Abstract
Background
Invasive aspergillosis (IA) and mucormycosis contribute to substantial mortality, especially among immunocompromised persons, including those with hematopoietic stem cell transplant (HSCT), hematologic malignancy (HM), and solid organ transplant (SOT).
Methods
Using International Classification of Diseases, Ninth Revision codes available in the National Inpatient Sample, a hospital discharge database, we estimated IA-related hospitalizations (IA-RH), mucormycosis-RH (M-RH), HSCT-RH, HM-RH, and SOT-RH during 2000–2013. United States census data were used to calculate overall M-RH and IA-RH rates and present trends; estimated annual numbers of HSCT-RH, HM-RH, and SOT-RH served as denominators to calculate M-RH and IA-RH rates occurring with these conditions. Weighted least-squares technique was used to test for linear trends and calculate average annual percentage change (APC).
Results
There were an estimated 169 110 IA-RH and 9966 M-RH during 2000–2013. Overall, IA-RH and M-RH rates per million persons rose from 32.8 to 46.0 (APC = +2.9; P < .001) and 1.7 to 3.4 (APC = +5.2%; P < .001), respectively, from 2000 to 2013. Among HSCT-RH, there was no significant change in M-RH rate, but a significant decline occurred in IA-RH rate (APC = −4.6%; P = .004). Among HM-RH, the rate of M-RH increased (APC = +7.0%; P < .001), but the IA-RH rate did not change significantly (APC = +1.2%; P = .073). Among SOT-RH, M-RH (APC = +6.3%; P = .038) and IA-RH rates (APC = +4.1%; P < .001) both increased.
Conclusions
Overall IA-RH and M-RH rates increased during 2000–2013, with a doubling of M-RH. Mucormycosis-related hospitalization occurring in conjunction with certain comorbidities increased, whereas IA-RH rates among patients with the comorbidities, decreased, remained stable, or increased to a lesser extent than M-RH.
Keywords: burden, immunocompromised population, invasive aspergillosis, mucormycosis, trends
Invasive mold infections (IMIs), including invasive aspergillosis (IA) and mucormycosis, contribute to substantial morbidity and mortality, especially among immunocompromised persons. Invasive aspergillosis is the most common IMI, and mucormycosis, although less frequent, is highly fatal even with the best available treatment [1]. Both are associated with lengthy and costly hospitalizations [2, 3]. Compared with the general population, the incidence of IA and mucormycosis is much higher among those with certain underlying conditions, such as hematologic malignancies (HMs), hematopoietic stem cell transplant (HSCT), and solid organ transplant (SOT) [4–6].
Surveillance for IA and mucormycosis in the United States has been limited; the only population-based surveillance for these IMIs was conducted in 3 counties in the San Francisco area during 1992–1993, where the reported annual incidence was 12.4 cases/per 1 million persons for IA and 1.7 cases per 1 million persons for mucormycosis [7]. Single-center studies subsequently conducted in the United States [8, 9] and studies of specific patient populations over relatively short periods [4, 6, 10] suggest a rise in the incidence of mucormycosis, and IA to a lesser extent. Several factors may be driving an increase in infections, particularly of mucormycosis. First, the number of susceptible persons has increased, due to increases in the number of stem cell and SOTs performed each year [11, 12]. Improved diagnostics, especially with introduction of the galactomannan assay in the last decade, may have altered detection of IA and hence observed trends in IA [13]. Awareness of the need to prevent IA in addition to Candida infections in certain high-risk groups has led to changes in antifungal prophylaxis practices and may have altered the relative frequency of mucormycosis and IA among patients at risk for both infections [14, 15].
Because IA and mucormycosis are not reportable diseases and there is no ongoing surveillance for these infections, no single data source exists to determine the burden of and trends in IA and mucormycosis in the United States. However, because most persons with these IMIs require hospitalization, national administrative hospital discharge data (HDD) can be used to estimate the incidence of IA-related hospitalizations (IA-RHs) and mucormycosis-related hospitalizations (M-RHs), a proxy for the incidence of IA and mucormycosis. In addition, HDD can provide insights into the trends in types of patients developing IMIs by evaluating other discharge diagnoses listed for each hospitalization.
Our primary objective was to estimate nationally representative incidence rates and trends in IA-RH M-RH in the United States during 2000–2013. We also sought to compare trends in IA-RHs and M-RHs among hospitalizations that were also related to certain comorbidities, such as HSCT, HM, and SOT, because these groups are at higher risk for these IMIs than the general population.
METHODS
Data Sources
We used data from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (HCUP), the largest database of publicly-available all-payer healthcare data in the United States. The HCUP’s National (Nationwide) Inpatient Sample (NIS) is a nationally representative database of hospitalizations derived from billing data [16]. Before 2012, the NIS contained all discharges from a sample of 20% (n = ~1000) of hospitals in the United States; starting in 2012, the NIS includes a 20% stratified sample of discharges from all participating hospitals (n = ~4500 hospitals). National estimates are produced by assigning specific sampling weights to discharges based on hospital census region, rural/urban location, teaching status, bed size, and ownership. The NIS contains more than 7 million unweighted discharges each year, corresponding to approximately 36 million weighted annual discharges. We used data from the US Census Bureau to obtain denominators for the US population by year, age group, sex, race, and census region.
Definitions
We identified IA-RH and M-RH and in the 2000–2013 NIS using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnosis codes 117.3 and 484.6 for IA-RH and 117.7 for M-RH. We identified selected underlying conditions using the following ICD-9 codes: HM (200–208), SOT (V42.0, V42.1, V42.2, V42.6, V42.7, V42.83, V42.84, V42.89, V42.9, and 996.80–996.89 excluding 996.85), HSCT (V42.81, V42.82, and 996.85), and diabetes mellitus (249.xx, 250.xx). “Diabetes only” was defined as presence of a diabetes mellitus ICD-9 code without mention of the ICD-9 codes for HM, HSCT, or SOT. For analysis looking at nonkidney transplant-related hospitalizations, we excluded ICD-9 codes V42.0 and 996.81 from the SOT-related hospitalization criteria. To be included, the ICD-9 code for any of these diagnoses could be listed anywhere on the discharge record.
Data Analysis
National estimates of the number of IA-RH and M-RH and 95% confidence intervals (CIs) were calculated from the NIS using the HCUP weighting methodology [17]. The unit of analysis was a hospitalization, and records were examined by age group, sex, census region, presence of underlying condition, and in-hospital mortality. We calculated annual rates per 1 million persons using population data from the US Census Bureau. For rates of IA-RHs and M-RHs occurring in conjunction with hospitalizations related to a specific underlying condition (ie, HM-RHs, HSCT-RHs, and SOT-RHs), the weighted estimate of the number of hospitalizations with 1 or more ICD-9 codes pertinent to that specific underlying condition were included in the denominator. Analyses specific to HSCT-RH are limited to hospitalizations occurring in 2001–2013 because of the limited number of M-RH in this group in the year 2000, which restricts our ability to report these numbers in accordance with the HCUP data use agreement.
We tested for linear trends in annual rates of overall IA-RH and M-RH and rates of IA-RHs and M-RHs occurring in conjunction with each underlying condition of interest using the weighted least-squares (WLS) technique [18]. The WLS was chosen to account for the NIS sample design and uncertainty around estimated rates for each year. To calculate average annual percentage change (APC), we performed the WLS on the log-transformed rates, and the delta method was used to approximate the variances used as weights. Logistic regression using survey data analysis was used to detect whether there is a significant change in underlying conditions among M-RH over time.
RESULTS
Trends in Invasive Aspergillosis-Related Hospitalizations
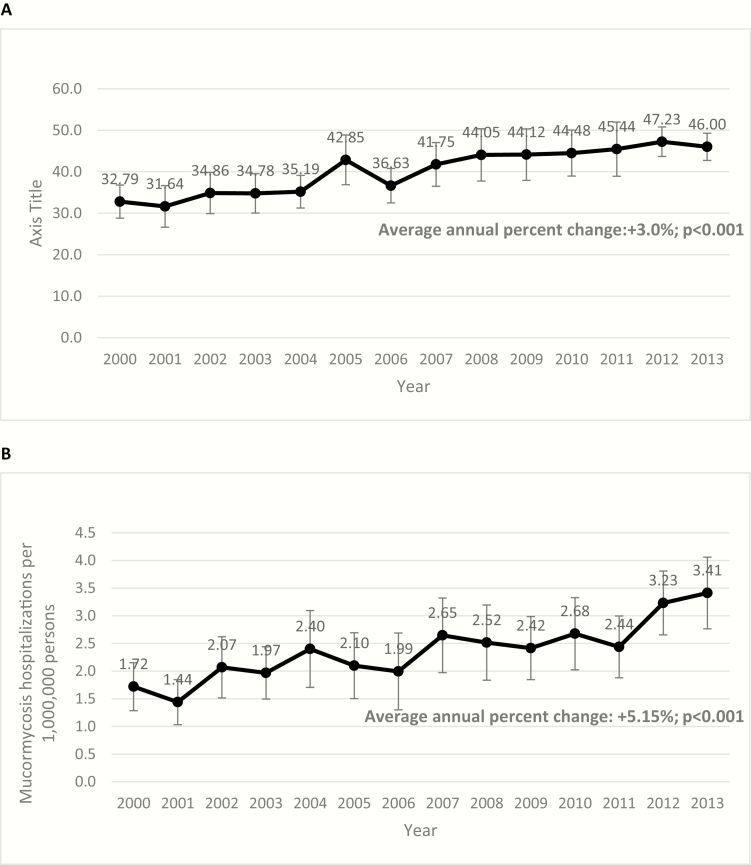
There were an estimated 169 110 (95% CI, 163 445–174 774) IA-RHs in the United States during 2000–2013. The weighted number of IA-RHS increased from 9252 (95% CI, 8129–10376) in 2000 to 14560 (95% CI, 13524–15596) in 2013. The overall rate of IA-RHS increased from 32.7/1 million persons (95% CI, 28.7–36.6) in 2000 to 45.7 (95% CI, 43.5–49.0) in 2013 with an APC of +3.0 (P < .001) (Figure 1A). In-hospital death occurred in 16.1% of IA-RHs.
Figure 1.
Annual rates of invasive aspergillosis related hospitalizations (IA-RH) (Panel A) and mucormycosis-associated hospitalizations (M-RH) (Panel B) per 1 million persons, United States, 2000–2013.
Trends in Mucormycosis-Related Hospitalizations
There were an estimated 9966 (95% CI, 9296–10636) M-RHs in the United States during 2000–2013. The estimated number of M-RHs rose from 485 (95% CI, 363–607) in 2000 to 1080 in 2013 (95% CI, 875–1285). The overall rate of M-RHs doubled from 1.7/1 million persons (95% CI, 1.3–2.2) in 2000 to 3.4 (95% CI, 2.7–4.1) in 2013 with an estimated APC of +5.2% (P < .001) (Figure 1B).
Overall, 32.5% of all M-RH had HM listed as an underlying condition: 10.9% listed SOT, 8.5% listed SCT, and 30.2% listed diabetes only. The proportion of M-RH with HM, SOT, or HSCT as an underlying condition increased significantly during 2001–2013 (Table 2), whereas the proportion of M-RH with diabetes listed as an underlying condition declined significantly during this time period (P = .02). In-hospital death occurred in 19.8% of M-RHs; the proportion of these deaths attributable to mucormycosis is not known.
Table 2.
Proportion of M-RH WITH Selected Underlying Conditions Listed, United States, 2000–2013
| Underlying Condition | Crude Proportion of M-RH With the Condition in 2000 | Crude Proportion of M-RH With the Condition in 2013 | P Valueb |
|---|---|---|---|
| Hematologic malignancy | 21.7 | 38.4 | .002 |
| Solid organ transplant | 5.8 | 13.9 | .02 |
| Hematopoietic stem cell transplanta | 8.5 | 10.6 | .04 |
| Diabetes only | 45.8 | 25.0 | .02 |
Abbreviations: HCUP, Healthcare Cost and Utilization Project; HSCT, hematopoietic stem cell transplant; IA-RH, invasive aspergillosis-related hospitalizations; M-RH, mucormycosis-related hospitalizations.
aStarts in 2001 because data for mucormycosis and HSCT were limited for the year 2000, and HCUP data use agreement restrict reporting in these instances.
b P value corresponds to logistic regression using survey analysis to test for significant change in underlying conditions among M-RH over time.
Invasive Aspergillosis-Related Hospitalizations and Mucormycosis-Related Hospitalizations Rates by Demographic Characteristics
The IA-RH and M-RH rates in 2013 by various demographic characteristics are shown in Table 1; differences in rates between groups in 2013 were similar to those noted in other years. The rate of both IA-RH and M-RH increased with age. Male sex was associated with almost twice the rate of M-RHs (4.4/1 million persons) compared with females (2.5/1 million persons). The Northeast census region had a lower rate of IA-RHs and M-RHs compared with rates in the West.
Table 1.
Rates of Invasive Aspergillosis-Related Hospitalizations and Mucormycosis-Related Hospitalizations by Demographic Characteristics, United States, 2013
| Characteristic | IA-RH Rate per 1 Million Persons | 95% Confidence Interval | M-RH Rate per 1 Million Persons | 95% Confidence Interval |
| Age group | ||||
| <18 years | 11.6 | 8.6–14.5 | 1.5 | 0.6–2.4 |
| 18 to 44 years | 18.1 | 15.2–20.9 | 1.7 | 1.1–2.2 |
| 45 to 64 years | 66.4 | 60.1–72.8 | 5.5 | 4.3–6.8 |
| ≥65 years | 136.4 | 125.9–146.9 | 7.2 | 4.9–9.4 |
| Sex | ||||
| Male | 52.7 | 48.2–57.1 | 4.4 | 3.4–5.4 |
| Female | 39.6 | 36.2–42.8 | 2.5 | 1.8–3.2 |
| Race | ||||
| Black | 44.6 | 38.5–50.7 | 3.4 | 2.1–4.7 |
| Non-Black | 42.9 | 39.5–46.2 | 3.1 | 2.4–3.7 |
| Hospital region | ||||
| Northeast | 42.2 | 34.0–50.5 | 1.5 | 0.6–2.4 |
| Midwest | 50.7 | 42.9–58.4 | 4.1 | 2.5–5.7 |
| South | 43.5 | 38.8–48.3 | 3.4 | 2.3–4.5 |
| West | 48.5 | 41.5–55.6 | 4.2 | 2.8–5.6 |
Abbreviations: IA-RH, invasive aspergillosis-related hospitalizations; M-RH, mucormycosis-related hospitalizations.
Comparison of Mucormycosis-Related Hospitalizations and Invasive Aspergillosis-Related Hospitalizations Trends by Underlying Condition
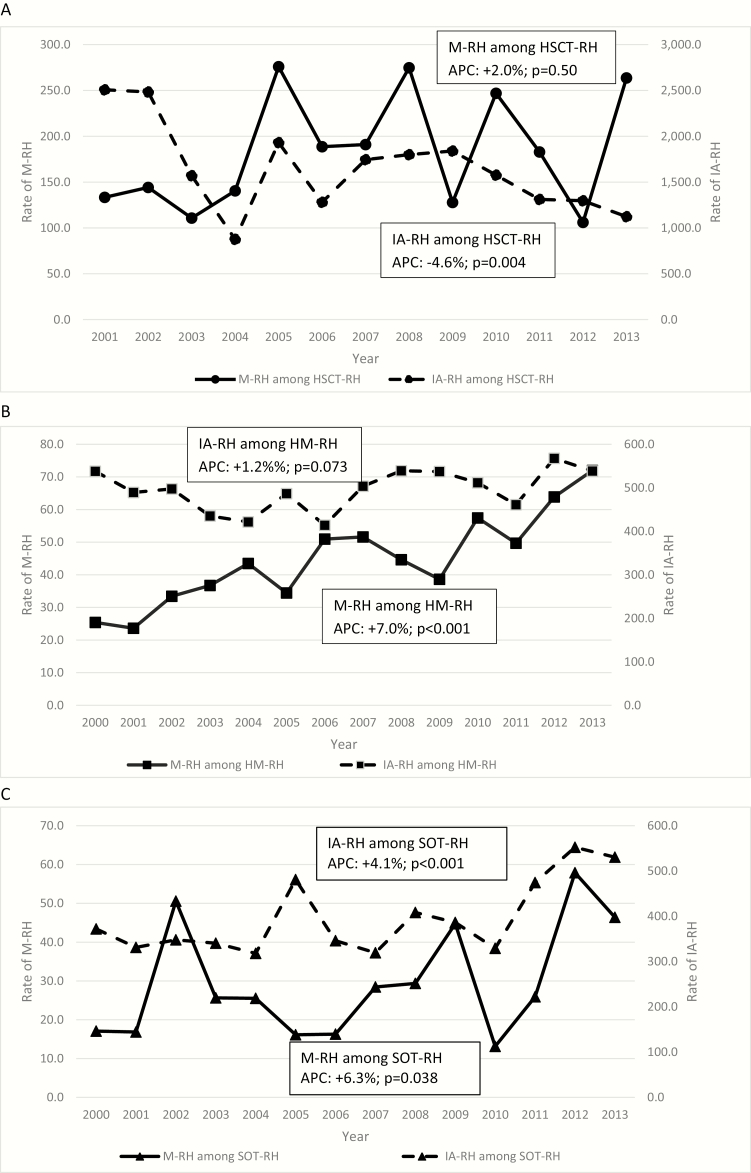
For all 3 underlying conditions evaluated (HM, HSCT, and SOT), the rates of IA-RHs were 10-fold higher than M-RH (Figure 2). Compared with HM-RHs and SOT-RHs, the rates of both IA-RHs and M-RHs were higher among HSCT-RHs. Although there was no significant change (APC = +2.0%; P = .50) in the rate of M-RHs occurring among HSCT-RHs, the rate of IA-RH declined significantly (APC = −4.6%; P = .004) (Figure 2A). Among HM-RHs, the rate of M-RHs increased (APC = +7.0%; P < .001), whereas no significant change was observed in the rate of IA-RHs (APC = +1.2%; P = .073) (Figure 2B). Among SOT-RHs, the rate of M-RHs (APC = +6.3%; P = .038) and IA-RHs (APC +4.1%; P < .001) both increased over the study period (Figure 2C). The IA-RH rates increased among both kidney SOT-RHs (APC = +2.7; P = .019) and non-kidney SOT (APC = +4.1; P < .001), but the increase was greater among non-kidney SOT. The increase in M-RHs was observed among non-kidney SOT-RH (APC = +8.4%; P = .007) but not in kidney SOT-RH.
Figure 2.
Annual rates of mucormycosis-related hospitalizations (M-RH) and invasive aspergillosis-related hospitalizations (IA-RH) per 100 000 hospitalizations for specific underlying conditions (Panel A: hematopoetic stem cell transplant [HSCT]; Panel B: hematologic malignancy [HM]; Panel C: solid organ transplant [SOT]), United States, 2000–2013. Panel A starts in 2001 because data for mucormycosis and hematopoietic stem cell transplant (HSCT) were limited for the year AQ4 2000, and Healthcare Cost and Utilization Project data use agreement restrict reporting in these instances. APC, annual percentage change.
DISCUSSION
With this analysis of the largest publicly available and nationally representative database of hospitalizations in the United States, we provide an estimate of the recent incidence of and trends in IA-RHs and M-RHs in the United States. The overall rate of IA-RHs increased annually by 3% during 2000–2013, and M-RH rates doubled during the study period from 1.7 in 2000 to 3.4 per million persons in 2013. These findings are consistent with the reported increases in IMI incidence from smaller US studies of specific patient populations and data from other parts of the world [8–10, 19, 20]. Because IA mucormycosis almost always requires hospitalization, the estimated 14560 (95% CI, 13524–15596) IA-RHs and 1080 (CI, 875–1285) M-RHs in 2013 serve as a proxy for the current overall burden of these infections in the United States.
Increases in the observed overall rates of hospitalization for these IMIs may be explained by increases in number of individuals receiving HSCT and SOT, which eventually put them at risk for IMIs [11, 12]. Furthermore, among patients with HMs, more aggressive chemotherapeutic regimens are being used and more frail patients are increasingly being accepted for chemotherapy treatment at select centers, which can increase a patient’s risk for IMIs. In our study, HM, HSCT, or SOT were listed as a comorbidity in a larger proportion of M-RHs in 2013 than in 2000; by 2013, approximately 2 of 3 M-RHs were related to 1 of these 3 comorbidities. Diabetes, on the other hand, declined from being listed as a comorbidity in >50% of M-RHs to approximately 15%. This shift in underlying conditions in mucormycosis has also been documented in Europe [19, 21], suggesting a wide-scale change in the epidemiology of mucormycosis. It is interesting to note that a recent analysis of a proprietary database containing discharge diagnoses at 560 participating US hospitals during 2005–2014 did not reveal an increasing trend in M-RHs [22]; the selection of hospitals included in this database may account for the difference in observed trends of mucormycosis.
In our study, trends in M-RHs and IA-RHs appear to differ from each other among hospitalizations related to HSCT and HM; mucormycosis remained stable among HSCT-RH, whereas IA decreased and mucormycosis increased significantly by 7% per year among HM-RHs, and there was a small and nonsignificant increase of 1.2% annually of IA-RHs in this group. These trends we observed in the United States are similar to those reported in a population-based French study of IMI hospitalizations during 2001–2010 [24]. In that study, the incidence of mucormycosis and IA ranged from 0.07 to 0.1 and 11 to 18 cases per 1 million persons, respectively. The average annual increase in the overall incidence of mucormycosis was higher (7.3%) than that observed for IA (4.4%), and an even greater difference between incidences of mucormycosis and IA was seen among patients with HM (8.7% vs 2.7%, respectively) [23]. Similarly, in a single-center autopsy study of deceased HM patients during 1989–2008, the prevalence of IA decreased significantly from 0.12 to 0.14 cases per 100 autopsies during 1989–2003 to 0.07 during 2004–2008 while mucormycete infections increased from 0.02 to 0.05 per 100 autopsies across these same 2 time periods [24].
It is particularly striking that the declining IA-RH trends among hospitalizations related to HSCT and the small and nonsignificant increase in IA-RHs related to HM (compared with a relatively large increase of mucormycosis among HM-RHs) in our study were seen despite advances in diagnostics for aspergillosis, such as use of galactomannan assay and chest computed tomography scans, and improved understanding of disease presentation during the period studied [13]; increased galactomannan assay use should have increase detection and diagnosis of IA, but we did not observe this in our study, suggesting that there may be a real decline or stabilization in IA associated with HSCT and HM. No similar advances in mucormycosis diagnostics occurred during this time [25, 26]. Thus, the true relative changes in M-RHs versus IA-RHs may be greater than the data suggest.
Declines of IA-RH among patients with HSCT, among other reasons, could potentially be due to heightened awareness among clinicians about the risk of IA and the use of prophylaxis targeted towards prevention of IA in these highly immunocompromised populations. Even though there was no difference in fungal disease-free survival with voriconazole when compared with fluconazole among HSCT recipients, there was a nonstatistically significant trend toward lower IFIs, fewer IA infections, and less frequent need for empiric antifungal therapy in the voriconazole arm [27]. Although fluconazole, with its proven survival benefit, remains the mainstay of antifungal prophylaxis for most patients with HM and HSCT, use of prophylaxis to prevent IA, in certain HM and HSCT patients during high-risk periods, is recommended [28]. Prophylaxis against IA may prevent IA but leave patients susceptible to mucormycosis infection, and it may partly explain the diverging trends in the 2 IMIs in this high-risk population [29, 30]. Alternatively, the relative increases in mucormycosis compared with IA that we observed could be unrelated to changing antifungal use practices and simply reflect the risk profile of patients or changes in the ecology of these molds.
Both M-RH and IA-RH rates increased in conjunction with hospitalizations related to SOT. Lung transplantation has the highest risk of IA [31]. In line with this, we found that IA-RH had increased more in non-kidney SOT-RH than in kidney SOT-RH. The risk of mucormycosis appears to be highest in liver transplant recipients, who have infection earlier after transplant than other SOT recipients [6, 32]. Lung transplant patients are also at high risk for mucormycosis; in one study, approximately half of SOT recipients with non-IA mold infections were lung transplant recipients, and most occurred less than 6 months after transplant [10]. In fact, we found that M-RH increased among non-kidney SOT-RH, whereas this increase was not observed among kidney SOT-RH. Small single-center trials, especially among lung transplant recipients, have shown some benefit of mold-active prophylaxis [33], but large randomized controlled trials in this population are lacking. Prescription practices around mold prophylaxis among SOT recipients are highly variable and depend on the particular transplant program [6, 34]. The increase in IA-RH and M-RH among SOT-RH is particularly notable after 2006, and reasons for this recent increase could have to do with changes in types of recipients transplanted, availability of transplant ID services at major medical centers, and improved use of galactomannan assay in the SOT population. This trend deserves further exploration.
The approximately 2-fold higher rates of M-RH among males was notable and consistent with, but more pronounced than, previous reports. Roden et al [35] observed a male to female rate ratio of 1.5. We also observed this sex discrepancy in IA-RH and outbreak investigations of IMIs [36]. We did not conduct a multivariable analysis that accounted for variability in underlying conditions by gender; it is possible that some of the difference could be explained by more males having some of the major risk factors for IA and mucormycosis including HSCT, HM, SOT, or diabetes. Another fungal infection, paracoccidioidomycosis, is at least 10 times more common in men than women, and it has been associated with the influence of sex hormones [37]. The reason for difference in 2-fold rates in M-RH by sex are not clear and need further study.
The regional variation in rates of IA-RH and M-RH we observed has been reported previously. In a study of non-Aspergillus mold infections, mostly due to mucormycosis, at 23 transplant centers all across the United States, hospitals in the Northeast only reported 7.1% of cases, whereas hospitals from the South reported 47.3% of cases [38]. The reason for these regional differences may be multifactorial, including variation in climate and ecology that may encourage mold growth, difference in distribution of underlying conditions such as diabetes [39], or regional differences in medical practices, including antifungal prophylaxis.
This study is limited by its reliance on hospital discharge coding, which can be subject to errors including misdiagnosis and inconsistent coding. However, in the absence of prospective surveillance for IMIs, this may currently be the best available source of data to estimate the burden and changing trends in IMIs. How the number of hospitalizations with an ICD-9 discharge code for mucormycosis relates to the actual number of M-RHs and the actual incidence of mucormycosis remains to be determined. On one hand, because of the inaccuracies and limitations of ICD-9 coding, this may be an overestimate of the actual number of IA and mucormycosis cases. Pulmonary and rhinocerebral mucormycosis is associated with a mortality rate of 50% or greater, and mortality for IA is 20%–50% depending on the underlying condition [1, 10]; however, in this study, in-hospital death occurred in only 16% of IA-RH and 20% of M-RHs, indicating that perhaps not all of the hospitalizations with an ICD-9 code for IA and mucormycosis were in fact related to these conditions. It is also possible (1) that a larger than expected proportion of mucormycosis infections were cutaneous, a less common form of mucormycosis that is associated with a much lower mortality rate, or (2) that other types of Aspergillus infection were coded as IA. In addition, because patients can be hospitalized for the same condition more than once, the number of patients with these IMIs could be lower than the number of hospitalizations for the condition. On the other hand, ICD-9 codes almost certainly underestimate the true frequency of this disease because mucormycosis is notoriously difficult to diagnose. Culture is insensitive, pathology-based diagnosis is challenging, and patients often rapidly decompensate before mucormycosis is suspected and diagnosis attempted. Diagnosis of mucormycosis, unlike diagnosis of aspergillosis, is not aided by the presence of galactomannan in serum or bronchial lavage fluid, and there are less clear pathognomonic radiologic findings for mucormycosis. According to one autopsy study, only 40% of invasive fungal infections were diagnosed pre-mortem [40].
CONCLUSIONS
Overall rates of IA-RH and M-RH have increased in the United States since 2000. Although the incidence of mucormycosis remains several-fold lower than that of IA, mucormycosis is becoming an increasingly important IMI, especially among highly immunocompromised patients such as those with HSCT, HMs, and SOTs. Ongoing surveillance to better understand the true burden of IA, mucormycosis, and other IMIs and strategies for the control these infections are needed.
Acknowledgments
We thank the state data organizations that contribute data to the Healthcare Cost and Utilization Project ([HCUP; https://www.hcup-us.ahrq.gov/db/hcupdatapartners.jsp).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lanternier F, Sun HY, Ribaud P, et al. Mucormycosis in organ and stem cell transplant recipients. Clin Infect Dis 2012; 54:1629–36. [Google Scholar]
- 2. Zaoutis TE, Heydon K, Chu JH, et al. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. Pediatrics 2006; 117:e711–6. [DOI] [PubMed] [Google Scholar]
- 3. Zilberberg MD, Shorr AF, Huang H, et al. Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infect Dis 2014; 14:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010; 50:1091–100. [DOI] [PubMed] [Google Scholar]
- 5. Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009; 48:265–73. [DOI] [PubMed] [Google Scholar]
- 6. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 7. Rees JR, Pinner RW, Hajjeh RA, et al. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin Infect Dis 1998; 27:1138–47. [PubMed] [Google Scholar]
- 8. Neofytos D, Treadway S, Ostrander D, et al. Epidemiology, outcomes, and mortality predictors of invasive mold infections among transplant recipients: a 10-year, single-center experience. Transpl Infect Dis 2013; 15:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002; 34:909–17. [DOI] [PubMed] [Google Scholar]
- 10. Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis 2011; 17:1855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Health Resources and Services Administration, U.S. Department of Health & Human Services. Organ Procurement and Transplantation Network: View Data Reports. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/. Accessed 4 December 2016.
- 12.Center for International Blood and Marrow Transplant Research. Center for International Blood and Marrow Transplant Research Transplant Activity Report Covering 2009–2013. Available at: http://bloodcell.transplant.hrsa.gov/research/transplant_data/transplant_activity_report/summary-total_tx_by_year.pdf. Accessed 4 December 2016.
- 13. Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis 2005; 5:609–22. [DOI] [PubMed] [Google Scholar]
- 14. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 15. Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant 2011; 46:709–18. [DOI] [PubMed] [Google Scholar]
- 16. HCUP National Inpatient Sample 2012 and Nationwide Inpatient Sample 2001-2011. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD: Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 4 December 2016. [Google Scholar]
- 17. Healthcare Utilization Project (HCUP). 2015. Agency for Healthcare Research and Quality Available at: www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed 4 December 2016.
- 18. Gillum BS, Graves EJ, Kozak LJ. Trends in hospital utilization: United States 1988–92. Vital Health Stat 1996; 13: 1–71. [PubMed] [Google Scholar]
- 19. Petrikkos G, Skiada A, Drogari-Apiranthitou M. Epidemiology of mucormycosis in Europe. Clin Microbiol Infect 2014; 20:67–73. [DOI] [PubMed] [Google Scholar]
- 20. Saegeman V, Maertens J, Meersseman W, et al. Increasing incidence of mucormycosis in University Hospital, Belgium. Emerg Infect Dis 2010; 16:1456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 2011; 17:1859–67. [DOI] [PubMed] [Google Scholar]
- 22. Kontoyiannis DP, Song J, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States. BMC Infect Dis 2016; 16:–730. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27905900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bitar D, Lortholary O, Le Strat Y, et al. Population-based analysis of invasive fungal infections, France, 2001-2010. Emerg Infect Dis 2014; 20:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis RE, Cahyame-Zuniga L, Leventakos K, et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses 2013; 56:638–45. [DOI] [PubMed] [Google Scholar]
- 25. Walsh TJ, Gamaletsou MN, McGinnis MR, et al. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis 2012; 54:S55–60. [DOI] [PubMed] [Google Scholar]
- 26. Robin C, Alanio A, Cordonnier C. Mucormycosis: a new concern in the transplant ward? Curr Opin Hematol 2014; 21:482–90. [DOI] [PubMed] [Google Scholar]
- 27. Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116:5111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trifilio SM, Bennett CL, Yarnold PR, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant 2007; 39:425–9. [DOI] [PubMed] [Google Scholar]
- 30. Siwek GT, Dodgson KJ, de Magalhaes-Silverman M, et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis 2004; 39:584–7. [DOI] [PubMed] [Google Scholar]
- 31. Minari A, Husni R, Avery RK, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis 2002; 4:195–200. [DOI] [PubMed] [Google Scholar]
- 32. Singh N, Aguado JM, Bonatti H, et al. Zygomycosis in solid organ transplant recipients: a prospective, matched case-control study to assess risks for disease and outcome. J Infect Dis 2009; 200:1002–11. [DOI] [PubMed] [Google Scholar]
- 33. Husain S, Paterson DL, Studer S, et al. Voriconazole prophylaxis in lung transplant recipients. Am J Transplant 2006; 6:3008–16. [DOI] [PubMed] [Google Scholar]
- 34. Neoh CF, Snell GI, Kotsimbos T, et al. Antifungal prophylaxis in lung transplantation–a world-wide survey. Am J Transplant 2011; 11:361–6. [DOI] [PubMed] [Google Scholar]
- 35. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41:634–53. [DOI] [PubMed] [Google Scholar]
- 36. Novosad SA, Vasquez AM, Nambiar A, et al. Notes from the field: probable mucormycosis among adult solid organ transplant recipients at an acute care hospital - Pennsylvania, 2014-2015. MMWR Morb Mortal Wkly Rep 2016; 65:481–2. [DOI] [PubMed] [Google Scholar]
- 37. Pinzan CF, Ruas LP, Casabona-Fortunato AS, et al. Immunological basis for the gender differences in murine Paracoccidioides brasiliensis infection. PLoS One 2010; 5:e10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis 2011; 17:1855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barker LE, Kirtland KA, Gregg EW, et al. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med 2011; 40:434–9. [DOI] [PubMed] [Google Scholar]
- 40. Sinkó J, Csomor J, Nikolova R, et al. Invasive fungal disease in allogeneic hematopoietic stem cell transplant recipients: an autopsy-driven survey. Transpl Infect Dis 2008; 10:106–9. [DOI] [PubMed] [Google Scholar]