Abstract
Background
Data on oseltamivir treatment among hospitalized community-acquired pneumonia (CAP) patients are limited.
Methods
Patients hospitalized with CAP at 6 hospitals during the 2010−2012 influenza seasons were included. We assessed factors associated with oseltamivir treatment using logistic regression.
Results
Oseltamivir treatment was provided to 89 of 1627 (5%) children (<18 years) and 143 of 1051 (14%) adults. Among those with positive clinician-ordered influenza tests, 39 of 61 (64%) children and 37 of 48 (77%) adults received oseltamivir. Among children, oseltamivir treatment was associated with hospital A (adjusted odds ratio [aOR], 2.76; 95% confidence interval [CI], 1.36−4.88), clinician-ordered testing performed (aOR, 2.44; 95% CI, 1.47−5.19), intensive care unit (ICU) admission (aOR, 2.09; 95% CI, 1.27−3.45), and age ≥2 years (aOR, 1.43; 95% CI, 1.16−1.76). Among adults, oseltamivir treatment was associated with clinician-ordered testing performed (aOR, 8.38; 95% CI, 4.64−15.12), hospitals D and E (aOR, 3.46−5.11; 95% CI, 1.75−11.01), Hispanic ethnicity (aOR, 2.06; 95% CI, 1.18−3.59), and ICU admission (aOR, 2.05; 95% CI, 1.34−3.13).
Conclusions
Among patients hospitalized with CAP during influenza season, oseltamivir treatment was moderate overall and associated with clinician-ordered testing, severe illness, and specific hospitals. Increased clinician education is needed to include influenza in the differential diagnosis for hospitalized CAP patients and to test and treat patients empirically if influenza is suspected.
Keywords: community-acquired pneumonia, influenza, oseltamivir
Community-acquired pneumonia (CAP) is a common cause of hospitalization in the United States [1–3]. Influenza viruses are a known cause of CAP [4, 5]. Pneumonia is the most common severe complication of influenza virus infection in all age groups [6–9]. The Advisory Committee on Immunization Practices (ACIP), Infectious Disease Society of America (IDSA), and Pediatric Infectious Disease Society (PIDS) guidelines currently recommend use of influenza antiviral treatment in all hospitalized patients with suspected or confirmed influenza [10–12], particularly during the influenza season [10, 12]. Furthermore, the IDSA guidelines recommend influenza testing for hospitalized patients with CAP during the influenza season [10, 12, 13]. Although studies have reported on influenza testing and antiviral treatment and factors associated with antiviral treatment among hospitalized patients with influenza, the factors associated with antiviral treatment specifically among hospitalized patients with CAP are not well described.
The Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study was a prospective, multicenter, population-based, active surveillance study of the incidence and etiology of CAP among children and adults requiring hospitalization in the United States [4, 5]. We describe the epidemiologic and clinical factors associated with oseltamivir treatment among patients enrolled in the EPIC study.
METHODS
Study Setting and Population
Children (<18 years) and adults hospitalized with clinical and radiographic CAP were prospectively enrolled between January 2010 and June 2012 at 8 hospitals in Chicago, Memphis, Nashville, and Salt Lake City; full details of the EPIC study have previously been described [4, 5]. Demographic and clinical data were collected by patient interview and medical chart abstraction using a standardized form and entered into a centralized database. Outpatient influenza antiviral data were derived from patient interview, and inpatient antiviral data were derived from medical chart abstraction. This study was approved by the institutional review boards at each institution and the CDC. For this analysis, the 6 hospitals (3 pediatric, 3 adult) with available data were included.
Influenza Laboratory Testing
Naso/oropharyngeal swabs were systematically obtained from all enrolled patients for influenza virus testing using real-time reverse-transcription polymerase chain reaction (PCR) in the research laboratory at each site (hereafter referred to as research-associated influenza tests). These results were not available to clinicians at any time during clinical care. Clinician-ordered influenza tests and oseltamivir use was based on the clinician’s judgment, independent of the study protocol. Influenza diagnostic tests types and availability differed among hospitals as reported in the results.
Statistical Analysis and Definitions
All patients who received an antiviral agent received oseltamivir; 4 patients received oseltamivir plus an additional influenza antiviral agent. Because oseltamivir was most common, we limited the analysis to oseltamivir specifically. We compared the demographic and clinical characteristics, including clinical testing practices, between patients treated and not treated with oseltamivir. Treatment was defined as receiving oseltamivir during hospitalization for any period of time, including if treatment was started as an outpatient before hospitalization. Patients who received oseltamivir before but not during hospitalization were excluded.
For each study year, influenza season was defined as October 1−April 30 except for the first year, which was defined as January 1, 2010−April 30, 2010 because enrollment began on January 1, 2010. Influenza season reflected periods when any influenza viruses were circulating based on laboratory-confirmed influenza surveillance in the study hospital regions [14] and laboratory-confirmed influenza positive tests in the EPIC study [4, 5]. We also performed a sensitivity analysis limiting the influenza season to peak periods of influenza circulation (January−March).
Documentation of clinician-ordered influenza testing was not a mandatory variable in the EPIC study. However, all 3 pediatric hospitals (hospitals A, B, C) recorded this consistently and are included. Data on clinician-ordered testing were incomplete for 2 adult hospitals (n = 436 patients) that were excluded, and thus 3 of 5 adult hospitals (D, E, F) are included.
We conducted bivariate analysis to assess associations between covariates and oseltamivir treatment using χ2 or Fisher’s exact test for categorical variables and the t test or one-way analysis of variance test for continuous variables; Wilcoxon rank-sum test was used to assess differences in distribution for nonnormally distributed variables (P < .05). Covariates of interest were based on published literature and epidemiological plausibility, and included age, sex, race and ethnicity, study hospital, household college education status, health insurance status, chronic conditions, obesity (in children), morbid obesity (in adults), current smoker (in adults), antibiotics before admission, self-reported influenza vaccination (reported for the study, not necessarily to clinicians), admission ≤48 hours from illness onset, clinician-ordered testing, intensive care unit (ICU) admission, invasive mechanical ventilation, and hypoxia on presentation. For those treated with oseltamivir, ICU admission and invasive mechanical ventilation were defined as ICU admission or invasive mechanical ventilation within 2 calendar days before or after oseltamivir initiation.
We used multivariable logistic regression to identify factors independently associated with oseltamivir treatment for children and adults separately. The multivariable models were developed using manual selection based on bivariate analyses (P < .20) and other epidemiologically or biologically plausible variables based on literature [11, 15–17]. Due to overfitting concerns in the pediatric model, we report a parsimonious model for children only that excluded nonsignificant covariates; when comparing the parsimonious model to a fuller model, the likelihood ratio test was P > .05, indicating that the dropped covariates did not significantly impact the outcome. Data were analyzed using SAS version 9.3 statistical software (SAS Institute, Cary, NC).
RESULTS
Study Population and Influenza Testing
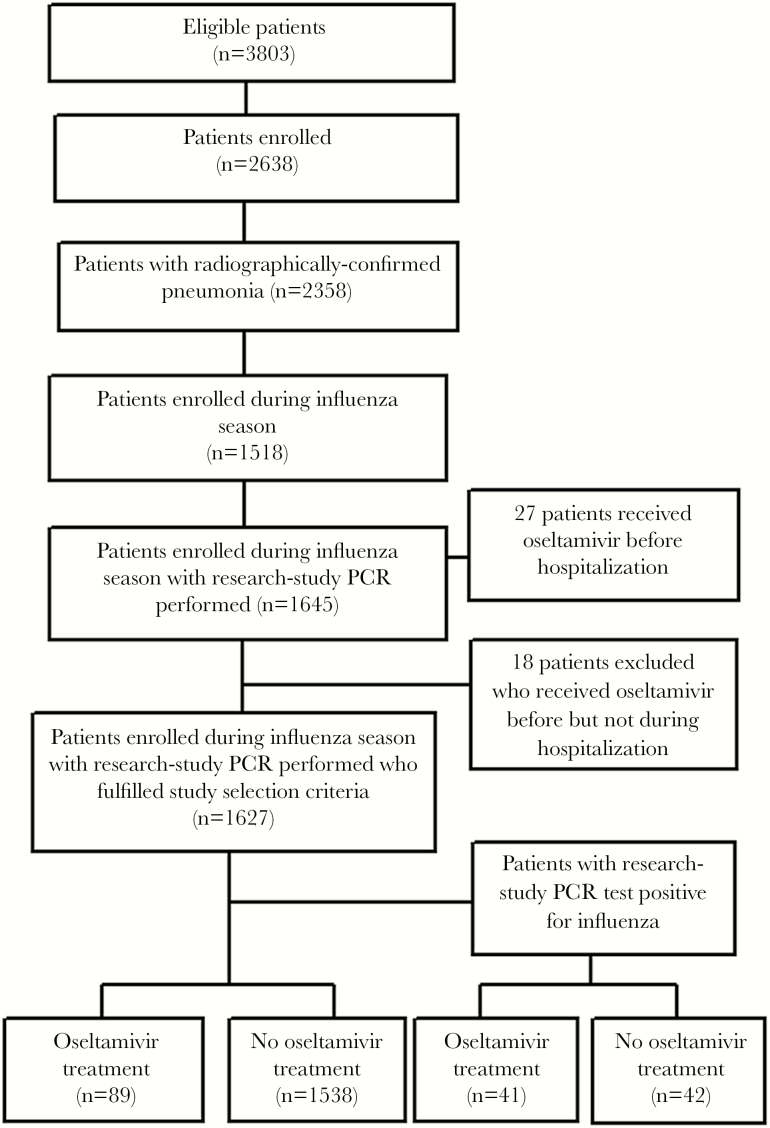
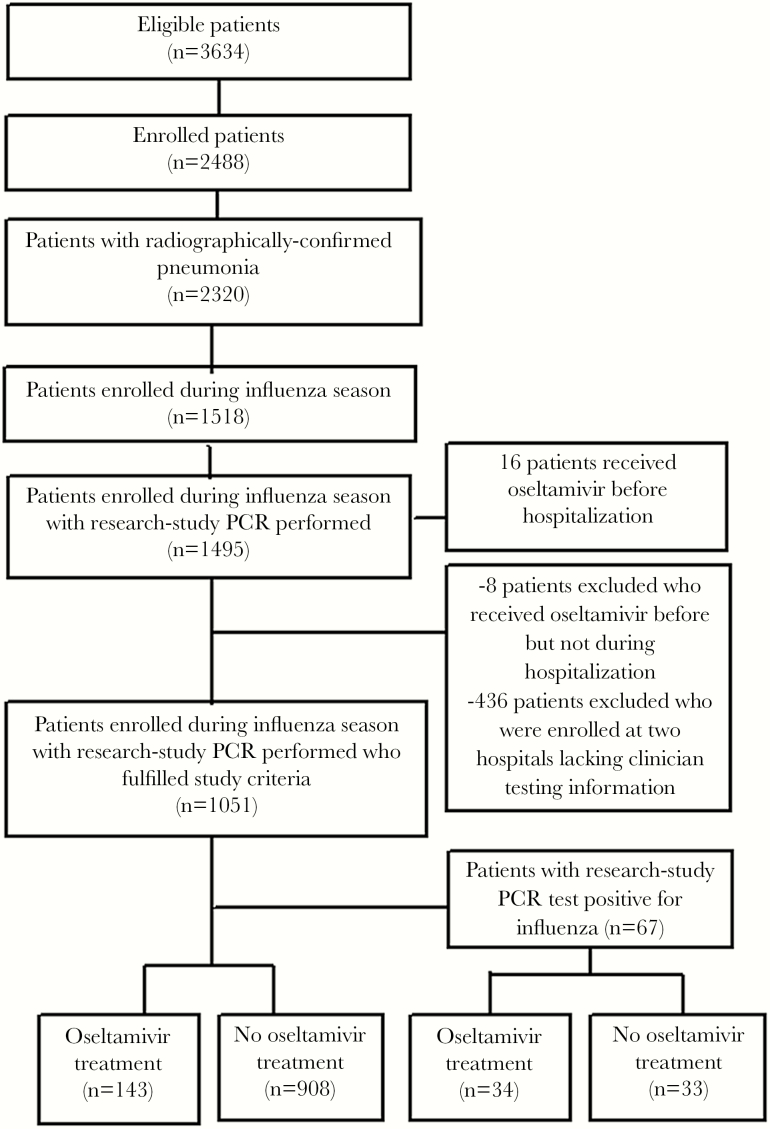
The final study population included 1627 children and 1051 adults (Figures 1–2). Clinician-ordered influenza tests were performed on 1134 of 1627 (70%) children, and 61 (5%) had positive results; the most common test types performed included both PCR and rapid influenza diagnostic tests (RIDTs) (31%), PCR alone (22%), direct fluorescent antibody (DFA) alone (21%), both PCR and DFA (16%), and RIDTs alone (6%). Clinician-ordered influenza tests were performed on 581 of 1051 (55%) adults, and 48 (8%) had positive results; the most common test types performed included PCR alone (95%), both RIDTs and DFA (2%), and RIDTs alone (1%) (Table 1). Test types differed by hospital (Table 2).
Figure 1.
Enrollment flow for children in the Etiology of Pneumonia in the Community (EPIC) study. PCR, real-time polymerase chain reaction.
Figure 2.
Enrollment flow for adults in the Etiology of Pneumonia in the Community (EPIC) study. PCR, real-time polymerase chain reaction.
Table 1.
Influenza Testing Methods Among Patients With a Clinician-Ordered Test Performed
| PCR | RIDT | DFA | Viral Culture | No. of Patients (%) | |
|---|---|---|---|---|---|
| Childrena | x | x | 350 (30.9) | ||
| x | 254 (22.4) | ||||
| x | 241 (21.3) | ||||
| x | x | 185 (16.3) | |||
| x | 65 (5.7) | ||||
| x | x | 12 (1.1) | |||
| x | x | x | 11 (1.0) | ||
| x | x | x | 6 (0.5) | ||
| x | x | 5 (0.4) | |||
| x | 3 (0.3) | ||||
| x | x | 1 (0.1) | |||
| x | x | 1 (0.1) | |||
| Adultsb | x | 553 (95.2) | |||
| x | x | 12 (2.1) | |||
| x | 8 (1.4) | ||||
| x | x | 3 (0.5) | |||
| x | x | x | 4 (0.7) | ||
| x | x | 1 (0.2) |
Abbreviations: DFA, direct fluorescent antibody; PCR, real-time polymerase chain reaction; RIDT, rapid influenza diagnostic test.
aA total of 1134 children had clinician-ordered tests performed.
bA total of 581 adults had clinician-ordered tests performed.
Table 2.
Oseltamivir Treatment and Type of Influenza Testing Method by Study Hospital Among Patients Who Had a Clinician-Ordered Test Performed
| Hospital (No. of Patients Enrolled at Hospital) | Oseltamivir Treatment No. (%)c | Specific Influenza Testing Method | ||||
|---|---|---|---|---|---|---|
| Total No. of Tests Performed | PCR No. (%)d | RIDT No. (%)d | DFA No. (%)d | Viral Culture No. (%)d | ||
| Pediatric hospitala | ||||||
| Hospital A (n = 585) | 46 (7.9) | 744 | 367 (49.3) | 364 (48.9) | 0 (0) | 13 (1.7) |
| Hospital B (n = 554) | 29 (5.2) | 645 | 223 (34.5) | 0 (0) | 421 (65.3) | 1 (0.2) |
| Hospital C (n = 488) | 14 (2.9) | 333 | 217 (65.2) | 85 (25.6) | 24 (7.2) | 7 (2.1) |
| Hospitals A, B, C (n = 1627) | 89 (5.5) | 1722 | 807 (46.9) | 449 (26.1) | 445 (25.8) | 21 (1.2) |
| Adult hospitalb | ||||||
| Hospital D (n = 603) | 98 (16.3) | 398 | 398 (100) | 0 (0) | 0 (0) | 0 (0) |
| Hospital E (n = 181) | 34 (18.8) | 79 | 79 (100) | 0 (0) | 0 (0) | 0 (0) |
| Hospital F (n = 267) | 11 (4.1) | 128 | 83 (64.8) | 28 (21.9) | 16 (12.5) | 1 (0.8) |
| Hospitals D, E, F (n = 1051) | 143 (13.6) | 605 | 560 (92.6) | 28 (4.6) | 16 (2.6) | 1 (0.2) |
Abbreviations: DFA, direct fluorescent antibody; PCR, real-time polymerase chain reaction; RIDT, rapid influenza diagnostic test.
aAmong 1134 of 1627 (70%) children who had at least 1 influenza test ordered by a clinician, there were a total of 1722 clinician-ordered influenza tests because any one patient could have had more than 1 test performed.
bAmong 581 of 1051 (55%) adults who had at least 1 influenza test ordered by a clinician, there were a total of 605 tests ordered because any one patient could have had more than 1 test performed.
cProportion refers to row percentage of total number who received oseltamivir of total number of patients enrolled at hospital(s).
dProportion refers to row percentage of clinician-ordered tests of total number of clinician-ordered tests performed at hospital(s).
Research-associated influenza tests identified 150 patients (83 of 1627 [5%] children, 67 of 1051 [6%] adults) with influenza-associated CAP during influenza season. Because clinician-ordered influenza testing was not routinely performed, influenza-associated CAP was missed in 13 of 83 (16%) children and 11 of 67 (16%) adults who were positive by research-associated influenza tests (Table 3); another 26 of 83 (31%) children and 18 of 67 (27%) adults had negative clinician-ordered tests but positive research-associated tests. Among the 26 children with negative clinician-ordered tests but positive research-associated tests, 9 (35%) were negative by both PCR and RIDTs, 8 (31%) by DFA alone, 5 (19%) by PCR alone, and 4 (15%) by both PCR and DFA. Among the 18 adults with negative clinician-ordered tests but positive research-associated tests, all clinician-ordered tests were PCR.
Table 3.
Comparison Between Clinician-Ordered Influenza Tests and Research-Associated PCR Tests Results Among Children and Adults
| Study PCR Test Positive n (%) | Study PCR Test Negative n (%) | Total n (%) | |
|---|---|---|---|
| Pediatric hospital | n = 83 | n = 1544 | n = 1627 |
| Clinician test positive | 44 (53.0) | 17 (1.1) | 61 (3.8) |
| Clinician test negative | 26 (31.3) | 1047 (67.8) | 1073 (66.0) |
| Clinician test not performed | 13 (15.7) | 480 (31.1) | 493 (30.3) |
| Adult hospital | n = 67 | n = 984 | n = 1051 |
| Clinician test positive | 38 (56.7) | 10 (1.0) | 48 (4.6) |
| Clinician test negative | 18 (26.9) | 515 (52.3) | 533 (50.7) |
| Clinician test not performed | 11 (16.4) | 459 (46.6) | 470 (44.7) |
Abbreviations: PCR, real-time polymerase chain reaction.
Of 61 children who tested positive by clinician-ordered testing, 17 (28%) were negative by research-associated influenza tests (Table 3), among whom 8 (47%) were only positive by RIDTs, 5 (29%) by PCR alone, 3 (18%) by DFA alone, and 1 (<1%) by both PCR and DFA. Of 48 adults who tested positive by clinician-ordered testing (Table 3), 10 (21%) were negative by research-associated tests but positive by clinician-ordered PCR alone.
Oseltamivir Treatment
During an influenza season (October–April), 89 of 1627 (5%) children received oseltamivir during their CAP hospitalization, ranging from 3% to 8% between the 3 hospitals; 143 of 1051 (14%) adults received oseltamivir during their CAP hospitalization, ranging from 4% to 19% between the 3 hospitals. When the influenza season was restricted to January–March for each study year, 63 of 899 (7%) children and 106 of 530 (20%) adults received oseltamivir.
Among patients with clinician-ordered tests performed, 75 of 1134 (7%) children and 129 of 581 (22%) adults received oseltamivir. Whereas, among patients without a clinician-ordered test performed, 14 of 493 (3%) children and 14 of 470 (3%) adults received oseltamivir. Among patients positive by clinician-ordered testing, 39 of 61 (64%) children and 37 of 48 (77%) adults received oseltamivir. In comparison, among patients positive by research-associated testing, 41 of 83 (49%) children and 34 of 67 (51%) adults received oseltamivir. Among the 39 children and 29 adults with influenza identified by research-associated but not clinician-ordered testing, 11 (28%) children and 6 (21%) adults received oseltamivir.
Characteristics Associated With Oseltamivir Treatment in Children
On bivariate analysis, compared with children not treated (n = 1538), children treated with oseltamivir (n = 89) were older (median age, 3 years; interquartile range [IQR], 1−8 years vs median age, 2 years; IQR, 0−2 years); and significantly more likely to be enrolled at hospital A (vs B or C), require ICU admission (29% vs 19%) or invasive mechanical ventilation (19% vs 6%), and less likely to be Hispanic (11% vs 21%) or have received influenza vaccination (14% vs 25%) (Table 4). Children treated with oseltamivir were significantly more likely than those not treated to have a clinician-ordered influenza test (84% vs 69%) and a positive result (44% vs 1%) (Table 4); 39 of 61 with a positive result (64%) were treated. Among children with negative results from a clinician-ordered influenza test, 36 of 1073 (3%) were treated, and among children with no clinician-ordered influenza test performed, 14 of 493 (3%) were treated. There was no significant difference in the median days from illness onset to admission (3 days in both groups) (Table 4). In particular, children who were clinically tested for influenza but not given oseltamivir (n = 1059) were as likely as those who did receive oseltamivir (n = 75) to have been admitted >48 hours from illness onset (62% vs 57%, P = .45).
Table 4.
Bivariate and Multivariate Analysis of Select Factors Associated With Oseltamivir Treatment Among Children Hospitalized With All-Cause CAP During Influenza Season
| Characteristic | Oseltamivir Treatment (n = 89) No. (%) | No Oseltamivir Treatment (n = 1538) No. (%) | P Valuea | Unadjusted Odds Ratio (95% CI) (n = 1627) | Adjusted Odds Ratio (95% CI) (n = 1627) |
|---|---|---|---|---|---|
| Age ≥2 years | 61 (68.5) | 814 (52.9) | <.01 | 1.94 (1.23–3.07) | 1.43 (1.16–1.76) |
| Female sex | 41 (46.1) | 720 (46.8) | .89 | 0.97 (0.63–1.49) | — |
| Hispanic race/ethnicity | 10 (11.2) | 320(20.8) | .03 | 0.48 (0.25–0.94) | 0.49 (0.24–0.99) |
| Study hospitals | <.01 | ||||
| Hospital A | 46 (51.7) | 539 (35.1) | 2.89 (1.57–5.32) | 2.76 (1.47–5.19) | |
| Hospital B | 29 (32.6) | 525 (34.1) | 1.87 (0.98–3.58) | 1.72 (0.88–3.37) | |
| Hospital C | 14 (15.7) | 474 (30.8) | Ref | Ref | |
| At least college education in household | 46 (51.7) | 811 (52.7) | .54 | 0.96 (0.63–1.47) | — |
| Had health insurance | 89 (100) | 1509 (98.4) | .40 | N/Af | — |
| Any chronic conditionb | 40 (44.9) | 772 (50.2) | .33 | 0.81 (0.53–1.24) | 0.68 (0.43–1.06) |
| Asthma/reactive airway disease | 29 (32.6) | 505 (32.8) | 1.0 | 0.99 (0.63–1.56) | — |
| Any preterm birth c | 6/28 (21.4) | 143/724 (19.8) | .83 | 1.11 (0.44–2.78) | — |
| Neurological conditions | 10 (11.2) | 117 (7.6) | .22 | 1.54 (0.78–3.05) | — |
| Congenital heart disease | 3 (3.4) | 108 (7.0) | .28 | 0.46 (0.14–1.49) | — |
| Obesity (BMI percentile ≥95) | 4 (4.5) | 114 (7.4) | .4 | 0.59 (0.21–1.63) | — |
| Antibiotics before admission | 20 (22.5) | 378 (24.6) | .65 | 0.89 (0.53–1.48) | — |
| Received influenza vaccine (self-report)d | 12/86 (14.0) | 331/1335 (25.0) | .02 | 0.49 (0.26–0.92) | — |
| Admission ≤48 hours from illness onset | 3 9 (43.8) | 583 (37.9) | .26 | 1.28 (0.83–1.97) | — |
| Median days from illness onset to admission (IQR) | 3 (2–5) | 3 (2–6) | .30 | — | — |
| Clinician-ordered influenza test | 75 (84.3) | 1059 (68.9) | <.01 | 2.42 (1.36–4.33) | 2.46 (1.35–4.49) |
| Positive test among those performed | 39/75 (43.8) | 22/1059 (1.4) | <.01 | — | — |
| Research study PCR test positive | 41 (46.1) | 42 (2.73) | <.01 | — | — |
| ICU admission | 26 (29.2) | 299 (19.4) | .03 | 1.71 (1.07–2.75) | 2.09 (1.27–3.45) |
| Invasive mechanical ventilation | 17 (19.1) | 88 (5.7) | <.01 | 4.06 (1.84–9.64) | — |
| Hypoxiae | 42 (47.2) | 590 (38.4) | .10 | 1.44 (0.94–2.21) | — |
Abbreviations: BMI, body mas index; CAP, community-acquired pneumonia; CI, confidence interval; EPIC, Etiology of Pneumonia in the Community; ICU, intensive care unit; IQR, interquartile range; N/A, not applicable; PCR, real-time polymerase chain reaction; Ref, reference.
a P value is comparing characteristics associated with oseltamivir treatment vs no oseltamivir treatment.
bAny underlying medical conditions included asthma/reactive airway disease, chromosomal disorders including Down syndrome, chronic kidney disease, chronic liver disease, congenital heart disease, diabetes mellitus, immunosuppression (either due to chronic condition or medication, malignancy [but not skin cancer], human immunodeficiency virus infection with CD4 count >200 cells/mm3), neurological disorders (including seizure disorder, cerebral palsy, scoliosis), preterm birth (defined as gestational age <37 weeks at birth for those children who were <2 years old at time of hospitalization, n = 752), and splenectomy.
cPreterm birth (defined as gestational age <37 weeks at birth for those children who were <2 years old at time of hospitalization), n = 752.
dReceived influenza vaccine, excludes children <6 months of age (n = 206) so overall denominator = 1421; patients were considered vaccinated if they received vaccine at least 2 weeks before admission by self-report to EPIC study. Influenza vaccine not added to multivariable model because vaccination status not necessarily reported to clinician.
eHypoxia: at presentation defined as oxygen saturation <92 or FiO2 liters >0 or percentage of supplemental oxygen use >21.
fAll children who received influenza antiviral agents had health insurance.
In multivariable analysis, enrollment at hospital A (adjusted odds ratio [aOR], 2.76; 95% confidence interval [CI], 1.36–4.88), clinician-ordered influenza testing (aOR, 2.46; 95% CI, 1.47–5.19), ICU admission (aOR, 2.09; 95% CI, 1.27–3.45), and age ≥2 years old (aOR, 1.43; 95% CI, 1.16–1.76) were associated with oseltamivir treatment (Table 4). Patients who were Hispanic were less likely to receive oseltamivir (aOR, 0.49; 95% CI, 0.24–0.99).
Characteristics Associated With Oseltamivir Treatment in Adults
On bivariate analysis, compared with adults not treated (n = 908), adults treated with oseltamivir (n = 143) were of similar age (median age, 55 years; IQR, 46−67 vs median age, 58 years; IQR, 47−71) but were significantly more likely to be Hispanic (18% vs 9%), enrolled at hospitals D or E, require ICU admission (39% vs 21%) or invasive mechanical ventilation (17% vs 5%), and have hypoxia on presentation (34% vs 24%) but less likely to have received influenza vaccination (11% vs 29%) (Table 5). Adults treated with oseltamivir were significantly more likely than those not treated to have a clinician-ordered influenza test (90% vs 50%) and shorter median days from illness onset to admission (3 days vs 4 days) (Table 5). Adults treated with oseltamivir were also more likely to have a positive clinician-ordered influenza test; among 48 adults with a positive influenza test, 37 (77%) were treated. Among adults with a negative clinician-ordered influenza test, 92 of 533 (17%) were treated. Among adults without a clinician-ordered influenza test performed, 14 of 470 (3%) were treated. In particular, adults who were clinically tested for influenza but not given oseltamivir (n = 452) were as likely as those who did receive oseltamivir (n = 129) to have been admitted >48 hours from illness onset (66% vs 64%, P = .52).
Table 5.
Bivariate and Multivariate Analysis of Select Factors Associated With Oseltamivir Treatment Among Adults Hospitalized With All-Cause CAP During Influenza Season
| Characteristic | Oseltamivir Treatment (n = 143) No. (%) | No Oseltamivir Treatment (n = 908) No. (%) | P Valuea | Unadjusted Odds Ratio (95% CI) (n = 1051) | Adjusted Odds Ratio (95% CI) (n = 1051) |
|---|---|---|---|---|---|
| Age ≥65 years | 42 (29.4) | 355 (39.1) | .03 | 0.65 (0.44–0.95) | 0.70 (0.45–1.08) |
| Female sex | 72 (50.4) | 476 (52.4) | .65 | 0.92 (0.65–1.31) | — |
| Hispanic ethnicity | 26 (18.2) | 84 (9.3) | <.01 | 2.18 (1.35–3.53) | 2.06 (1.18–3.59) |
| Study hospital | <.01 | ||||
| Hospital D | 98 (68.5) | 505 (55.6) | 4.52 (2.38–8.58) | 3.46 (1.75–6.83) | |
| Hospital E | 34 (23.8) | 147 (16.2) | 5.38 (2.65−10.9) | 5.11 (2.37–11.01) | |
| Hospital F | 11 (7.7) | 256 (28.2) | Ref | Ref | |
| College education or more | 90 (62.9) | 552 (60.8) | .27 | 1.10 (0.76−1.58) | 1.07 (0.71–1.61) |
| Had insurance | 130 (90.9) | 836 (92.5) | .51 | 0.81 (0.45–1.51) | — |
| Any chronic conditionb | 109 (76.2) | 731 (80.5) | .23 | 0.78 (0.51−1.18) | 0.74 (0.46–1.18) |
| Chronic lung disease | 52 (36.4) | 375 (41.3) | .26 | 0.81 (0.56–1.17) | — |
| Immunosuppression | 46 (32.2) | 290 (31.9) | .96 | 1.01 (0.69–1.47) | — |
| Diabetes | 43 (30.1) | 227 (25.0) | .21 | 1.29 (0.88–1.90) | — |
| Chronic kidney disease | 23 (16.1) | 143 (15.8) | .92 | 1.03 (0.63–1.66) | — |
| Chronic heart disease | 50 (35.0) | 361 (40.0) | .28 | 0.82 (0.56–1.18) | — |
| Morbid obesity (BMI ≥40 kg/m2) | 18 (12.6) | 76 (8.4) | .10 | 1.58 (0.91−2.72) | 1.63 (0.87–3.04) |
| Current smoker | 34 (23.8) | 214 (23.6) | .96 | 1.01 (0.67–1.53) | — |
| Antibiotics before admission | 24 (16.8) | 201 (22.1) | .15 | 0.71 (0.45−1.13) | 0.91 (0.55–1.51) |
| Received influenza vaccine (self-report)c | 15 (10.5) | 262 (28.9) | <.01 | 0.29 (0.17–0.50) | — |
| Admission ≤48 hours from illness onset | 54 (37.8) | 326 (35.9) | .69 | 1.08 (0.75–1.56) | 1.03 (0.69–1.54) |
| Median days from illness onset to admission (IQR) | 3 (2–6) | 4 (2–8) | .05 | — | — |
| Clinician-ordered influenza test | 129 (90.2) | 452 (49.8) | <.01 | 9.30 (5.27–16.38) | 8.38 (4.64–15.12) |
| Positive test/test done | 37/129 (28.7) | 11/452 (2.4) | <.01 | — | — |
| Research study PCR test positive | 34 (23.8) | 33 (3.6) | <.01 | — | — |
| ICU admission | 55 (38.5) | 187 (20.6) | <.01 | 2.41 (1.66–3.50) | 2.05 (1.34–3.13) |
| Invasive mechanical ventilation | 24 (16.8) | 47 (5.2) | <.01 | 3.70 (2.18–6.26) | — |
| Hypoxiad | 48 (33.6) | 218 (24.0) | .02 | 1.60 (1.10–2.34) | 1.47 (0.94–2.29) |
| Median PSI scoree (IQR) | 79 (49–102) | 78.5 (52–106) | .54 | — | — |
Abbreviations: BMI, body mas index; CAP, community-acquired pneumonia; CI, confidence interval; EPIC, Etiology of Pneumonia in the Community; ICU, intensive care unit; IQR, interquartile range; PCR, real-time polymerase-chain-reaction; PSI, pneumonia severity index; Ref, reference.
a P value is comparing characteristics associated with oseltamivir treatment vs no oseltamivir treatment.
bUnderlying medical conditions included chronic lung disease (asthma, chronic obstructive pulmonary disease, obstructive sleep apnea), chronic heart disease (ie, coronary artery disease, congestive heart failure, but not hypertension), immunosuppression (either due to chronic condition or medication, malignancy [but not skin cancer], human immunodeficiency virus infection with CD4 count >200 cells/mm3), diabetes mellitus, chronic kidney disease (with or without dialysis), neurological disorders (epilepsy, cerebral palsy, dementia, history of stroke), chronic liver disease (hepatitis, cirrhosis, hepatic failure), and splenectomy.
cPatients were considered vaccinated if they received vaccine at least 2 weeks before admission by self-report to EPIC study. Influenza vaccine not added to multivariable model because vaccination status not necessarily reported to clinician.
dHypoxia: at presentation defined as oxygen saturation <92 or FiO2 liters >0 or percentage supplemental oxygen use >21.
ePneumonia severity index is a clinical prediction rule for CAP-related mortality based on gender, age, nursing home status, mental status, heart rate, respiratory rate, blood pressure, temperature, select underlying medical conditions, select laboratory values, and presence of pleural effusion [40].
In multivariable analysis, having a clinician-ordered influenza test (aOR, 8.38; 95% CI, 4.64–15.12), enrollment at hospitals D or E (aOR, 3.46−5.11; 95% CI, 1.75–11.01), Hispanic ethnicity (aOR, 2.06; 95% CI, 1.18–3.59), and ICU admission (aOR, 2.05; 95% CI, 1.34–3.13) were associated with oseltamivir treatment (Table 5).
DISCUSSION
During the 2010−12 influenza seasons, oseltamivir treatment was provided to 5% children and 14% adults hospitalized with CAP and enrolled in the EPIC study. Treatment was significantly associated with clinician-ordered influenza testing, and more strongly when there was a positive result with two thirds of these patients treated. However, only half of patients with influenza-associated CAP identified by research-associated testing were treated, likely due to missing or negative clinician-ordered tests. Severe illness and enrollment at specific hospitals were also significantly associated with oseltamivir treatment.
The proportion of patients with a CAP hospitalization during an influenza season who received oseltamivir treatment was low in our study, and it was only slightly higher but still suboptimal when the influenza season was restricted to 3 months during peak influenza circulation. In the United States, influenza antiviral use increased sharply during the 2009 H1N1 pandemic [16, 18, 19] and declined subsequently, particularly in children [20, 21]. Previous reports of the use of influenza antiviral treatment have included patients with all-cause acute respiratory illness and/or laboratory-confirmed influenza but not CAP. Data from the New Vaccine Surveillance Network from 2004 to 2009 demonstrated that only 1.5% of children hospitalized with acute respiratory illness who were positive for influenza by research-associated testing (not disclosed to clinicians) received influenza antiviral treatment [22]. In contrast, in studies that specifically focused on hospitalized patients with laboratory-confirmed influenza based on tests performed at a clinician’s discretion, the proportion of patients who received antiviral treatment was higher, ranging from 21% to 84% in children and 54% to 82% in adults [7, 16, 18–21]. Direct comparisons between studies are difficult because our study focused on CAP hospitalization rather than all-cause acute respiratory illness or only laboratory-confirmed influenza.
During the influenza season, the IDSA influenza and IDSA/PIDS CAP guidelines encourage influenza testing in all patients hospitalized with suspected influenza, and IDSA also recommends testing for hospitalized patients with CAP. Thus, the modest levels of influenza testing and oseltamivir use we observed among hospitalized CAP patients during influenza season suggest it is worth exploring potential reasons why physicians did not prescribe oseltamivir. This may be because physicians did not consider influenza in the differential diagnosis for CAP, were reluctant to treat patients without a positive influenza test (who may be positive for other pathogens), lacked awareness of local influenza circulation, or perceived a lack of antiviral efficacy, particularly when patients present >48 hours from illness onset [23–26]. In addition, not all influenza tests are equal and PCR tests are expensive, leading to barriers to accessing sensitive, specific, and timely influenza tests, which can hinder treatment [27]. Due to low sensitivity and negative predictive value of RIDTs, negative RIDT results alone do not exclude influenza virus infection; antiviral treatment should not be withheld from these patients if influenza is suspected, and further testing with molecular assays is recommended when available, because they have higher sensitivity and specificity [12, 13]. Continued efforts for the improved development of influenza diagnostics including affordable molecular-based diagnostic tests are needed to help inform testing and treatment practice [28]. In addition, improvements in hospital-based algorithms for influenza testing and for empiric treatment, similar to conventional CAP standing orders for antibiotics, may improve adherence with IDSA/PIDS guidelines.
Observational studies of hospitalized adults with laboratory-confirmed influenza have demonstrated that patients with positive RIDTs were more likely to receive influenza antiviral treatment [16, 29]. Likewise, in a study of hospitalized adults with influenza virus infection from 2006 to 2012 across 4 hospitals, clinician-ordered laboratory-confirmed influenza by RIDTs, PCR, or viral culture was independently associated with antiviral treatment [17]. Although a positive influenza test was strongly associated with oseltamivir treatment in our study, just having a clinician-ordered influenza test performed regardless of the result was also significantly associated with treatment. The diagnosis of influenza based purely on clinical signs and symptoms has modest sensitivity and specificity [30–32], and thus it is challenging to rely on clinical diagnosis alone for testing and treatment decisions, including in patients hospitalized with CAP. Our findings that clinician-ordered influenza testing strongly correlated with oseltamivir treatment underscores the importance of increasing adherence to current PIDS, IDSA, and ACIP guidelines, which recommend influenza testing in hospitalized persons with CAP during the influenza season and empiric antiviral use while awaiting results.
We found enrollment at specific hospitals influenced clinician-ordered testing, type of influenza test ordered, and influenza antiviral treatment in children and adults, even between hospitals in the same city. Other observational studies have also noted variability in antiviral prescribing across ambulatory and hospital sites [15, 23, 33]. This variation in influenza antiviral prescribing patterns between clinicians, and between hospitals, highlights the need for understanding the factors associated with this heterogeneity.
In our study, being of Hispanic race/ethnicity was associated with an increased probability of oseltamivir treatment among adults but a decreased probability among children; the reasons for which are unclear but may have been related to study hospital. A recent study found that being of Hispanic ethnicity or other or unknown race/ethnicity increased the odds of a person with laboratory-confirmed influenza receiving a clinical influenza diagnosis [34]. In addition, (1) racial-ethnic differences in vaccination coverage and (2) access to care were reported during the 2009 H1N1 pandemic among Spanish-speaking Hispanics and warrants further study [35, 36].
In our study, children ≥2 years old hospitalized with CAP were more likely to receive influenza antiviral treatment than younger children. Similar findings were reported from a previous population-based study of children hospitalized with laboratory-confirmed influenza during the 2010−2011 influenza season [20]. This may be due to lack of recognition of influenza in children <2 years old with CAP or in children who test positive for respiratory syncytial virus, which is a more frequent cause of CAP in this age group [5, 26]. Providers may also be less comfortable using influenza antiviral agents in children <2 years. Oseltamivir was not approved by the US Food and Drug Administration to treat influenza infection in children <1 year old until December 2012 [37]. In our analysis, after excluding children <1 years of age, the association between age ≥2 years and oseltamivir treatment remained significant (Supplemental Table 1).
Intensive care unit admission was also associated with oseltamivir treatment in both children and adults hospitalized with CAP. This may indicate that influenza may be more likely to be considered in the differential diagnosis of ICU patients hospitalized with CAP, or patients with more severe disease or who do not improve, which then prompts influenza testing and subsequent treatment. The benefits of oseltamivir in critically ill children and adults with influenza have been demonstrated [38, 39].
This study has limitations. First, although oseltamivir treatment was associated with clinician-ordered testing, the factors that led to testing cannot be determined directly. Second, we were unable to evaluate the timing of the availability of clinician-ordered testing results compared with oseltamivir initiation and other factors because we lacked complete information on dates and times of the clinician testing, particularly among children, because this was independent of the study protocol. Third, the study hospitals were mostly urban medical centers in 3 geographic regions, and thus our findings may not be generalizable to other settings. Furthermore, we lacked clinician-ordered testing information from 2 of 5 adult hospitals, which may further limit the generalizability. Finally, the true influenza status of the patient was unknown in situations when the research-study PCR and clinician-ordered PCR results were discordant.
CONCLUSIONS
In conclusion, 5% of children and 14% of adults hospitalized with CAP during an influenza season received oseltamivir, and approximately half of patients hospitalized with influenza-associated CAP were treated with oseltamivir despite ACIP and IDSA recommendations for treatment of all patients hospitalized with suspected influenza. Oseltamivir treatment was associated with clinician-ordered testing, severe disease, and study hospital. Studies are needed to better understand the reasons why oseltamivir prescribing among patients hospitalized with CAP is not higher. In addition, increased clinician education is needed to include influenza in the differential diagnosis for hospitalized patients with CAP and to test and treat patients empirically if influenza is suspected.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank the patients who graciously consented to participate in this study and all members of the Etiology of Pneumonia in the Community (EPIC) Study Team for their contributions.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was funded by the Centers for Disease Control and Prevention. W. H. S. received fees for serving on an advisory board from BioFire Diagnostics, and Venaxis, grant support through his institution from bioMérieux, Affinium Pharmaceuticals, Astute Medical, Thermo Fisher, Pfizer, Rapid Pathogen Screening, Venaxis, BioAegis Inc and Sphingotec GmbH, and consulting fees from Abbott Point of Care, and Cempra Pharmaceuticals. R.G.W. received consulting fees from bioMerieux and GenMark, grant support through his institution from bioMerieux, and fees for serving on a data safety monitoring board from Vertex. C. G. G. received grant support through his institution from CDC and consulting fees from Pfizer. E. J. A. received grant support from MedImmune, editorial support for an unrelated study from Roche, and consulting fees from AbbVie. D. M. C. received grant support through his institution from CDC. S. R. A received grant support through her institution from CDC GlaxoSmithKline. K. A. received grant support through his institution from CDC, receiving fees through his institution from GlaxoSmithKline, Cubist Pharmaceuticals, and National Institutes of Health for the enrollment of patients in other studies. A. T. P. received grant support through his institution from CDC, National Institute of Allergy and Infectious Diseases and BioFire, received fees for serving on a data safety monitory board for Alios Pharmaceutical, received fees for the preparation of educational material from Medscape, and royalties from Antimicrobial Therapy. K. M. E. served on a data and safety monitoring board for Novartis for which her institution received fees. D. J. W., J. A. M., and Y. Z. received grant support through their institutions from CDC. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pfuntner A, Wier LM, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2011: Statistical Brief #162. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality, 2013. [PubMed] [Google Scholar]
- 2. Yu H, Wier LM, Elixhauser A. Hospital Stays for Children, 2009: Statistical Brief #118. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality, 2011. [PubMed] [Google Scholar]
- 3. Lee GE, Lorch SA, Sheffler-Collins S, et al. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics 2010; 126:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaves SS, Perez A, Farley MM, et al. The burden of influenza hospitalizations in infants from 2003-2012, United States. Pediatr Infect Dis J 2014; 33:912–9. [DOI] [PubMed] [Google Scholar]
- 7. Dawood FS, Fiore A, Kamimoto L, et al. Burden of seasonal influenza hospitalization in children, United States, 2003 to 2008. J Pediatr 2010; 157:808–14. [DOI] [PubMed] [Google Scholar]
- 8. Dao CN, Kamimoto L, Nowell M, et al. Adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis 2010; 202:881–8. [DOI] [PubMed] [Google Scholar]
- 9. Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis 2006; 6:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53:e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–24. [PubMed] [Google Scholar]
- 12. Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children–diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention (CDC). Rapid Influenza Diagnostic Tests. Available at: http://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm Accessed 1 January 2017. [Google Scholar]
- 14. Centers for Disease Control and Prevention (CDC). National and Regional Level Outpatient Illness and Viral Surveillance. Available at: http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html Accessed 21 August 2014. [Google Scholar]
- 15. Havers F, Thaker S, Clippard JR, et al. Use of influenza antiviral agents by ambulatory care clinicians during the 2012–2013 influenza season. Clin Infect Dis 2014; 59:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doshi S, Kamimoto L, Finelli L, et al. Description of antiviral treatment among adults hospitalized with influenza before and during the 2009 pandemic: United States, 2005–2009. J Infect Dis 2011; 204:1848–56. [DOI] [PubMed] [Google Scholar]
- 17. Lindegren ML, Griffin MR, Williams JV, et al. Antiviral treatment among older adults hospitalized with influenza, 2006–2012. PLoS One 2015; 10:e0121952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox CM, D’Mello T, Perez A, et al. Increase in rates of hospitalization due to laboratory-confirmed influenza among children and adults during the 2009-10 influenza pandemic. J Infect Dis 2012; 206:1350–8. [DOI] [PubMed] [Google Scholar]
- 19. Dawood FS, Chaves SS, Perez A, et al. Complications and associated bacterial coinfections among children hospitalized with seasonal or pandemic influenza, United States, 2003–2010. J Infect Dis 2013; 209:686–94. [DOI] [PubMed] [Google Scholar]
- 20. Garg S, Chaves SS, Perez A, et al. Reduced influenza antiviral treatment among children and adults hospitalized with laboratory-confirmed influenza infection in the year after the 2009 pandemic. Clin Infect Dis 2012; 55:e18–21. [DOI] [PubMed] [Google Scholar]
- 21. Chaves SS, Aragon D, Bennett N, et al. Patients hospitalized with laboratory-confirmed influenza during the 2010–2011 influenza season: exploring disease severity by virus type and subtype. J Infect Dis 2013; 208:1305–14. [DOI] [PubMed] [Google Scholar]
- 22. Poehling KA, Edwards KM, Griffin MR, et al. The burden of influenza in young children, 2004–2009. Pediatrics 2013; 131:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothberg MB, Bonner AB, Rajab MH, et al. Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza. Clin Infect Dis 2006; 42:95–9. [DOI] [PubMed] [Google Scholar]
- 24. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385:1729–37. [DOI] [PubMed] [Google Scholar]
- 25. Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:Cd008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 27. Nelson RE, Stockmann C, Hersh AL, et al. Economic analysis of rapid and sensitive polymerase chain reaction testing in the emergency department for influenza infections in children. Pediatr Infect Dis J 2015; 34:577–82. [DOI] [PubMed] [Google Scholar]
- 28. Caliendo AM, Gilbert DN, Ginocchio CC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57Suppl 3:S139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mueller MR, Smith PJ, Baumbach JP, et al. Influenza testing and antiviral prescribing practices among emergency department clinicians in 9 states during the 2006 to 2007 influenza season. Ann Emerg Med 2010; 55:32–9. [DOI] [PubMed] [Google Scholar]
- 30. Dugas AF, Valsamakis A, Atreya MR, et al. Clinical diagnosis of influenza in the ED. Am J Emerg Med 2015; 33:770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stein J, Louie J, Flanders S, et al. Performance characteristics of clinical diagnosis, a clinical decision rule, and a rapid influenza test in the detection of influenza infection in a community sample of adults. Ann Emerg Med 2005; 46:412–9. [DOI] [PubMed] [Google Scholar]
- 32. Ohmit SE, Monto AS. Symptomatic predictors of influenza virus positivity in children during the influenza season. Clin Infect Dis 2006; 43:564–8. [DOI] [PubMed] [Google Scholar]
- 33. Havers F, Flannery B, Clippard JR, et al. Use of influenza antiviral medications among outpatients at high risk for influenza-associated complications during the 2013-2014 influenza season. Clin Infect Dis 2015; 60:1677–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller MR, Peters TR, Suerken CK, et al. Predictors of influenza diagnosis among patients with laboratory-confirmed influenza. J Infect Dis 2015; 212:1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quinn SC, Kumar S, Freimuth VS, et al. Racial disparities in exposure, susceptibility, and access to health care in the US H1N1 influenza pandemic. Am J Public Health 2011; 101:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Setse RW, Euler GL, Gonzalez-Feliciano AG, et al. Influenza vaccination coverage - United States, 2000–2010. MMWR Suppl 2011; 60:38–41. [PubMed] [Google Scholar]
- 37. U.S. Food and Drug Administration. FDA expands Tamiflu’s use to treat children younger than 1 year. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm333205.htm Accessed 17 June 2015. [Google Scholar]
- 38. Coffin SE, Leckerman K, Keren R, et al. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J 2011; 30:962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.