Abstract
Background
South Africa has dual epidemics of human immunodeficiency virus (HIV) and tuberculosis (TB). Nurse-focused training was combined with onsite mentoring for nurses to improve HIV and TB care. A pre-/postevaluation was conducted in 3 districts in South Africa to assess the effects of the course on clinical patient monitoring and integration of TB and HIV care.
Methods
Two cross-sectional, unmatched samples of patient charts at 76 primary healthcare facilities were collected retrospectively in 2014 to evaluate the impact of training on treatment monitoring. Proportions of HIV patients receiving a viral load test 6 months after initiating antiretroviral therapy (ART) and TB patients receiving end of intensive phase sputum testing were compared pre- and posttraining. Analysis of creatinine clearance testing and integration of TB and HIV care were also performed.
Results
Data were analyzed from 1074 pretraining and 1048 posttraining records among patients initiating ART and from 1063 pretraining and 1008 posttraining among patients initiating TB treatment. Documentation of a 6-month viral load test was 36.3%, and a TB test at end of intensive phase was 70.7%, and neither increased after training. Among patients with a viral load test, the percentage with viral load less than 50 copies/mL increased from 48.6% pretraining compared with 64.2% posttraining (P = .001). Integration of TB and HIV care such as isoniazid preventive therapy increased significantly.
Conclusions
The primary outcome measures did not change after training. However, the evaluation documented many other improvements in TB and HIV care that may have been supported by the course.
Keywords: HIV, integration of care, tuberculosis
The Republic of South Africa has dual epidemics of human immunodeficiency virus (HIV) and tuberculosis (TB). According to UNAIDS, South Africa had an estimated 6.8 million people living with HIV in 2014, with 140000 acquired immune deficiency syndrome-related deaths [1]. In 2014, South Africa also had an estimated 450000 incident cases of TB, representing 834 cases per 100000 people. An estimated 61% of these cases were among people living with HIV [2].
The South African government has expanded policies and access to treatment [3, 4]. In 2013, fixed-dose combination was rolled out as first-line antiretroviral therapy (ART). By 2014, an estimated 2.6 million people were on ART [5]. As of January 2015, all pregnant women and people with a CD4 count of ≤500 cells/mm3 were eligible for lifelong ART. The 2012–2016 National Strategic Plan calls for integration of HIV and TB treatment, and the country is poised to adopt the 2015 World Health Organization guidelines indicating ART for all people living with HIV [6].
South Africa adopted a public health approach to TB and HIV care. Access for treatment is available at all primary healthcare (PHC) clinics, and community health workers and district outreach teams provide additional services. Training programs have been scaled up to support Nurse Initiated Management of ART (NIMART), which is the cornerstone of integrated care [7, 8]. Three pragmatic trials have been conducted on outreach interventions for TB and pulmonary diseases [9], ART in addition to TB and pulmonary diseases [10], and a combination of educational outreach and organizational support in partnership with the rollout of NIMART [11–13]. A mentoring guide was developed by the National Department of Health (NDOH), specific to HIV- and TB-related competencies [14]; nurses should be mentored through a specified number of patient cases to achieve competency in new skills, and they should receive authorization from the Nursing and Pharmacy Councils to prescribe ART.
To support expanded policies and access to treatment, NDOH worked with the (1) International Training & Education Center for Health (I-TECH) to develop a 5-day advanced “TB and HIV Care and Management Course for Health Care Workers” and (2) other partners to provide onsite mentoring programs in diagnosis and management of patients on ART and/or TB treatment. The course provided an update on ART guidelines, newer ART regimens [5], and viral load scale up [15], and it supported the rollout of GeneXpert testing for TB. District health personnel, I-TECH, and partners conducted an evaluation with a pre-/postdesign to test the effects of the course on monitoring of HIV and TB treatment and integration of TB/HIV care. I-TECH did not have the mandate or resources to evaluate the mentoring intervention.
Our hypothesis was that the course would improve monitoring of patients with TB and/or HIV. The precourse assessments found large gaps in patient monitoring. The context supported the success of the training program as described above, and evidence suggests that in-service training in combination with guidelines can improve provider performance [16, 17]. Our 2 primary outcomes were as follows: (1) viral load test 6 months after ART initiation and (2) TB sputum test at the end of intensive phase of TB treatment. To measure them accurately, we collected additional data on the quality of HIV and TB care.
METHODS
Study Population
The course was taught in Frances Baard District in Northern Cape Province and 2 subdistricts in the Eastern Cape: Qaukeni in OR Tambo District and Inxuba-Yethemba in Chris Hani District. National and Provincial Department of Health officials selected the districts, which represented high prevalence of HIV (antenatal prevalence of 23% in Francis Baard, 29% in Chris Hani, and 23% in OR Tambo) and TB (692, 789, and 828 cases per 100000 persons, respectively) [18, 19]. The district health management teams selected the subdistricts. Facilities included all public sector PHC clinics, community health centers (CHCs), mobile/satellite clinics, and district hospitals that routinely provide HIV and TB services.
Course participants were nurses and doctors who were currently managing patients with HIV, TB, and/or drug-resistant TB in clinical practice. Many had previous training in NIMART and other foundational trainings in HIV or TB.
Anonymous patient data were collected for people diagnosed with HIV who started ART during the evaluation time periods (see Supplemental File 1), and whose recorded age was 15 or more years. Anonymous patient data were also collected for people diagnosed with TB who started TB treatment during the evaluation time periods, whose recorded age was 8 or more years, and whose weight qualified for adult TB treatment guidelines.
Intervention
The objective of the 5-day TB and HIV Care and Management Course for Health Care Workers was to strengthen diagnosis and management of patients infected with HIV and TB including drug-resistant TB in public facilities. The curriculum contained 11 sessions (Table 1), and it was delivered from February to July 2013 with adult learning methods and standardized materials including case studies, role plays, large- and small-group work and discussions, individual work, demonstration and practice, and simulated patient stations. The content could be adapted to emphasize specific concerns in each district/subdistrict that were identified during precourse assessments, such as isoniazid preventive therapy (IPT) in the Eastern Cape.
Table 1.
Topics of the “TB and HIV Care and Management Course for Health Care Workers”
| Session | Topic | Suggested Duration |
|---|---|---|
| 1 | Introduction and TB and HIV Review | 2 hours |
| 2 | Diagnosis of HIV in Adults and Children | 1 hour, 15 minutes |
| 3 | Diagnosis and Management of Other Opportunistic Infections | 2 hours, 35 minutes |
| 4 | Antiretroviral Therapy for the Treatment of HIV Infection | 6 hours |
| 5 | Diagnosis of TB and Drug-Resistant TB in Adults and Children | 3 hours, 15 minutes |
| 6 | Management and Treatment of Pulmonary and Extra-Pulmonary TB | 3 hours, 45 minutes |
| 7 | Drug Resistance and Multidrug-Resistant TB | 3 hours |
| 8 | Management of TB in an HIV-Infected Person and Adherence | 2 hours, 20 minutes |
| 9 | Infection Control and Prevention | 2 hours, 35 minutes |
| 10 | Community-Based Care and Patient Education | 2 hours |
| 11 | Putting it all together - Using Simulated Patient stations to apply learning | 2 hours |
Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis.
The course was taught by I-TECH in collaboration with Regional Training Centers and provinces at training venues in district/subdistricts. It was offered more than once in consecutive weeks so that health professionals who missed the first course could attend a later one. It was delivered 11 times and attended by 327 health professionals: 3 in Inxuba Yethemba (N = 120), 6 in Frances Baard (N = 158), and 2 in Qaukeni (N = 49). Among participants, 287 (87.8%) were nurses, 10 (3.1%) were doctors, and 30 (9.2%) listed as “other”. There were 587 staff in 66 of 76 (87%) facilities, 432 full-time and 155 part-time. We estimated that 56% were trained with 3 caveats: 1) data were missing for 10 facilities, 2) parttime professionals who worked at multiple facilities may have been double-counted, and 3) staffing may have changed between training and data collection.
Mentoring was provided after the course by district support partners at public facilities between March 2013 and May 2014. The purpose was to support transfer of knowledge from the course to clinical practice, assist facilities in developing continuous quality improvement plans, and support infection control at clinics.
Outcomes
The primary outcome of ART patient chart reviews was an improvement in monitoring viral load 6 months after initiation. It was measured as the pre- and posttraining difference in the percentage of patients with a documented test 4 to 8 months after ART initiation. Among patients with viral load test results, the percentage with a detectable viral load was defined as <50 copies/mL, and suppressed viral load was defined as <1000 copies/mL [5]. Additional outcome measures were creatinine clearance (CrCl) monitoring, TB screening, and initiation of IPT. Screening for TB as well as IPT followed national TB guidelines. For TB, patients were screened for a cough more than 2 weeks, weight loss, fever, and night sweats, and patients received a sputum test when they screened positive for one of the symptoms [20]. Two types of HIV patients were eligible for IPT: (1) patients without TB symptoms and (2) patients who screened positive for TB symptoms and whose TB sputum test was negative.
For TB chart reviews, the primary outcome was an improvement in monitoring at the end of the intensive treatment phase. It was measured as the pre- and posttraining difference in the percentage of patients with a documented sputum test 1 to 4 months after TB treatment initiation. Tuberculosis treatment monitoring was performed using sputum microscopy or GeneXpert with confirmatory sputum cultures for positive patients. GeneXpert was more common during the posttraining periods. Additional outcome measures were TB diagnosis by sputum test, HIV testing, and ART initiation.
Sample Size
Using assessment data, sample size calculations accounted for (1) variation in the number of records available per facility and (2) clustering at the facility level [21]. All sample size calculations assumed 80% power, a 2-tailed 5% type-I error rate, an intraclass correlation coefficient of 0.2, and 10% loss to follow-up of facilities across time periods. For HIV patients, we anticipated a fixed number of 71 clusters. Assuming a pretraining percentage of patients who received a 6-month viral load test of 57.2%, and an average of 16.0 patient records per facility with a coefficient of variation of 0.70, this evaluation was powered to detect a 15.0% absolute increase in the proportion of patients who received the test. For TB patients, we anticipated 73 clusters. Assuming a pretraining percentage of patients who received an end of intensive phase sputum test of 65.6%, and an average of 16.0 patient records per facility with a coefficient of variation of 0.86, this evaluation was powered to detect a 15.0% absolute increase in the proportion of patients who received the test.
Data Collection
Data collection teams visited facilities from February 10 to May 30, 2014 and collected clinical data from ART and TB patient charts and registers during time periods in which patient monitoring occurred pre- and posttraining (see Supplementary File 1). For ART, we selected patients initiating ART at least 8 months before the time period. For example, to measure 6-month viral load monitoring in Francis Baard district from September 2012 to January 2013, we selected a sample that initiated treatment from January to May 2012. Likewise, we selected patients who initiated TB treatment at least 3 months before the time period for TB monitoring.
Human immunodeficiency virus registers were used to identify newly diagnosed HIV patients from each time period, starting from the most recent month and moving into the more distant past with the goal of collecting data on 16 patients during 5 months. In facilities with more HIV patients, we stopped data collection at a maximum of 25 patients, which meant the time period was less than 5 months. No clinic had a sample of patients per time period with more than 25 patients or 2.5% of the sample. In facilities with fewer HIV patients, we stopped data collection at a maximum of 12 months, which meant that data on fewer than 16 patients were collected. Tuberculosis registers were used in the same way to identify newly diagnosed TB patients, and the same maxima were applied.
Data were simultaneously collected and entered electronically by data collection teams using Open Data Kit (Open Data Kit, 2013 Seattle, WA) [22]. Data were collected retrospectively by 3 teams, composed of 2 I-TECH study staff, and 1 representative from the local department of health district/subdistrict and/or the partner responsible for mentoring. All team members attended a 1-day training session on the data collection forms. Registers, charts, and laboratory records with monitoring results differed across facilities. In addition, some clinics used electronic medical systems, and the teams had to request electronic data. The teams collected data from the most reliable source at the facility and entered the source on the collection forms. Data were uploaded onto FormHub (Modi Research Group, 2012/13, New York, NY) each evening. Data collection methods were piloted at 12 facilities in Emalahleni subdistrict.
Data Analysis
Descriptive statistics were calculated to compare study outcomes pre- and posttraining. We report the number and percentage of services that were documented in the patient files or registers. Statistical significance was determined using a χ2 test of the difference in the proportion of patients with appropriate treatment pre- and posttraining adjusting for clustering at the facility level. Among HIV patients with a documented 6-month viral load test, we calculated the median and interquartile range (IQR) of viral load results and compared them between the pre- and posttraining samples using a Kruskal-Wallis equality-of-populations test.
We performed multivariate logistic regression analyses to test the main effect of the time period with covariates for district/subdistrict, patient age, and patient gender. Regression analyses were clustered on the facility with robust standard errors using the binomial family and a logit link to estimate the odds ratio (OR). Patient age and gender did not alter the main effects across time periods and were not statistically significant, and consequently these were not included in the multivariate analyses reported below. Results were presented with 95% confidence intervals (CIs). All analyses were performed with Stata version 11 (StataCorp, College Station, TX).
Ethical Considerations
The evaluation protocol was approved by the Walter Sisulu University Faculty of Health Sciences, Human Research Committee, Protocol Number 050/2012. An informed consent process was not necessary for health professionals, because they were invited to participate in the course by NDOH as part of their normal responsibilities. It was not necessary for patients, because no identifying information was collected and all data were reported in aggregate. The Human Subjects Division of the University of Washington determined that the evaluation did not meet the regulatory definition of research under 45 CFR 46.102 (d).
RESULTS
Participant Flow
Data were collected from 76 facilities including 63 PHCs, 9 CHCs, 3 mobile/satellite clinics, and 1 district hospital. Data were analyzed from facilities that had at least 3 records in both the pretraining and posttraining periods. Among 67 facilities, 1074 (93%) pretraining ART patient records and 1048 (93%) posttraining were analyzed (Figure 1). Most patient records that were excluded had missing or out-of-range ART treatment start dates. Four months after ART initiation, 111 (10.3%) pretraining records and 192 (18.3%) posttraining were not eligible for a viral load test due to loss to follow-up, transfer, or death. Among 64 facilities, 1063 (97%) pretraining TB patient records and 1008 (95%) posttraining were analyzed. At the end of the intensive phase, 31 (2.9%) pretraining records and 34 (3.4%) posttraining were not eligible for sputum test due to treatment default, transfer, or death. Samples are described in Table 2.
Figure 1.
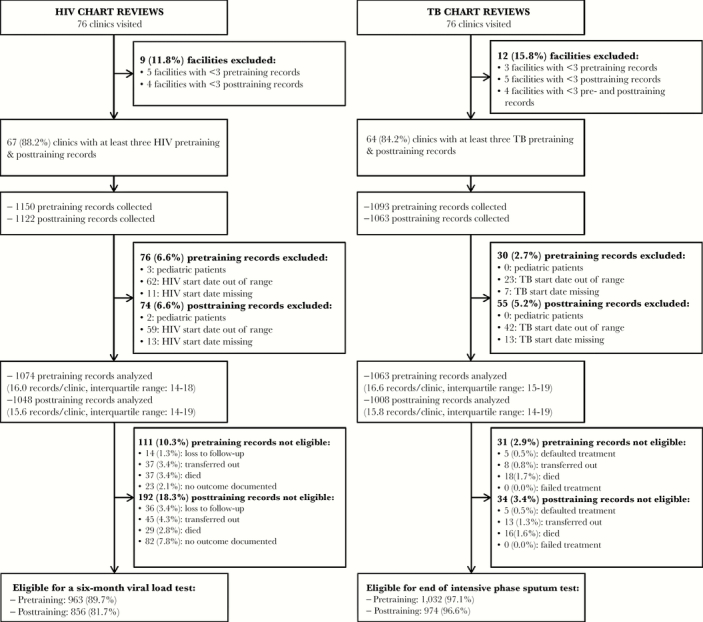
Participant flow chart for data collection for human immunodeficiency virus (HIV) and tuberculosis (TB) chart reviews.
Table 2.
Description of the Evaluation Samples
| ART Chart Reviews (N = 2122) | TB Chart Reviews (N = 2071) | |||||
|---|---|---|---|---|---|---|
| Total | Pretraining | Posttraining | Total | Pretraining | Posttraining | |
| Demographic Factor | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Gender | ||||||
| Female | 1455 (68.6) | 698 (65.0) | 757 (72.2) | 897 (43.3) | 463 (43.6) | 434 (43.1) |
| Male | 661 (31.1) | 373 (34.7) | 288 (27.5) | 1166 (56.3) | 596 (56.1) | 570 (56.5) |
| Not documented | 6 (0.3) | 3 (0.3) | 3 (0.3) | 8 (0.4) | 4 (0.4) | 4 (0.4) |
| Age (years) | ||||||
| 8–14a | – | – | – | 34 (1.6) | 17 (1.6) | 17 (1.7) |
| 15–24 | 312 (14.7) | 115 (10.7) | 197 (18.8) | 305 (14.7) | 165 (15.5) | 140 (13.9) |
| 25–34 | 822 (38.7) | 414 (38.6) | 408 (38.9) | 598 (28.9) | 312 (29.4) | 286 (28.4) |
| 35–44 | 553 (26.1) | 296 (27.6) | 257 (24.5) | 503 (24.3) | 249 (23.4) | 254 (25.2) |
| ≥45 | 421 (19.8) | 240 (22.3) | 181 (17.3) | 552 (26.7) | 278 (26.2) | 274 (27.2) |
| Unknown | 14 (0.7) | 9 (0.8) | 5 (0.5) | 79 (3.8) | 42 (4.0) | 37 (3.7) |
| Year of diagnosisb | ||||||
| Before 2011 | 599 (28.2) | 336 (31.3) | 263 (25.1) | – | – | – |
| 2011 | 246 (11.6) | 177 (16.5) | 69 (6.6) | – | – | – |
| 2012 | 525 (24.7) | 361 (33.6) | 164 (15.7) | – | – | – |
| 2013 | 449 (21.2) | 0 (0.0) | 449 (42.8) | – | – | – |
| Not documented | 303 (14.3) | 200 (18.6) | 103 (9.8) | – | – | – |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; TB, tuberculosis.
aPediatric HIV patients (<15 years) were not assessed in this evaluation.
bYear of TB diagnosis was not documented. HIV patients were diagnosed with HIV as early as 1993.
Human Immunodeficiency Virus Monitoring After Treatment Initiation
Six-month viral load testing was documented for 37.0% of patients pretraining and 35.6% posttraining (P = .678) (Table 3A). After adjusting for district/subdistrict, the adjusted odds of a documented viral load test were the same across time periods (OR = 1.0; 95% CI, 0.7–1.3; P = .716). There was relatively lower variation between facilities compared with within facilities, with an intraclass correlation coefficient of 0.156 in the pretraining sample and 0.172 in the posttraining. Among patients with a documented test, median viral load decreased from 75 copies/mL (IQR, <50–604) pretraining to <50 copies/mL (IQR, <50–118) posttraining (P = .003). Undetectable viral load (<50 copies/mL) increased from 48.6% pretraining to 64.2% posttraining (P = .002). However, viral suppression (<1000 copies/mL) did not increase significantly (80.6% pretraining vs. 86.3% posttraining; P = .116).
Table 3.
Receipt of HIV and TB Monitoring Services After Treatment Initiation Among Eligible Participants
| Total | Pretraining | Posttraining | ||
|---|---|---|---|---|
| Services Received | n (%) | n (%) | n (%) | P a |
| A. ART CHART REVIEWS | ||||
| Receipt of an HIV viral load test 4–8 months after ART initiation (N = 1819)b | ||||
| Yes | 661 (36.3) | 356 (37.0) | 305 (35.6) | .678 |
| No | 1158 (63.7) | 607 (63.0) | 551 (64.4) | |
| Among those on a tenofovir-based regimen, receipt of a creatinine clearance test 5–7 months after ART initiation (N = 1765) | ||||
| Yes | 352 (19.9) | 166 (17.6) | 186 (22.6) | .089 |
| No | 1413 (80.1) | 777 (82.4) | 636 (77.4) | |
| B. TB CHART REVIEWS | ||||
| Initial TB test documented at diagnosis (N = 2071) | ||||
| Yes | 1818 (87.8) | 911 (85.7) | 907 (90.0) | .011 |
| No | 253 (12.2) | 152 (14.3) | 101 (10.0) | |
| Initial TB test documented positive at diagnosis (N = 1818)c | ||||
| Yes | 1394 (76.7) | 629 (69.0) | 765 (84.3) | <.001 |
| No | 424 (23.3) | 282 (31.0) | 142 (15.7) | |
| End of intensive phase TB test documented 1–4 months after TB treatment initiation (N = 2006)d | ||||
| Yes | 1418 (70.7) | 726 (70.3) | 692 (71.0) | .810 |
| No | 588 (29.3) | 306 (29.7) | 282 (29.0) | |
| If end of intensive phase TB was positive, additional TB test documented 0.5–1.5 months later (N = 167) | ||||
| Yes | 65 (38.9) | 25 (32.9) | 40 (44.0) | .185 |
| No | 102 (61.1) | 51 (67.1) | 51 (56.0) | |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; TB, tuberculosis.
a χ 2 test adjusting for clustering at the facility level. Statistically significant differences pre- and posttraining (p < .05) are labeled in bold.
bExcludes 303 patients (111 pretraining [10.3%] and 192 posttraining [18.3%]) who were not in ART treatment during the 6-month monitoring period due to death, transfer to another facility, treatment default, or lost to follow-up.
cExcludes 253 patients with no documentation of a TB test at diagnosis.
dExcludes 65 patients (31 pretraining [2.9%] and 34 posttraining [3.4%]) who were not in TB treatment at the end of the intensive phase due to death, transfer to another facility, treatment default, or lost to follow-up.
Initiation of patients on a fixed-dose combination regimen of tenofovir/lamivudine/efavirenz increased from 2.8% pretraining to 63.7% posttraining (P < .001). Documentation of CrCl testing among patients who initiated ART and were on a regimen with tenofovir was measured at 5 to 7 months after treatment initiation and increased from 17.6% pretraining to 22.6% posttraining (P = .089).
Tuberculosis Monitoring After Treatment Initiation
At TB treatment initiation, documentation of sputum testing increased from 85.7% pretraining to 90.0% posttraining (P = .011) (Table 3B). Among patients with a sputum test result at initiation, 69.0% pretraining and 84.3% posttraining (P < .001) had a positive test result documented. The percentage of tests performed with GeneXpert increased from 27.3% to 67.7% (P < .001).
Documentation of sputum testing 1 to 4 months after initiation of TB treatment was 70.3% pretraining and 71.0% posttraining (P = .810). After adjusting for district/subdistrict, the odds of having a documented TB test were the same across time periods (OR = 1.0; 95%CI, 0.8–1.4; P = .793). The interclass correlation coefficient was 0.162 pretraining and posttraining 0.104. Among patients with a positive test result at the end of intensive phase (n = 167), the percentage who received a second sputum test 0.5 to 1.5 months later increased from 32.9% to 44.0% (P = .185).
Integration of Tuberculosis/Human Immunodeficiency Virus Care
Documentation of TB screening among patients who initiated ART increased from 71.8% pretraining to 81.3% posttraining (P = .003) (Table 4A). Documentation of IPT screening increased from 41.2% pretraining to 63.1% posttraining (P < .001), and IPT initiation increased from 32.8% pretraining to 41.0% posttraining (P = .005). Among 892 patients with a documented TB sputum test, 122 (13.7%) had a positive smear. There were 53 (4.9%) new TB diagnoses pretraining and 69 (6.6%) posttraining (P = .098). Documentation of TB treatment initiation in HIV charts was 75.5% pretraining compared with 78.3% posttraining among patients who tested positive for TB (P = .719).
Table 4.
Integration of Care Between TB/HIV Services, Before and After Training
| Total | Pretraining | Posttraining | ||
|---|---|---|---|---|
| Services Received | n (%) | n (%) | n (%) | P a |
| A. ART CHART REVIEWS | ||||
| Documented TB symptom screen (N = 2122) | ||||
| Yes | 1623 (76.5) | 771 (71.8) | 852 (81.3) | .003 |
| No | 499 (23.5) | 303 (28.2) | 196 (18.7) | |
| Screened for IPT (N = 2122) | ||||
| Yes | 1103 (52.0) | 442 (41.2) | 661 (63.1) | <.001 |
| No | 1019 (48.0) | 632 (58.8) | 387 (36.9) | |
| Documented initating IPT (N = 2122) | ||||
| Yes | 782 (36.9) | 352 (32.8) | 430 (41.0) | .005 |
| No | 1340 (63.1) | 722 (67.2) | 618 (59.0) | |
| Documented new TB diagnoses (N = 2122) | ||||
| Yes | 122 (5.7) | 53 (4.9) | 69 (6.6) | .098 |
| No | 2000 (94.3) | 1021 (95.1) | 979 (93.4) | |
| Documented initiating TB treatment, among those with a positive TB test result (N = 122) | ||||
| Yes | 94 (77.0) | 40 (75.5) | 54 (78.3) | .719 |
| No | 28 (23.0) | 13 (24.5) | 15 (21.7) | |
| B. TB CHART REVIEWS | ||||
| Has a documented HIV test, among those not known positive at diagnosis (N = 1629)b | ||||
| Yes | 1285 (78.9) | 629 (77.6) | 656 (80.2) | .396 |
| No | 344 (21.1) | 182 (22.4) | 162 (19.8) | |
| Documented initiating ART, among those who are HIV positive (N = 1138)c | ||||
| Yes | 754 (66.3) | 391 (66.0) | 363 (66.5) | .903 |
| No | 384 (33.7) | 201 (34.0) | 183 (33.5) | |
| Documented initiating ART before or within 8 weeks of diagnosis, among those who tested HIV positive (N = 989)d | ||||
| Yes | 516 (52.2) | 250 (49.5) | 266 (55.0) | .179 |
| No | 473 (47.8) | 255 (50.5) | 218 (45.0) | |
| ART start date documented, if documented initiating ART (N = 754) | ||||
| Yes | 657 (87.1) | 335 (85.7) | 322 (88.7) | .303 |
| No | 97 (12.9) | 56 (14.3) | 41 (11.3) | |
| CD4 test documented, if documented initiating ART (N = 754) | ||||
| Yes | 657 (87.1) | 348 (89.0) | 309 (85.1) | .246 |
| No | 97 (12.9) | 43 (11.0) | 54 (14.9) | |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; TB, tuberculosis.
a χ 2 test adjusting for clustering at the facility level. Statistically significant differences pre- and posttraining (p < .05) are labeled in bold.
bA total of 445 (21.5%) of individuals were known to be HIV positive at TB diagnosis.
cIncludes N = 442 known positives at the time of diagnosis and 696 patients who tested positive for HIV.
dExcluding 149 individuals who were documented initiating ART but have no ART start date documented (N = 97) or an incomplete ART start date (N = 52).
The percentage of TB patients with a documented HIV test, ART initiation, ART start date, and CD4 test were compared between time periods (Table 4B). Documentation of an HIV test among those not known to be HIV positive was 77.6% pretraining and 80.2% posttraining (P = .396). Among patients with test results, 54% were positive for HIV. Among patients who tested positive for HIV, documentation of ART initiation was 66.0% pretraining and 66.5% posttraining (P = .903).
DISCUSSION
Documentation of 6-month viral load test after initiation of ART and TB sputum test at the end of the intensive phase did not increase after training. Documentation of CrCl testing increased within 5 to 7 months of initiation, but the effect was not statistically significant. Among patients who initiated TB treatment, the increase in documentated sputum test before TB treatment initiation was statistically significant and may be partially attributable to the course. Among patients who initiated ART, statistically significant increases were noted in screening for TB or IPT eligibility and initiation, but these changes would not be attributable to the course when the screening occured at initiation of ART. Most ART patients in the posttraining sample initiated ART before the course, because the evaluation was designed for the 6-month viral load test.
The proportion of ART patients with an undetectable viral load increased significantly posttraining. The course provided an update on the guidelines that expanded ART eligibility to patients at a CD4 count ≤350 cells/mm3 and fixed-dose combination treatment. However, the improvements may be attributable to patients initiating treatment with lower viral load, better adherence to a simpler regimen, the course, or some combination.
South African guidelines are stated in terms of viral supression, and we did not observe a significant change in it. Likewise, the trial of educational outreach and organizational support for the rollout of NIMART [11] reported no significant improvement in viral suppression defined as viral load <400 copies/mL among the 62% of patients with at least 6 months of ART and test results. The trial of educational outreach for TB and pulmonary disease plus ART reported no improvement in viral suppression among the 15% of patients with test results [10]. In contrast, a prospective, observational study of home-based HIV testing reported an improvement in viral suppression within 12 months of ART among all participants [23]. The percentage of ART patients in our sample who had a viral load <1000 copies/mL was 80.6% pretraining, making it difficult to observe a significant increase. Given that only 36.3% had a documented 6-month viral load test, the high rate of viral suppression may not be representative if the patients who adhere to testing were more likely to adhere to treatment or be treated in high-performing facilities.
We also observed an increase in new TB diagnoses similar to Fairall et al [11] and Zwarenstein et al [10]. In our evaluation, however, the difference was not statistically significant.
The lack of improvement in monitoring outcomes may be partially explained by supply logistics. Facility managers reported stock-outs of the following supplies in approximately 20% of facilities: HIV tests, specimen containers for blood, sputum containers, and dried blood spot forms. Shorter, more frequent trainings and offering participants an opportunity to implement their new knowledge in a phased approach may also be more effective, as demonstrated in an onsite training evaluation [24]. The course curriculum provided instructions for shorter, facility-based educational outreach.
Summarizing the strengths, the course complimented existing national training programs and focused on training new guidelines, advances in HIV and TB testing, treatment monitoring, and integration of TB/HIV care. Within each district/subdistrict, the course was adapted to specific concerns that were identified during assessments. The evaluation measured the effects of training on a large sample of facilities and patients. The electronic data collection method was innovative and accommodated the heterogeneity of record keeping methods.
A first limitation to the evaluation was that we did not collect data from patient records about the health professionals who treated them at each visit and link it to training records. Some posttraining patients may have been treated by professionals who did not attend the training or transferred to the facility afterwards. Second, the pre-/postdesign did not control for guideline changes and other facility improvements. Third, onsite mentoring was initiated at some facilities during the posttraining period, and its partial effects could have been reflected in these results. However, attribution is irrelevant in the absence of effects on the primary outcomes. Fourth, although many patients were coinfected with HIV and TB, the majority of facilities maintained separate TB and HIV files, and it was not possible to link them. Finally, we relied on documentation in registers and patient charts, and we could not distinguish between missing information and missing services.
CONCLUSIONS
The course was not associated with improvements in monitoring among HIV patients using 6-month viral load testing or among TB patients using sputum testing at the end of the intensive phase. The pre-/postevaluation design provided evidence of improvements in treatment for people with HIV, TB, or coinfection that may have been supported by the course.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
Supplementary Material
Acknowldgments
International Training & Education Center for Health expresses its gratitude to the South African National Department of Health, the Northern Cape Department of Health, the Eastern Cape Department of Health, and district/subdistrict mentoring partners Health Systems Trust and Africare for their support of this work. We also acknowledge Annatjie Peters, Omphemetse Mokgatlhe, and Satvinder Singh at the Centers for Disease Control and Prevention for technical expertise and leadership. We also thank Statistical Consulting Services at the Departments of Biostatistics and Statistics (University of Washington) for advice in developing the sample size calculations.
Disclaimer. The contents of the report do not represent the views of the US Department of Health and Human Services, Health Resources and Services Administration (HRSA) or the US Government.
Financial support. This study was funded by Cooperative Agreement U91HA06801-06-00 from the HRSA.
Potential conflicts of interest. All authors: No reported conflicts.All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. HIV and AIDS Estimates. Available at: http://www.unaids.org/en/regionscountries/countries/southafrica/ Accessed 11 April 2016.
- 2. World Health Organization. Global Tuberculosis Report, 2015. Geneva, Switzerland: World Health Organization; Available at: http://www.who.int/tb/publications/global_report/en/ Accessed 20 February 2016. [Google Scholar]
- 3. Abdool Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 2009; 374:921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klausner JD, Serenata C, OʼBra H, et al. Scale-up and continuation of antiretroviral therapy in South African treatment programs, 2005–2009. J Acquir Immune Defic Syndr 2011; 56:292–5. [DOI] [PubMed] [Google Scholar]
- 5. National Department of Health, South Africa. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. Pretoria, South Africa: National Department of Health; 2014. [Google Scholar]
- 6. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: World Health Organization; Available at: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ Accessed 11 April 2016. [PubMed] [Google Scholar]
- 7. Nyasulu JC, Muchiri E, Mazwi SL, Ratshefola M. NIMART rollout to primary healthcare facilities increases access to antiretrovirals in Johannesburg: An interrupted time series analysis. S Afr Med J 2013; 103:232–6. [DOI] [PubMed] [Google Scholar]
- 8. Cameron D, Gerber A, Mbatha M, et al. Nurse-initiation and maintenance of patients on antiretroviral therapy: are nurses in primary care clinics initiating ART after attending NIMART training? S Afr Med J 2012; 102:98–100. [DOI] [PubMed] [Google Scholar]
- 9. Fairall LR, Zwarenstein M, Bateman ED, et al. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ 2005; 331:750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zwarenstein M, Fairall LR, Lombard C, et al. Outreach education for integration of HIV/AIDS care, antiretroviral treatment, and tuberculosis care in primary care clinics in South Africa: PALSA PLUS pragmatic cluster randomised trial. BMJ 2011; 342:d2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet 2012; 380:889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colvin CJ, Fairall L, Lewin S, et al. Expanding access to ART in South Africa: the role of nurse-initiated treatment. S Afr Med J 2010; 100:210–2. [DOI] [PubMed] [Google Scholar]
- 13. Georgeu D, Colvin CJ, Lewin S, et al. Implementing nurse-initiated and managed antiretroviral treatment (NIMART) in South Africa: a qualitative process evaluation of the STRETCH trial. Implement Sci 2012; 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Department of Health, South Africa. Clinical Mentorship Manual for Integrated Services. National Department of Health; Available at: http://www.sahivsoc.org Accessed 20 February 2016. [Google Scholar]
- 15. Centers for Disease Control and Prevention. Scale-up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6446a3.htm Accessed April 11, 2016. [Google Scholar]
- 16. Zurovac D, Rowe AK. Quality of treatment for febrile illness among children at outpatient facilities in sub-Saharan Africa. Ann Trop Med Parasitol 2006; 100:283–96. [DOI] [PubMed] [Google Scholar]
- 17. Zurovac D, Rowe AK, Ochola SA, et al. Predictors of the quality of health worker treatment practices for uncomplicated malaria at government health facilities in Kenya. Int J Epidemiol 2004; 33:1080–91. [DOI] [PubMed] [Google Scholar]
- 18. Massyn N, Day C, Dombo M, et al. District Health Barometer 2014/15. Durban, South Africa: Health Systems Trust; Available at: http://www.hst.org.za/publications/district-health-barometer-201415-1 Accessed 25 September 2016. [Google Scholar]
- 19. Massyn N, Peer N, Padarath A, et al. District Health Barometer 2012/13. Durban, South Africa: Health Systems Trust; Available at: http://www.hst.org.za/publications/district-health-barometer-201213 Accessed 25 September 2016. [Google Scholar]
- 20. National Department of Health (NDOH), South Africa. Guidelines for Tuberculosis Preventative Therapy among HIV Infected Individuals in South Africa. Pretoria, South Africa: National Department of Health (NDOH), South Africa; Available at: http://www.who.int/hiv/pub/guidelines/south_africa_hiv_tb.pdf. [Google Scholar]
- 21. Hemming K, Girling AJ, Sitch AJ, et al. Sample size calculations for cluster randomised controlled trials with a fixed number of clusters. BMC Med Res Methodol 2011; 11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brunette W, Sundt M, Dell N, Chaudhri R, Breit N, Borriello G. Open Data Kit 2.0: Expanding and Refining Information Services for Developing Regions In HotMobile.; 2013. http://dl.acm.org/citation.cfm?id=2444790. [Google Scholar]
- 23. Barnabas RV, van Rooyen H, Tumwesigye E, et al. Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV 2014; 1:e68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manabe YC, Zawedde-Muyanja S, Burnett SM, et al. Rapid improvement in passive tuberculosis case detection and tuberculosis treatment outcomes after implementation of a bundled laboratory diagnostic and on-site training intervention targeting mid-level providers. Open Forum Infect Dis 2015; 2:ofv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


