist. Cartilage is bathed in synovial fluid that, together with the synovial lining of the joint capsule, acts as an intermediary conduit of biochemical signals and metabolites between cartilage and the rest of the body. The cells of the articular cartilage are embedded in a hydrated viscoelastic matrix, and joint movement (compressive and tensile forces) is critical to nutrient and metabolite transport through the ECM. In addition, as the depth of the cartilage increases, the oxygen tension dramatically decreases to 2–4% in the deep zones of cartilage, and this presumably has consequences on cell metabolism and homeostasis.141,142 On the other hand, bone is in immediate contact with the body vasculature and the bone marrow space. This environment is normoxic and the matrix is much stiffer.143 Thus, in practice, a biomimetic reproduction of these different environments requires dramatically different growth media and scaffold structure (e.g. porosity) and a close monitoring of the interface if the changes connected with OA are to be observed.
The second challenge is achieving the correct balance in the mechanical properties between the chondral and osseous components of the micro-tissue. Within the musculoskeletal system, cartilage distributes load on the joint surfaces and ensures low friction joint movement, while bone acts as a load transducer allowing for body movement and ensures shape maintenance. Each tissue must therefore possess very different mechanical properties, determined by their matrix composition, structure and water content.144
The biological relevance of the unique mechanical properties of the tissue-specific scaffolds becomes evident when the constructs are mechanically stimulated. It is well established in the literature that the homeostasis of both cartilage and bone is highly dependent on mechanobiological stimulation, and an imbalance in mechanical properties has pathological consequences.145,146 This is true for engineered tissues as well.147,148 Therefore, the third major challenge to osteochondral tissue development in vitro is the application of a regimen of mechanical forces, generally in the form of controlled compression, to the osteochondral construct. These must be applied in such a manner that cells in each compartment are anabolically stimulated, which emphasizes the importance of the specific mechanical properties of each compartment. The importance of this is seen in vivo where the osteoarthritic degeneration of cartilage is often coincident with the thickening and hardening of the subchondral bone, whereas osteoporosis is coincident with thickening of articular cartilage.149
Bone and cartilage are connected at the osteochondral junction that is believed to mediate the interaction of the subchondral bone and articular cartilage, and it is recently implicated as a locus of the disease as well as a potential target of future disease modifying osteoarthritis drugs (DMOADs).150 It is hypothesized that the formation and maintenance of this junction are a consequence of the interaction of the tissues layers, as a function of the different structural (scaffold) and biochemical/biophysical (i.e. cytokines, O2 tension, etc.) conditions in which cartilage and bone exist and of the mechanical environment. The osteochondral junction is characterized by the tidemark, a histologically detectable feature distinguished by strong basophilic staining that marks the transition to calcified cartilage adjacent to the subchondral bone. In healthy joints, the tidemark is avascular, whereas in OA it is often breached by the vasculature. Furthermore, in OA the tidemark is often duplicated and advanced into the normally non-calcified cartilage itself,151 indicative of a changing relationship between cartilage and bone. Recreating the tidemark in vitro is probably one of the key challenges in reproducing the osteochondral complex, but it could represent a crucial step in understanding the mechanism of cartilage-bone interaction in OA.
Approaches to recreating the osteochondral junction
As there are limited differentiated cell sources available for cartilage and bone tissue engineering, MSCs, with their well-characterized ability to differentiate into chondrocyte-and osteoblast-like cells, represent a natural candidate cell source for engineering these tissues.152,153 A large number of studies have been performed on in vitro engineering of osteochondral tissues from MSC-seeded scaffolds. Although the methods of production vary greatly, they can be generally categorized as single phase constructs and multiphase constructs by the number of layers initially employed to induce the formation of osteochondral tissue. Single phase constructs are composed of a single scaffold type (such as PVA, chitosan, gelatin) with regional differentiation induced by the seeding or encapsulation of different cells types (i.e. pre-differentiated MSCs) and by the inclusion of chondro- and osteoinductive agents in a spatially specific manner. Spatially defined cytokine release can be achieved through incorporation of the cytokine within the scaffold (ionically or covalently) or by incorporation of cytokine-laden micro- or nanoparticles.154 In a recent study, opposing gradients were used to generate an osteochondral tissue in a single-phase PEG hydrogel, reporting spatially restricted differentiation and a gradual transition between chondral and osseous tissues.155 The use of particles is attractive because the cytokine is protected and the local concentration can be controlled based upon the degradation rate of the particle material. In general, the simplicity of a single-phase scaffold for osteochondral engineering is appealing. However, hurdles for this technique include the very different mechanical environments in which the two tissues optimally develop and the requirement for vascularization (generally promoted by increased porosity) in the osseous portion of the construct. In some cases, these challenges have been overcome by varying the construct stiffness during crosslinking or the construct porosity by porogen leaching, freeze-drying, gas foaming and direct 3D printing.156
However, the most frequently tested scaffolds for osteochondral engineering are the biphasic constructs that can provide stiff and porous constructs for osteogenesis and hydrated, viscoelastic environments for chondrogenesis.157 For instance, for cartilage, scaffold-free layers of cells as well as cell-laden hydrogels comprised of synthetics (e.g. PVA),158 proteins (e.g., gelatin, collagen, and hyaluronic acid),159 glycosaminoglycans (chitosan)160 have been employed in various forms. For bone, stiffer and porous scaffolds have been produced using polymers (PCL, PLLA, PGA, PLGA),161,162 hydroxyapatite or other ceramics,163 and metals.164 A popular approach exploits the mechanical properties of hydroxyapatite but mixes it with the biomimetic structures formed by polymers, such as nanofibers, and especially popular in this context are bio-printing approaches.165 Often, the two phases are fabricated separately (permitting specific cell seeding to each material) and subsequently pressed or fused together.157 When purposed for in vivo application, the bone side of the constructs is often not seeded with cells, relying on the intrinsic healing capacity of bone and the ability of endogenous osteoblasts to populate hydroxyapatite scaffolds.166 However, in biphasic scaffolds, the different mechanical properties of the two phases pose a significant risk of the two construct parts separating once implanted in vivo. In fact, integration of the layers to form a structural unit represents a major challenge in biphasic/multiphasic construct fabrication. The advent of in situ fabrication techniques such as layered fabrication, and chemical and photo-crosslinking have served to generate greater cohesion between layers.167 Nevertheless, although after differentiation and maturation most biphasic constructs exhibit a clear distinction in histochemical reactivity of the chondral and osseous components, often the interface is limited to “a transition” from one phase to the other. A more desirable mimic of the osteochondral junction would include a basophilic boundary between the two tissues and histomorphometric changes in chondrocyte organization (i.e. columnar arrangement) near the boundary.
Towards this goal, triphasic or multiphasic scaffolds have also been attempted,168 with the intervening layer(s) designed as a transition state between the osseous and chondral phases, upon which trophic factors from the cartilage and bone create opposing gradients that can promote a functional biological osteochondral junction. Alternatively, bioactive intervening layers have been attempted using, for instance, platelet-rich plasma (PRP)169 and MSC-laden collagen.170 One interesting challenge for these approaches based on MSCs-laden scaffolds is the idea, put forward by some investigators, that the chondrocytes of each cartilage zone and the osteochondral junction have unique developmental histories that cannot be easily replicated by matrix composition or cytokine gradients alone. In one set of studies, it has been shown that the cells in each zone produce unique matrices171 and respond to external stimuli and ECM components in zone-specific manners.172,173 This suggests that MSC and/or the surrounding matrix and biophysical parameters require more sophisticated manipulation in order to achieve the zonal qualities of native articular cartilage and a functional osteochondral unit. Hydrogels with different mechanical properties systems in combination with advanced fabrication techniques such as bio-printing and solid free-form fabrication offer the opportunity to construct scaffolds with zone-specific properties for better osteochondral engineering. In any case, the communication of the osseous and chondral halves of the construct across the transition zone is almost always an unexplored phenomenon.
While many studies provide microscopic and/or histological evidence of transition zones,174–177 few studies have focused on the establishment and functional characterization of the inter-tissue signaling at osteochondral interface in vitro. Studies using co-culture of chondrocytes and osteo-blasts in 2D Transwell cultures have shown mitotic potential of chondrocytes mediated via TGFβ1 secretion.178 A more recent study using micromass co-cultures of osteo-blasts and chondrocytes found both collagen types I and II at the interface, and the authors suggest that co-cultured chondrocytes and osteoblasts are capable of forming an osteochondral-like interface.179 However, how this osteochondral interface is formed – whether it is a result of the direct cell–cell physical contact or of the paracrine signaling between the neighboring osteoblast and chondrocyte populations – and how it is maintained remain undetermined from the study.
Another recent study generated an osteochondral interface using MSC-seeded collagen microspheres.180 MSCs seeded in collagen microspheres were first differentiated into chondrogenic (cartilage-like) and osteogenic (bone-like) tissues. Then layers of these functional subunits were separated by a thin interfacial layer of undifferentiated MSC-collagen gel in a trilayered configuration for 3D co-culture. The resulting construct showed presence of hyper-trophic chondrocytes, calcium phosphate deposits, collagen types II and X, proteoglycans, and vertically running collagen bundles in this interface region. The authors suggested that the middle undifferentiated MSCs were under influence from the microenvironment created by the neighboring osteogenic and chondrogenic layers. The osteogenic layer was previously shown to secrete BMP2180 that may stimulate chondrogenic maturation181 and hypertrophy of MSCs,182 and other soluble factors that might contribute to the formation of the calcified cartilage in the trilayer co-culture system. Co-culture with articular cartilage tissue or derived chondrocytes can also enhance chondrogenesis of MSCs.183–184 Taken together, these findings suggest that when undifferentiated MSCs are simultaneously stimulated by chondrogenic and osteogenic tissue layers, they would give rise to a calcified cartilage-like interface, and this could serve as the basis for the in vitro fabrication of a physiologically relevant osteochondral complex.
In another investigation, cells isolated from OA cartilage and bone tissues were incubated in the presence or absence of interleukin-1β, interleukin-6 or oncostatin, factors that are important in bone remodeling. When compared to osteoblasts from non-sclerotic zones of human OA subchondral bone, osteoblasts from sclerotic zones induced a marked decrease in aggrecan gene expression and an increase in matrix metalloproteinase-3 and -13 gene expression in chondrocytes from OA cartilage. This strongly suggests that an altered OA osteoblast phenotype contributes to OA pathology through actions on nearby chondrocytes.
Bioreactors
The experimental findings described above suggest that communication between chondrocytes and osteoblasts across the osteochondral junction can play a key role, but its rigorous elucidation requires an in vitro culture system that supports native or engineered osseous and chondral components of an osteochondral unit in a way that communication between two tissues can be monitored. In fact, most studies of osteochondral tissue engineering involve the use of in vivo models of articular surface repair. While the use of such models can give the impression of translation readiness, it is also a necessity as conventional in vitro culture systems such as static cultures, spinner flasks, rotating wall vessels and flow perfusion fail to provide physiological and mechanical conditions sufficiently close to the in vivo conditions.
Bioreactors have been developed that attempt to replicate in vivo physiological conditions and permit controlled manipulation of the system and its critical variables for scientific investigation. The design of a bioreactor changes as understanding of tissue physiology evolves. In the case of OA, there is a renewed focus upon the osteochondral unit and the integrity of the osteochondral junction as a locus of the disease.150,187 Among the difficulties in culturing the osteochondral unit, the divergent environments in which cartilage and bone develop are a particularly relevant one. As discussed earlier, features such as growth factors and supplements, oxygen tension and pH, and mechanical stimulation are known to be both important to histogenesis and tissue-specific for bone or cartilage. The application of these variables while maintaining intimate contact between the developing tissues can benefit greatly from a rationally designed bioreactor.
As pointed out earlier, complex bioreactors have not been frequently explored in the development of skeletal tissues. Bone and cartilage were often studied in isolation. Bioreactors for living cartilage were often limited to different modes of mechanical stimulation of native or engineered tissues in confined or unconfined conditions.188 These types of studies have revealed the importance of chondro-inductive forces versus chondro-destructive forces. The osteochondral plug harvested from an articular joint specimen has been most commonly employed in studying the mechanical properties of the articular surface, because the osteochondral junction and the subchondral bone are known to confer significant protective mechanical properties to the overlying cartilage.189,190 Specifically, the subchondral bone reduces impact-induced fissuring and reduces chondrocyte cell death and matrix degradation, all of which are hallmarks of pre-OA cartilage degeneration.191,192 Studies of chronic joint overuse have suggested the involvement of subchondral bone changes in the etiology of OA, but these changes have not been successfully modeled in vitro.193,194
Early bioreactors specifically for bone were almost nonexistent. Some osteogenic studies were carried out within the context of HSC and bone marrow cultures in Koller reactors or similar devices in which osteogenic differentiation was evident, but not the focus of the studies.195,196 As attempts at bone tissue engineering increased, bioreactors have been developed primarily aimed at improving cell seeding, increasing nutrients and oxygen exchange and metabolite removal,197 and even providing mechanical stimulation to the seeded cells,198,199 all of which proved to be important in promoting good osteogenesis.200 The use of spinner flask bioreactors generally entailed enhanced expression of osteogenic markers and mineralization.201,202 However, the beneficial effect of increased medium movement is often limited to the outer surface of the construct where a dense cellular layer forms, impeding efficient exchange with the inner part of the scaffold.203 Rotating wall vessels are often considered an improvement over spinner flasks,204 as the laminar flow generated within the bioreactor may offer better medium exchange conditions and a degree of mechanical stimulation to the cells.205,206 Nevertheless, optimal conditions in rotating wall vessels pose some limitations as they require specific fluid densities, chamber diameters, and rotational speeds to ensure both good perfusion and continuous suspension of the constructs to avoid damage by bouncing against the vessel walls.207–209 Nevertheless, perfusion bioreactors offer the opportunity of mass transport of culture medium throughout the scaffold, with the continuous supply of nutrients and oxygen and the removal of metabolites. Furthermore, they can be used to obtain more uniform cell seeding throughout the scaffold by suspending cells in the medium that is continuously circulated through the scaffold.210–212 Overall enhanced cell proliferation, seeding density, expression of osteogenic markers, and deposition of mineralized matrix can be achieved by perfusion bioreactors, although this is often dependent on the choice of fluid flow regimen, e.g. oscillating flow, steady flow, etc.197,199,213–220
Cell culture within spinner flasks, rotating wall vessels and perfusion chambers, largely employed homogeneous cultures of stem cells or osteoblasts. Inclusion of endothelial cells or promotion of vascularization has been studied within perfusion chambers implanted in vivo,221 whereas in vitro studies are still limited but represent the object of progressively growing interest.222–225
Static cultures, while simple to achieve, suffer significant set-backs, such as inhomogeneous cell seeding at fabrication, and poor nutrient and metabolic waste movement through larger constructs (>2 μm thick). The application of mechanical loading can improve the nutrient/waste movement. Optimal loading regimen for 3D cartilage/chondral construct homeostasis is approximately 2–5% strain at 1 Hz for >1 h per day, while bone/osseous construct homeostasis is best maintained using 0.1–0.5% strain at approximately 0.5–2 Hz for >1 h per day. While these differences can be accommodated within a single loading device by choosing materials with suitable mechanical properties for the chondral and osseous components, the two tissues are usually still maintained within a common medium. Recently, a novel loading system was used to assess the effect of subchondral bone permeability on overlying cartilage using native porcine osteochondral tissue.226 While the biochemistry of native samples was apparently unaffected, restricted permeability of subchondral bone significantly reduced the mechanical properties of the overlying cartilage. Compared to static culture, constructs cultured in spinner flasks or rotating wall vessels have higher cell densities, more uniform distribution of cells, and enhanced biochemical and mechanical properties compared to static cultures.227 This is thought to be a function of greater nutrient perfusion into the cultured tissues or scaffolds. Interestingly, the rotating wall vessel culture is more favorable to chondrogenesis, while the spinner flask is apparently more favorable for osteogenesis. It is hypothesized that the spinner flask provides predominantly shear stresses, while the rotating wall vessel creates a hydrodynamic environment favorable to chondrogenesis. More multifunctional bioreactors to culture osteochondral constructs or explants have thus been developed with the recognition that the two tissues exist in very different environments while being intimately connected to each other. Instrumentations must be developed that are able to accommodate the differentiation and maintenance of tissue-engineered osteochondral constructs.
Flow perfusion bioreactors use a pump to percolate medium continuously through the scaffold’s interconnected pores, which results in improved mass transfer through the sample, not just around it because it forces the medium to flow through the interior of the scaffold. These systems can be used to ensure uniform cell seeding, proliferation, and enhancement of biochemical and mechanical properties of bone228 and also of cartilage.229 Cartilage constructs grown in flow perfusion reactors accumulated significantly more collagen and proteoglycans and displayed some evidence of the stratified morphology of native cartilage. Recently, flow reactors have been used to culture osteochondral constructs.230 Using such a system, different effects of perfusion culture upon differentiation or tissue elaboration by undifferentiated MSCs and predifferentiated chondrocytes and osteocytes were observed. Of particular interest with respect to the osteochondral junction, the authors showed enhanced integration of the osseous and chondral components of the construct.
There are many techniques with which to form the osteochondral junction, including assembly of predifferentiated or naïve osteochondral components, the assembly of tissue-specific scaffolds for chondro- and osteogenesis, and the utilization of scaffold or growth factor gradients. To accommodate these approaches, different bioreactors may be employed. In the case of the assembly of predifferentiated or naïve osteochondral components into a bilayered or multilayered constructs, it is vital that the nutrients and growth factors be supplied in a spatially restricted way. Recently, investigators have begun developing in vitro dual-chamber bioreactors that provide tissue-specific media for cartilage and bone to the two halves of native or engineered osteochondral tissues, such as the platform developed by the authors (Figure 6). In order to test biphasic chitosan-based scaffolds for osteochondral tissue engineering, investigators have employed a silicone septum to separate simulated body fluid and simulated synovial fluid to the bone and cartilage components, respectively.231 The effect of mechanical loading of the samples was assayed as well, as a first attempt at providing the full spectrum of tissue-specific growth and mechanical conditions. Comparisons of constructs grown in homogeneous versus tissue-specific media were not made; however, the anabolic effect of mechanical loading of this system was clearly demonstrated. More recently, a similar system providing separate tissue-specific growth media was used to assess the differentiation of MSCs in a biphasic scaffold.232 In the absence of mechanical stimulation in this system, medium perfusion was enhanced using magnetic stir bars in each chamber. Again, no comparison with homogeneous culture conditions was performed; however, tissue-specific differentiation was observed in the opposing halves of the construct.
Figure 6.
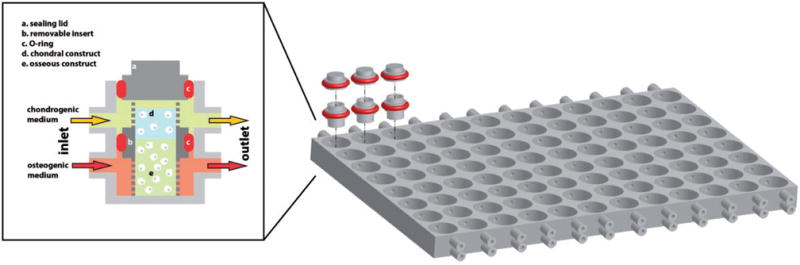
Schematics of a multiwell, dual chamber bioreactor system (modified from Lozito et al.233). On the left, a representation of a single bioreactor well. A removable, non-permeable insert (b) hosts the chondral tissue (d) and the bone tissue (e) constructs. The insert with the osteochondral construct is placed within a well of the bioreactor platform as shown on the right, and the O-rings (c) seal the separation of the well in an upper and lower chamber. A stream of chondrogenic medium (yellow) is perfused through the upper compartments in communication with the chondral tissue, while osteogenic medium (orange) is perfused through the lower chamber in communication with the bone tissue. Sealing is further ensured by a lid (a) equipped with an O-ring (c). On the right, a representation of the multiwell bioreactor platform for the simultaneous culture of 96 osteochondral constructs. The multiwell platform replicates the well dimensions and arrangement of standard 96-well tissue culture plates. Representative inserts and sealing lids with O-rings depicted in red are shown. The microfluidics connects eight wells in each column allowing for averaging of the experimental conditions along each array. (A color version of this figure is available in the online journal.)
In general, investigators have attempted to isolate the chondral and osseous portions of the scaffold and feed them with tissue-appropriate media and supplements,234–236 and added axial237 or both axial and shear stress238,239 mechanical stimulations. In the case of matrix or scaffold gradients, the scaffold provides the cues for differentiation, and several studies employing predifferentiated cell-laden microspheres in vitro or undifferentiated stem cells within scaffold gradients fabricated in situ have reported the formation of osteochondral junctions at the interface of the cartilage and bone components.240–242 The advantage of such an approach is that complex multitissue chambers and microfluidics are not required. Finally, growth factors and nutrients may be supplied in a gradient to affect cell differentiation to bone or cartilage and produce an interfacial osteochondral junction. The bioreactor thus acts as culture chamber in which media of different formulations are injected at specific locations. While the approach requires a sophisticated medium delivery system and chamber, the bioreactor itself could be of relatively simple design. This particular system has been generated within a microscale bioreactor on a chip.243
Conclusions
The development of an osteochondral MPS presents a high utility opportunity to better understand the initiation and development of osteoarthritis, as it allows to coordinately study the pathophysiology of bone and cartilage and the communication between the two tissues. A number of experimental systems have been developed to focus on either cartilage or bone, but there is a growing interest on both developmental models of the osteochondral junction and tissue engineering model of composite constructs that can mimic the cartilage/bone unit. For these endeavors to be successful, a number of hurdles need to be overcome. Among these, determining the minimal amount of tissue necessary to reasonably mimic the native tissue becomes prominent in a system such as cartilage and bone where most of the volume in the native tissue is taken up by the ECM. In general, the specific biological mechanisms under investigation will determine the level of complexity required for an in vitro osteochondral MPS. This may vary from the simplest co-culture systems to complex bioreactors to generate close-to-native osteochondral constructs, which may have the capability of incorporating other joint tissues, such as the synovial lining, the fat pad, or vasculature.
The current growing interest in the use of induced pluripotent stem cells (iPSCs), coupled with the most recent osteochondral MPSs being developed, can offer the exciting opportunity of generating genuine, high throughput research platforms to screen candidate therapeutic compounds as well as developing personalized medicine approaches to musculoskeletal disorders.
Acknowledgments
The authors gratefully thank Dr Jian Tan for his help with histology. This work was supported by grants from the Commonwealth of Pennsylvania, Department of Health, the National Institutes of Health (1U18 TR000532), the U.S. Department of Defense (W81XWH-08-2-0032 and W81XWH-10-1-0850), and the Ri.MED Foundation.
Footnotes
Author contribution: All authors participated in the writing and review of the manuscript; PGA and RG contributed equally to this manuscript.
References
- 1.Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–9. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 2.Hoemann CD, Lafantaisie-Favreau C-H, Lascau-Coman V, Chen G, Guzmán-Morales J. The cartilage-bone interface. J Knee Surg. 2012;25:85–97. doi: 10.1055/s-0032-1319782. [DOI] [PubMed] [Google Scholar]
- 3.Kouri JB, Lavalle C. Do chondrocytes undergo “activation” and “transdifferentiation” during the pathogenesis of osteoarthritis? A review of the ultrastructural and immunohistochemical evidence. Histol Histopathol. 2006;21:793–802. doi: 10.14670/HH-21.793. [DOI] [PubMed] [Google Scholar]
- 4.Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 5.Coates EE, Fisher JP. Phenotypic variations in chondrocyte subpopulations and their response to in vitro culture and external stimuli. Ann Biomed Eng. 2010;38:3371–88. doi: 10.1007/s10439-010-0096-1. [DOI] [PubMed] [Google Scholar]
- 6.Hoemann CD, Lafantaisie-Favreau C-H, Lascau-Coman V, Chen G, Guzmán-Morales J. The cartilage-bone interface. J Knee Surg. 2012;25:85–97. doi: 10.1055/s-0032-1319782. [DOI] [PubMed] [Google Scholar]
- 7.Thompson WR, Gottardi R, Stearns KM, Rubin J, Ambrosio F, Tuan RS. Biologics in Cartilage, Bone Repair, and Regeneration. In: Hughes C, editor. ISC 232, Applications of regenerative medicine to orthopaedic physical therapy. La Crosse, WI: Orthopaedic Section APTA; 2013. [Google Scholar]
- 8.Kouri JB, Lavalle C. Do chondrocytes undergo “activation” and “transdifferentiation” during the pathogenesis of osteoarthritis? A review of the ultrastructural and immunohistochemical evidence. Histol Histopathol. 2006;21:793–802. doi: 10.14670/HH-21.793. [DOI] [PubMed] [Google Scholar]
- 9.Keeney M, Pandit A. The osteochondral junction and its repair via bi-phasic tissue engineering scaffolds. Tissue Eng Part B Rev. 2009;15:55–73. doi: 10.1089/ten.teb.2008.0388. [DOI] [PubMed] [Google Scholar]
- 10.Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16:2247–70. doi: 10.1111/j.1582-4934.2012.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215–28. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18:436–44. doi: 10.1089/ten.teb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. The clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1:237–47. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40:750–65. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng. 2011;13:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- 16.Astashkina A, Mann B, Grainger DW. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol Ther. 2012;134:82–106. doi: 10.1016/j.pharmthera.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639–42. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller MB, Tuan RS. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PMR. 2011;3:S3–11. doi: 10.1016/j.pmrj.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Fukui N, Purple CR, Sandell LJ. Cell biology of osteoarthritis: the chondrocyte’s response to injury. Curr Rheumatol Rep. 2001;3:496–505. doi: 10.1007/s11926-001-0064-8. [DOI] [PubMed] [Google Scholar]
- 20.Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008;16(3):S1–3. doi: 10.1016/j.joca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Lynn SK, Reid SM, Costigan PA. The influence of gait pattern on signs of knee osteoarthritis in older adults over a 5–11 year follow-up period: a case study analysis. Knee. 2007;14:22–8. doi: 10.1016/j.knee.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Alexander PG, McCarron JA, Levine MJ, Melvin GM, Murray PJ, Manner PA, Tuan RS. An in vivo lapine model for impact-induced injury and osteoarthritic degeneration of articular cartilage. Cartilage. 2012;3:323–33. doi: 10.1177/1947603512447301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21:10–5. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–23. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, Buckwalter JA. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–9. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bijlsma JWJ, Knahr K. Strategies for the prevention and management of osteoarthritis of the hip and knee. Best Pract Res Clin Rheumatol. 2007;21:59–76. doi: 10.1016/j.berh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–32. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Lotz M, Martel-Pelletier J, Christiansen C, Brandi M-L, Bruyère O, Chapurlat R, Collette J, Cooper C, Giacovelli G, Kanis JA, Karsdal MA, Kraus V, Lems WF, Meulenbelt I, Pelletier J-P, Raynauld J-P, Reiter-Niesert S, Rizzoli R, Sandell LJ, Van Spil WE, Reginster J-Y. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72:1756–63. doi: 10.1136/annrheumdis-2013-203726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckland-Wright C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthritis Cartilage. 2004;12:S10–9. doi: 10.1016/j.joca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 33.Muraoka T, Hagino H, Okano T, Enokida M, Teshima R. Role of subchondral bone in osteoarthritis development: a comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. 2007;56:3366–74. doi: 10.1002/art.22921. [DOI] [PubMed] [Google Scholar]
- 34.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–43. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Sugano N, Noble PC, Kamaric E, Salama JK, Ochi T, Tullos HS. The morphology of the femur in developmental dysplasia of the hip. J Bone Joint Surg Br. 1998;80:711–9. doi: 10.1302/0301-620x.80b4.8319. [DOI] [PubMed] [Google Scholar]
- 36.Laborie LB, Lehmann TG, Engesæter IØ, Eastwood DM, Engesæter LB, Rosendahl K. Prevalence of radiographic findings thought to be associated with femoroacetabular impingement in a population-based cohort of 2081 healthy young adults. Radiology. 2011;260:494–502. doi: 10.1148/radiol.11102354. [DOI] [PubMed] [Google Scholar]
- 37.Steppacher SD, Tannast M, Ganz R, Siebenrock KA. Mean 20-year follow-up of Bernese periacetabular osteotomy. Clin Orthop Relat Res. 2008;466:1633–44. doi: 10.1007/s11999-008-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan J, Zhou X, Li W, Novotny JE, Doty SB, Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res. 2009;27:1347–52. doi: 10.1002/jor.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imhof H, Breitenseher M, Kainberger F, Trattnig S. Degenerative joint disease: cartilage or vascular disease? Skeletal Radiol. 1997;26:398–403. doi: 10.1007/s002560050254. [DOI] [PubMed] [Google Scholar]
- 40.Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzadeh S, Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol. 2000;35:581–8. doi: 10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Kuliwaba JS, Findlay DM, Atkins GJ, Forwood MR, Fazzalari NL. Enhanced expression of osteocalcin mRNA in human osteoarthritic trabecular bone of the proximal femur is associated with decreased expression of interleukin-6 and interleukin-11 mRNA. J Bone Miner Res. 2000;15:332–41. doi: 10.1359/jbmr.2000.15.2.332. [DOI] [PubMed] [Google Scholar]
- 42.Hayami T, Pickarski M, Wesolowski GA, McLane J, Bone A, Destefano J, Rodan GA, Duong LT. The role of subchondral bone remodeling in osteoarthritis: reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 43.Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–6. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 44.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 45.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthritis Cartilage. 2004;12:S20–30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Ciani C, Doty SB, Fritton SP. An effective histological staining process to visualize bone interstitial fluid space using confocal microscopy. Bone. 2009;44:1015–7. doi: 10.1016/j.bone.2009.01.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–74. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umansky R. The effect of cell population density on the developmental fate of reaggregating mouse limb bud mesenchyme. Dev Biol. 1966;13:31–56. doi: 10.1016/0012-1606(66)90048-0. [DOI] [PubMed] [Google Scholar]
- 49.Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60:69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 50.Solursh M, Ahrens PB, Reiter RS. A tissue culture analysis of the steps in limb chondrogenesis. In Vitro. 1978;14:51–61. doi: 10.1007/BF02618173. [DOI] [PubMed] [Google Scholar]
- 51.Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977;60:69–82. doi: 10.1016/0012-1606(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 52.Kosher RA, Savage MP, Chan SC. In vitro studies on the morphogenesis and differentiation of the mesoderm subjacent to the apical ectodermal ridge of the embryonic chick limb-bud. J Embryol Exp Morphol. 1979;50:75–97. [PubMed] [Google Scholar]
- 53.Newman SA, Pautou MP, Kieny M. The distal boundary of myogenic primordia in chimeric avian limb buds and its relation to an accessible population of cartilage progenitor cells. Dev Biol. 1981;84:440–8. doi: 10.1016/0012-1606(81)90413-9. [DOI] [PubMed] [Google Scholar]
- 54.DeLise AM, Stringa E, Woodward WA, Mello MA, Tuan RS. Embryonic limb mesenchyme micromass culture as an in vitro model for chondrogenesis and cartilage maturation. Methods Mol Biol. 2000;137:359–75. doi: 10.1385/1-59259-066-7:359. [DOI] [PubMed] [Google Scholar]
- 55.Mello MA, Tuan RS. High density micromass cultures of embryonic limb bud mesenchymal cells: an in vitro model of endochondral skeletal development. In Vitro Cell Dev Biol Anim. 1999;35:262–9. doi: 10.1007/s11626-999-0070-0. [DOI] [PubMed] [Google Scholar]
- 56.Tufan AC, Daumer KM, DeLise AM, Tuan RS. AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res. 2002;273:197–203. doi: 10.1006/excr.2001.5448. [DOI] [PubMed] [Google Scholar]
- 57.Tufan AC, Daumer KM, Tuan RS. Frizzled-7 and limb mesenchymal chondrogenesis: effect of misexpression and involvement of N-cadherin. Dev Dyn. 2002;223:241–53. doi: 10.1002/dvdy.10046. [DOI] [PubMed] [Google Scholar]
- 58.Tufan AC, Tuan RS. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J. 2001;15:1436–8. doi: 10.1096/fj.00-0784fje. [DOI] [PubMed] [Google Scholar]
- 59.San Antonio JD, Tuan RS. Chondrogenesis of limb bud mesenchyme in vitro: stimulation by cations. Dev Biol. 1986;115:313–24. doi: 10.1016/0012-1606(86)90252-6. [DOI] [PubMed] [Google Scholar]
- 60.Woodward WA, Tuan RS. N-Cadherin expression and signaling in limb mesenchymal chondrogenesis: stimulation by poly-L-lysine. Dev Genet. 1999;24:178–87. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<178::AID-DVG16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 61.Coleman CM, Tuan RS. Functional role of growth/differentiation factor 5 in chondrogenesis of limb mesenchymal cells. Mech Dev. 2003;120:823–36. doi: 10.1016/s0925-4773(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 62.Coleman CM, Tuan RS. Growth/differentiation factor 5 enhances chondrocyte maturation. Dev Dyn. 2003;228:208–16. doi: 10.1002/dvdy.10369. [DOI] [PubMed] [Google Scholar]
- 63.Mello MA, Tuan RS. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095–105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- 64.Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120:177–87. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 65.Tufan AC, Tuan RS. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J. 2001;15:1436–8. doi: 10.1096/fj.00-0784fje. [DOI] [PubMed] [Google Scholar]
- 66.Denker AE, Nicoll SB, Tuan RS. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor-beta 1. Differentiation. 1995;59:25–34. doi: 10.1046/j.1432-0436.1995.5910025.x. [DOI] [PubMed] [Google Scholar]
- 67.Daumer KM, Tufan AC, Tuan RS. Long-term in vitro analysis of limb cartilage development: involvement of Wnt signaling. J Cell Biochem. 2004;93:526–41. doi: 10.1002/jcb.20190. [DOI] [PubMed] [Google Scholar]
- 68.Woods A, Khan S, Beier F. C-type natriuretic peptide regulates cellular condensation and glycosaminoglycan synthesis during chondrogenesis. Endocrinology. 2007;148:5030–41. doi: 10.1210/en.2007-0695. [DOI] [PubMed] [Google Scholar]
- 69.Spézia F, Barrow PC. The teratology testing of cosmetics. Meth Mol Biol. 2013;947:91–4. doi: 10.1007/978-1-62703-131-8_8. [DOI] [PubMed] [Google Scholar]
- 70.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–40. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738–49. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 72.Wang W-G, Lou S-Q, Ju X-D, Xia K, Xia J-H. In vitro chondrogenesis of human bone marrow-derived mesenchymal progenitor cells in mono-layer culture: activation by transfection with TGF-beta2. Tissue Cell. 2003;35:69–77. doi: 10.1016/s0040-8166(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 73.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 74.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Su P, Xu C, Yang J, Yu W, Huang D. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32:1339–46. doi: 10.1007/s10529-010-0293-x. [DOI] [PubMed] [Google Scholar]
- 76.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Charbord P, Livne E, Gross G, Häupl T, Neves NM, Marie P, Bianco P, Jorgensen C. Human bone marrow mesenchymal stem cells: a systematic reappraisal via the genostem experience. Stem Cell Rev. 2011;7:32–42. doi: 10.1007/s12015-010-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 79.Steinert AF, Nöth U, Tuan RS. Concepts in gene therapy for cartilage repair. Injury. 2009;39:S97–113. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dickhut A, Pelttari K, Janicki P, Wagner W, Eckstein V, Egermann M, Richter W. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–26. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 81.Steck E, Fischer J, Lorenz H, Gotterbarm T, Jung M, Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969–78. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 82.Studer D, Millan C, Öztürk E, Maniura-Weber K, Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118–35. doi: 10.22203/ecm.v024a09. (discussion 135) [DOI] [PubMed] [Google Scholar]
- 83.Little CJ, Bawolin NK, Chen X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng Part B Rev. 2011;17:213–27. doi: 10.1089/ten.TEB.2010.0572. [DOI] [PubMed] [Google Scholar]
- 84.Ge Z, Li C, Heng BC, Cao G, Yang Z. Functional biomaterials for cartilage regeneration. J Biomed Mater Res A. 2012;100:2526–36. doi: 10.1002/jbm.a.34147. [DOI] [PubMed] [Google Scholar]
- 85.Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. 2011;17:281–99. doi: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials. 2011;32:8771–82. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Solursh M. Extracellular matrix and cell surface as determinants of connective tissue differentiation. Am J Med Genet. 1989;34:30–4. doi: 10.1002/ajmg.1320340108. [DOI] [PubMed] [Google Scholar]
- 88.Mathieu PS, Loboa EG. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 2012;18:436–44. doi: 10.1089/ten.teb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dickhut A, Gottwald E, Steck E, Heisel C, Richter W. Chondrogenesis of mesenchymal stem cells in gel-like biomaterials in vitro and in vivo. Front Biosci. 2008;13:4517–28. doi: 10.2741/3020. [DOI] [PubMed] [Google Scholar]
- 90.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen FH, Rousche KT, Tuan RS. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–82. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 92.Li W-J, Cooper JA, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–85. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 93.Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nano-fibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 94.Li W-J, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 95.Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–9. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 96.Quintana L, zur Nieden NI, Semino CE. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15:29–41. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–9. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 98.Quintana L, zur Nieden NI, Semino CE. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B Rev. 2009;15:29–41. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grad S, Eglin D, Alini M, Stoddart MJ. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 2011;469:2764–72. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Conor CJ, Case N, Guilak F. Mechanical regulation of chondrogenesis. Stem Cell Res Ther. 2013;4:61. doi: 10.1186/scrt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303–11. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Studer D, Millan C, Öztürk E, Maniura-Weber K, Zenobi-Wong M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118–35. doi: 10.22203/ecm.v024a09. discussion 135. [DOI] [PubMed] [Google Scholar]
- 103.Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater. 2010;22:3484–94. doi: 10.1002/adma.200904179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. 2011;17:281–99. doi: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chaudhari AMW, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215–22. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 106.Sun F, Zhou H, Lee J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011;7:3813–28. doi: 10.1016/j.actbio.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 107.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–33. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 108.Dahlin RL, Kasper FK, Mikos AG. Polymeric nanofibers in tissue engineering. Tissue Eng Part B Rev. 2011;17:349–64. doi: 10.1089/ten.teb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moutos FT, Guilak F. Composite scaffolds for cartilage tissue engineering. Biorheology. 2008;45:501–12. [PMC free article] [PubMed] [Google Scholar]
- 110.Appelman TP, Mizrahi J, Seliktar D. A finite element model of cell-matrix interactions to study the differential effect of scaffold composition on chondrogenic response to mechanical stimulation. J Biomech Eng. 2011;133:041010. doi: 10.1115/1.4003314. [DOI] [PubMed] [Google Scholar]
- 111.Sudarmadji N, Chua CK, Leong KF. The development of computer-aided system for tissue scaffolds (CASTS) system for functionally graded tissue-engineering scaffolds. Methods Mol Biol. 2012;868:111–23. doi: 10.1007/978-1-61779-764-4_7. [DOI] [PubMed] [Google Scholar]
- 112.Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245–56. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 113.Soltanoff CS, Yang S, Chen W, Li Y-P. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit Rev Eukaryot Gene Expr. 2009;19:1–46. doi: 10.1615/critreveukargeneexpr.v19.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gerber I, ap Gwynn I, Alini M, Wallimann T. Stimulatory effects of creatine on metabolic activity, differentiation and mineralization of primary osteoblast-like cells in monolayer and micromass cell cultures. Eur Cell Mater. 2005;10:8–22. doi: 10.22203/ecm.v010a02. [DOI] [PubMed] [Google Scholar]
- 115.Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Szpalski C, Wetterau M, Barr J, Warren SM. Bone tissue engineering: current strategies and techniques–part I: Scaffolds. Tissue Eng Part B Rev. 2012;18:246–57. doi: 10.1089/ten.TEB.2011.0427. [DOI] [PubMed] [Google Scholar]
- 117.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 119.Wu C, Chang J. A review of bioactive silicate ceramics. Biomed Mater. 2013;8:032001. doi: 10.1088/1748-6041/8/3/032001. [DOI] [PubMed] [Google Scholar]
- 120.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996;17:31–5. doi: 10.1016/0142-9612(96)80752-6. [DOI] [PubMed] [Google Scholar]
- 122.Gosain AK, Song L, Riordan P, Amarante MT, Nagy PG, Wilson CR, Toth JM, Ricci JL. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: part I. Plast Reconstr Surg. 2002;109:619–30. doi: 10.1097/00006534-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 123.Habibovic P, Gbureck U, Doillon CJ, Bassett DC, van Blitterswijk CA, Barralet JE. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 2008;29:944–53. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 124.Silver FH, Bradica G, Tria A. Relationship among biomechanical, biochemical, and cellular changes associated with osteoarthritis. Crit Rev Biomed Eng. 2001;29:373–91. doi: 10.1615/critrevbiomedeng.v29.i4.10. [DOI] [PubMed] [Google Scholar]
- 125.Thompson ID, Hench LL. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc Inst Mech Eng H. 1998;212:127–36. doi: 10.1243/0954411981533908. [DOI] [PubMed] [Google Scholar]
- 126.Wei J, Jia J, Wu F, Wei S, Zhou H, Zhang H, Shin J-W, Liu C. Hierarchically microporous/macroporous scaffold of magnesium-calcium phosphate for bone tissue regeneration. Biomaterials. 2010;31:1260–9. doi: 10.1016/j.biomaterials.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 127.Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JAC, Clark SG, Wheeler MB, Jamison RD, Wagoner Johnson AJ. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 2007;28:45–54. doi: 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 128.Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys. 2011;49:832–64. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bueno EM, Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol. 2009;5:685–97. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 130.Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 131.Puppi D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010;35:403–40. [Google Scholar]
- 132.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 133.Kim S-S, Park MS, Gwak S-J, Choi CY, Kim B-S. Accelerated bonelike apatite growth on porous polymer/ceramic composite scaffolds in vitro. Tissue Eng. 2006;12:2997–3006. doi: 10.1089/ten.2006.12.2997. [DOI] [PubMed] [Google Scholar]
- 134.Kim H-W, Kim H-E, Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials. 2005;26:5221–30. doi: 10.1016/j.biomaterials.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, Ni M, Zhang M, Ratner B. Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng. 2003;9:337–45. doi: 10.1089/107632703764664800. [DOI] [PubMed] [Google Scholar]
- 136.Chesnutt BM, Yuan Y, Buddington K, Haggard WO, Bumgardner JD. Composite chitosan/nano-hydroxyapatite scaffolds induce osteocalcin production by osteoblasts in vitro and support bone formation in vivo. Tissue Eng Part A. 2009;15:2571–9. doi: 10.1089/ten.tea.2008.0054. [DOI] [PubMed] [Google Scholar]
- 137.Rodrigues CVM, Serricella P, Linhares ABR, Guerdes RM, Borojevic R, Rossi MA, Duarte MEL, Farina M. Characterization of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials. 2003;24:4987–97. doi: 10.1016/s0142-9612(03)00410-1. [DOI] [PubMed] [Google Scholar]
- 138.Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater. 2006;11:43–56. doi: 10.22203/ecm.v011a06. [DOI] [PubMed] [Google Scholar]
- 139.Sharma AR, Jagga S, Lee S-S, Nam J-S. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14:19805–30. doi: 10.3390/ijms141019805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guévremont M, Martel-Pelletier J, Massicotte F, Tardif G, Pelletier J-P, Ranger P, Lajeunesse D, Reboul P. Human adult chondrocytes express hepatocyte growth factor (HGF) isoforms but not HgF: potential implication of osteoblasts on the presence of HGF in cartilage. J Bone Miner Res. 2003;18:1073–81. doi: 10.1359/jbmr.2003.18.6.1073. [DOI] [PubMed] [Google Scholar]
- 141.Grimshaw MJ, Mason RM. Modulation of bovine articular chondrocyte gene expression in vitro by oxygen tension. Osteoarthritis Cartilage. 2001;9:357–64. doi: 10.1053/joca.2000.0396. [DOI] [PubMed] [Google Scholar]
- 142.Silver IA. Measurement of pH and ionic composition of pericellular sites. Philos Trans R Soc Lond B Biol Sci. 1975;271:261–72. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- 143.Gibson JS, Milner PI, White R, Fairfax TPA, Wilkins RJ. Oxygen and reactive oxygen species in articular cartilage: modulators of ionic homeostasis. Pflugers Arch. 2008;455:563–73. doi: 10.1007/s00424-007-0310-7. [DOI] [PubMed] [Google Scholar]
- 144.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15:127–41. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kerin A, Patwari P, Kuettner K, Cole A, Grodzinsky A. Molecular basis of osteoarthritis: biomechanical aspects. Cell Mol Life Sci. 2002;59:27–35. doi: 10.1007/s00018-002-8402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Madry H, Luyten FP, Facchini A. Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20:407–22. doi: 10.1007/s00167-011-1705-8. [DOI] [PubMed] [Google Scholar]
- 147.Darling EM, Athanasiou KA. Biomechanical strategies for articular cartilage regeneration. Ann Biomed Eng. 2003;31:1114–24. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- 148.Little CJ, Bawolin NK, Chen X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng Part B Rev. 2011;17:213–27. doi: 10.1089/ten.TEB.2010.0572. [DOI] [PubMed] [Google Scholar]
- 149.Henrotin Y, Pesesse L, Sanchez C. Subchondral bone and osteoarthritis: biological and cellular aspects. Osteoporos Int. 2012;23:S847–51. doi: 10.1007/s00198-012-2162-z. [DOI] [PubMed] [Google Scholar]
- 150.Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–9. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 151.Haut RC, Ide TM, De Camp CE. Mechanical responses of the rabbit patello-femoral joint to blunt impact. J Biomech Eng. 1995;117:402–8. doi: 10.1115/1.2794199. [DOI] [PubMed] [Google Scholar]
- 152.Kraus KH, Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet Surg. 2006;35:232–42. doi: 10.1111/j.1532-950X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 153.Chen FH, Rousche KT, Tuan RS. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–82. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 154.Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering–Part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev. 2013;19:308–26. doi: 10.1089/ten.teb.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ferretti M, Marra KG, Kobayashi K, Defail AJ, Chu CR. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006;12:2657–63. doi: 10.1089/ten.2006.12.2657. [DOI] [PubMed] [Google Scholar]
- 157.Keeney M, Pandit A. The osteochondral junction and its repair via bi-phasic tissue engineering scaffolds. Tissue Eng Part B Rev. 2009;15:55–73. doi: 10.1089/ten.teb.2008.0388. [DOI] [PubMed] [Google Scholar]
- 158.Baker MI, Walsh SP, Schwartz Z, Boyan BD. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res B Appl Biomater. 2012;100:1451–7. doi: 10.1002/jbm.b.32694. [DOI] [PubMed] [Google Scholar]
- 159.Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303–11. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 161.Gloria A, De Santis R, Ambrosio L. Polymer-based composite scaffolds for tissue engineering. J Appl Biomater Biomech. 2010;8:57–67. [PubMed] [Google Scholar]
- 162.Frenkel SR, Bradica G, Brekke JH, Goldman SM, Ieska K, Issack P, Bong MR, Tian H, Gokhale J, Coutts RD, Kronengold RT. Regeneration of articular cartilage–evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthritis Cartilage. 2005;13:798–807. doi: 10.1016/j.joca.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 163.Bueno EM, Glowacki J. Cell-free and cell-based approaches for bone regeneration. Nat Rev Rheumatol. 2009;5:685–97. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 164.Ryan G, Pandit A, Apatsidis DP. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials. 2006;27:2651–70. doi: 10.1016/j.biomaterials.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 165.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jiang C-C, Chiang H, Liao C-J, Lin Y-J, Kuo T-F, Shieh C-S, Huang Y-Y, Tuan RS. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J Orthop Res. 2007;25:1277–90. doi: 10.1002/jor.20442. [DOI] [PubMed] [Google Scholar]
- 167.Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18:1304–12. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marquass B, Somerson JS, Hepp P, Aigner T, Schwan S, Bader A, Josten C, Zscharnack M, Schulz RM. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res. 2010;28:1586–99. doi: 10.1002/jor.21173. [DOI] [PubMed] [Google Scholar]
- 169.Marquass B, Somerson JS, Hepp P, Aigner T, Schwan S, Bader A, Josten C, Zscharnack M, Schulz RM. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res. 2010;28:1586–99. doi: 10.1002/jor.21173. [DOI] [PubMed] [Google Scholar]
- 170.Cheng H-W, Luk KDK, Cheung KMC, Chan BP. In vitro generation of an osteochondral interface from mesenchymal stem cell-collagen microspheres. Biomaterials. 2011;32:1526–35. doi: 10.1016/j.biomaterials.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 171.Schuurman W, Klein TJ, Dhert WJA, Weeren PR, Van Hutmacher DW, Malda J. Cartilage regeneration using zonal chondrocyte subpopulations: a promising approach or an overcomplicated strategy? J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1638. [DOI] [PubMed] [Google Scholar]
- 172.Hwang NS, Varghese S, Lee HJ, Theprungsirikul P, Canver A, Sharma B, Elisseeff J. Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS Lett. 2007;581:4172–8. doi: 10.1016/j.febslet.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Coates EE, Fisher JP. Phenotypic variations in chondrocyte subpopulations and their response to in vitro culture and external stimuli. Ann Biomed Eng. 2010;38:3371–88. doi: 10.1007/s10439-010-0096-1. [DOI] [PubMed] [Google Scholar]
- 174.Nöth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002;8:131–44. doi: 10.1089/107632702753503126. [DOI] [PubMed] [Google Scholar]
- 175.Tuli R, Nandi S, Li W-J, Tuli S, Huang X, Manner PA, Laquerriere P, Nöth U, Hall DJ, Tuan RS. Human mesenchymal progenitor cell-based tissue engineering of a single-unit osteochondral construct. Tissue Eng. 2004;10:1169–79. doi: 10.1089/ten.2004.10.1169. [DOI] [PubMed] [Google Scholar]
- 176.Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11:953–63. doi: 10.1089/ten.2005.11.953. [DOI] [PubMed] [Google Scholar]
- 177.Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, Griffith LG, Landeen LK, Ratcliffe A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–51. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 178.Lacombe-Gleize S, Grégoire M, Demignot S, Hecquet C, Adolphe M. Implication of TGF beta 1 in co-culture of chondrocytes-osteoblasts. In Vitro Cell Dev Biol Anim. 1995;31:649–52. doi: 10.1007/BF02634083. [DOI] [PubMed] [Google Scholar]
- 179.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338:762–70. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 180.Cheng H-W, Luk KDK, Cheung KMC, Chan BP. In vitro generation of an osteochondral interface from mesenchymal stem cell-collagen microspheres. Biomaterials. 2011;32:1526–35. doi: 10.1016/j.biomaterials.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 181.Leboy PS, Sullivan TA, Nooreyazdan M, Venezian RA. Rapid chondrocyte maturation by serum-free culture with BMP-2 and ascorbic acid. J Cell Biochem. 1997;66:394–403. doi: 10.1002/(sici)1097-4644(19970901)66:3<394::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 182.Steinert AF, Proffen B, Kunz M, Hendrich C, Ghivizzani SC, Nöth U, Rethwilm A, Eulert J, Evans CH. Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthritis Res Ther. 2009;11:R148. doi: 10.1186/ar2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Acharya C, Adesida A, Zajac P, Mumme M, Riesle J, Martin I, Barbero A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J Cell Physiol. 2012;227:88–97. doi: 10.1002/jcp.22706. [DOI] [PubMed] [Google Scholar]
- 184.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137–45. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Giovannini S, Diaz-Romero J, Aigner T, Heini P, Mainil-Varlet P, Nesic D. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater. 2010;20:245–59. doi: 10.22203/ecm.v020a20. [DOI] [PubMed] [Google Scholar]
- 186.Park JS, Yang HN, Woo DG, Chung H-M, Park K-H. In vitro and in vivo chondrogenesis of rabbit bone marrow-derived stromal cells in fibrin matrix mixed with growth factor loaded in nanoparticles. Tissue Eng Part A. 2009;15:2163–75. doi: 10.1089/ten.tea.2008.0532. [DOI] [PubMed] [Google Scholar]
- 187.Madry H, Luyten FP, Facchini A. Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20:407–22. doi: 10.1007/s00167-011-1705-8. [DOI] [PubMed] [Google Scholar]
- 188.Responte DJ, Lee JK, Hu JC, Athanasiou KA. Biomechanics-driven chondrogenesis: from embryo to adult. FASEB J. 2012;26:3614–24. doi: 10.1096/fj.12-207241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Silyn-Roberts H, Broom ND. Fracture behaviour of cartilage-on-bone in response to repeated impact loading. Connect Tissue Res. 1990;24:143–56. doi: 10.3109/03008209009152430. [DOI] [PubMed] [Google Scholar]
- 190.Shieh AC, Athanasiou KA. Principles of cell mechanics for cartilage tissue engineering. Ann Biomed Eng. 2003;31:1–11. doi: 10.1114/1.1535415. [DOI] [PubMed] [Google Scholar]
- 191.Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 192.Alexander PG, Song Y, Taboas JM, Chen FH, Melvin GM, Manner PA, Tuan RS. Development of a spring-loaded impact device to deliver injurious mechanical impacts to the articular cartilage surface. Cartilage. 2012;4:52–62. doi: 10.1177/1947603512455195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg Am. 1977;59:1068–76. [PubMed] [Google Scholar]
- 194.Haut RC, Ide TM, De Camp CE. Mechanical responses of the rabbit patello-femoral joint to blunt impact. J Biomech Eng. 1995;117:402–8. doi: 10.1115/1.2794199. [DOI] [PubMed] [Google Scholar]
- 195.Koller MR, Emerson SG, Palsson BO. Large-scale expansion of human stem and progenitor cells from bone marrow mononuclear cells in continuous perfusion cultures. Blood. 1993;82:378–84. [PubMed] [Google Scholar]
- 196.Winet H, Bao JY. Fibroblast growth factor-2 alters the effect of eroding polylactide-polyglycolide on osteogenesis in the bone chamber. J Biomed Mater Res. 1998;40:567–76. doi: 10.1002/(sici)1097-4636(19980615)40:4<567::aid-jbm8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 197.Amini AR, Laurencin CT, Nukavarapu SP. Bone Tissue Engineering: Recent Advances and Challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mauney JR, Sjostorm S, Blumberg J, Horan R, O’Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74:458–68. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- 199.Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–74. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rauh J, Milan F, Günther K-P, Stiehler M. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011;17:263–80. doi: 10.1089/ten.TEB.2010.0612. [DOI] [PubMed] [Google Scholar]
- 201.Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–22. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 202.Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136–48. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 203.Stiehler M, Bünger C, Baatrup A, Lind M, Kassem M, Mygind T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2009;89:96–107. doi: 10.1002/jbm.a.31967. [DOI] [PubMed] [Google Scholar]
- 204.Song K, Liu T, Cui Z, Li X, Ma X. Three-dimensional fabrication of engineered bone with human bio-derived bone scaffolds in a rotating wall vessel bioreactor. J Biomed Mater Res A. 2008;86:323–32. doi: 10.1002/jbm.a.31624. [DOI] [PubMed] [Google Scholar]
- 205.Yu X, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Bioreactor-based bone tissue engineering: the influence of dynamic flow on osteoblast phenotypic expression and matrix mineralization. Proc Natl Acad Sci U S A. 2004;101:11203–8. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171–81. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 207.Falsafi S, Koch RJ. Growth of tissue-engineered human nasoseptal cartilage in simulated microgravity. Arch Otolaryngol Head Neck Surg. 2000;126:759–65. doi: 10.1001/archotol.126.6.759. [DOI] [PubMed] [Google Scholar]
- 208.Botchwey EA, Pollack SR, El-Amin S, Levine EM, Tuan RS, Laurencin CT. Human osteoblast-like cells in three-dimensional culture with fluid flow. Biorheology. 2003;40:299–306. [PubMed] [Google Scholar]
- 209.Sikavitsas VI, Bancroft GN, Mikos AG. Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002;62:136–48. doi: 10.1002/jbm.10150. [DOI] [PubMed] [Google Scholar]
- 210.Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, Mikos AG. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99:12600–5. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 212.Gaspar DA, Gomide V, Monteiro FJ. The role of perfusion bioreactors in bone tissue engineering. Biomatter. 2012;2:167–75. doi: 10.4161/biom.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9:549–54. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 214.Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22:1279–88. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 215.Holtorf HL, Jansen JA, Mikos AG. Modulation of cell differentiation in bone tissue engineering constructs cultured in a bioreactor. Adv Exp Med Biol. 2006;585:225–41. doi: 10.1007/978-0-387-34133-0_16. [DOI] [PubMed] [Google Scholar]
- 216.Temple JP, Yeager K, Bhumiratana S, Vunjak-Novakovic G, Grayson WL. Bioreactor cultivation of anatomically shaped human bone grafts. Methods Mol Biol. 2013 doi: 10.1007/7651_2013_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107:3299–304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gardel LS, Serra LA, Reis RL, Gomes ME. Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng Part B Rev. 2014;20:126–46. doi: 10.1089/ten.TEB.2013.0010. [DOI] [PubMed] [Google Scholar]
- 219.Gardel LS, Correia-Gomes C, Serra LA, Gomes ME, Reis RL. A novel bidirectional continuous perfusion bioreactor for the culture of large-sized bone tissue-engineered constructs. J Biomed Mater Res B Appl Biomater. 2013;101:1377–86. doi: 10.1002/jbm.b.32955. [DOI] [PubMed] [Google Scholar]
- 220.Gaspar DA, Gomide V, Monteiro FJ. The role of perfusion bioreactors in bone tissue engineering. Biomatter. 2012;2:167–75. doi: 10.4161/biom.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 222.Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, Jia X, Grayson WL. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A. 2014 doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 223.Rao RR, Stegemann JP. Cell-based approaches to the engineering of vascularized bone tissue. Cytotherapy. 2013;15:1309–22. doi: 10.1016/j.jcyt.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kokemüller H, Jehn P, Spalthoff S, Essig H, Tavassol F, Schumann P, Andreae A, Nolte I, Jagodzinski M, Gellrich N-C. En bloc prefabrication of vascularized bioartificial bone grafts in sheep and complete workflow for custom-made transplants. Int J Oral Maxillofac Surg. 2014;43:163–72. doi: 10.1016/j.ijom.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 225.Cenni E, Perut F, Baldini N. In vitro models for the evaluation of angiogenic potential in bone engineering. Acta Pharmacol Sin. 2011;32:21–30. doi: 10.1038/aps.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hoenig E, Leicht U, Winkler T, Mielke G, Beck K, Peters F, Schilling AF, Morlock MM. Mechanical properties of native and tissue-engineered cartilage depend on carrier permeability: a bioreactor study. Tissue Eng Part A. 2013;19:1534–42. doi: 10.1089/ten.TEA.2012.0538. [DOI] [PubMed] [Google Scholar]
- 227.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–8. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 228.Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9:549–54. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 229.Khan AA, Suits JMT, Kandel RA, Waldman SD. The effect of continuous culture on the growth and structure of tissue-engineered cartilage. Biotechnol Prog. 2009;25:508–15. doi: 10.1002/btpr.108. [DOI] [PubMed] [Google Scholar]
- 230.Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthritis Cartilage. 2010;18:714–23. doi: 10.1016/j.joca.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Malafaya PB, Reis RL. Bilayered chitosan-based scaffolds for osteochondral tissue engineering: influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater. 2009;5:644–60. doi: 10.1016/j.actbio.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 232.Liu X, Jiang H. Preparation of an osteochondral composite with mesenchymal stem cells as the single-cell source in a double-chamber bioreactor. Biotechnol Lett. 2013;35:1645–53. doi: 10.1007/s10529-013-1248-9. [DOI] [PubMed] [Google Scholar]
- 233.Lozito TP, Alexander PG, Lin H, Gottardi R, Cheng A, Tuan RS. Three-dimensional osteochondral microtissue to model pathogenesis of osteoarthritis. Stem Cell Res Ther. 2013;4:S6. doi: 10.1186/scrt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A. 2010;92:1078–93. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 235.Chen K, Shi P, Teh TKH, Toh SL, Goh JC. In vitro generation of a multilayered osteochondral construct with an osteochondral interface using rabbit bone marrow stromal cells and a silk peptide-based scaffold. J Tissue Eng Regen Med. 2013;8:1902–11. doi: 10.1002/term.1708. [DOI] [PubMed] [Google Scholar]
- 236.Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthritis Cartilage. 2010;18:714–23. doi: 10.1016/j.joca.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wartella KA, Wayne JS. Bioreactor for biaxial mechanical stimulation to tissue engineered constructs. J Biomech Eng. 2009;131:044501. doi: 10.1115/1.3049859. [DOI] [PubMed] [Google Scholar]
- 238.Shahin K, Doran PM. Tissue engineering of cartilage using a mechanobioreactor exerting simultaneous mechanical shear and compression to simulate the rolling action of articular joints. Biotechnol Bioeng. 2012;109:1060–73. doi: 10.1002/bit.24372. [DOI] [PubMed] [Google Scholar]
- 239.Schätti O, Grad S, Goldhahn J, Salzmann G, Li Z, Alini M, Stoddart MJ. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 2011;22:214–25. doi: 10.22203/ecm.v022a17. [DOI] [PubMed] [Google Scholar]
- 240.Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A. 2012;100:162–70. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng. 2010;38:2167–82. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mohan N, Gupta V, Sridharan B, Sutherland A, Detamore MS. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds. Biotechnol Bioeng. 2013;111:829–41. doi: 10.1002/bit.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shi X, Zhou J, Zhao Y, Li L, Wu H. Gradient-regulated hydrogel for interface tissue engineering: steering simultaneous osteo/chondro-genesis of stem cells on a chip. Adv Healthc Mater. 2013;2:846–53. doi: 10.1002/adhm.201200333. [DOI] [PubMed] [Google Scholar]


