Abstract
Schemes for classifying skin and soft tissue infections (SSTIs) pose limitations for clinicians and regulatory agencies. Diabetic foot infections (DFIs) are a subset of SSTIs. We developed and are proposing a classification to harmonize current schemes for SSTIs and DFIs. Existing schemes for classifying SSTIs are limited in both their usefulness to clinicians and to regulatory agencies. The guidelines on SSTI from the Infectious Diseases Society of America (IDSA) and the guidance from the US Food and Drug Administration do not adequately address many types of wound infections. However, guidelines developed by the IDSA for DFIs provide a classification scheme that has been validated and widely used. Diabetic foot infections are similar to SSTIs in pathophysiology, microbiology, and treatment and can be seen as a subset of SSTI. Thus, based on the documents noted above, and our review of the literature, we have developed a proposed classification scheme for SSTI that harmonizes well with the DFI classification. We believe this new scheme will assist clinicians in classifying most wound infections and potentially aid regulatory agencies in testing and approving new antimicrobials for these infections.
Keywords: classification, diabetic foot, skin and soft tissue, topical therapy
Various authorities and organizations have proposed classification schemes for skin and soft tissue infections (SSTIs). These institutions organize SSTIs by such variables as anatomic location, causative pathogen(s), rate of progression, depth of extension, and clinical presentation or severity [1]. Unfortunately, each has key limitations both in assisting clinical management and in providing guidance for developing new therapeutic agents. Important deficiencies of these schemes include the omission of discussion of diabetic foot ulcers (DFUs) and superficial wounds, the categorization of wounds by parameters that lack proven therapeutic relevance, and a lack of validation in clinical practice. One exception to this general situation is a classification for foot infections in persons with diabetes. For this type of soft tissue (and often bone) infection, clinicians and researchers have used an internationally recognized and validated classification scheme for over a decade [2, 3]. Of note, diabetic foot infections (DFIs) share most pathophysiologic, etiologic, and therapeutic characteristics with other types of SSTIs. Thus, we believe it may be appropriate to use the DFI classification as a model for categorizing wound severity to develop a more general nosology of SSTI. Such a scheme, if generally accepted, could also inform treatment decisions and other aspects of clinical care, guide research studies, and aid in regulatory evaluation of novel therapeutic agents.
We have had a long-standing interest in SSTIs, and we conducted a nonsystematic review of the literature on classification schemes for these infections. This review included searching PubMed for all published papers up until September 23, 2016, EMBASE, and the Cochrane Database of Systematic Reviews, and the bibliographies of all retrieved papers. In this study, we offer an analysis of the overlap between common types of SSTIs and DFIs and propose a new classification scheme for SSTIs based on that used for DFI. We term this scheme the Consolidated Classification of Skin and Soft Tissue Infections (COCLASSTI).
PATHOPHYSIOLOGY AND EPIDEMIOLOGY OF SKIN AND SOFT TISSUE INFECTIONS
The skin, especially in conjunction with the immediately contiguous subcutaneous tissues, is the largest organ in the body. The epidermis has no blood vessels and is protected against infection by the mechanical barrier posed by the stratum corneum [4]. Infections of the skin and underlying soft tissues, which occur when microbial invasion of various layers overwhelm host defenses, are clinical entities with variable presentations, causes, and levels of clinical severity [5]. Skin and soft tissue infections range from relatively mild and superficial, such as impetigo, to deeper and more severe, such as necrotizing fasciitis. They are common in ambulatory and inpatient settings, accounting for more than 14 million outpatient visits and 850 000 hospitalizations annually in the United States alone [6]. Although the reported estimates for incidence of SSTIs in the United States and the United Kingdom are 49.6 per 1000 persons and 16.4 per 1000 persons, respectively, the true prevalence is likely higher because mild cases may resolve without medical care [7]. A study in the United States reported an estimated 869 800 hospital admissions for SSTIs in 2004, and the incidence increased over the next several years by 29% in the inpatient setting and by 50% in the outpatient setting [8, 9]. A survey across European countries found that SSTI was the second most common indication, after lower respiratory tract infection, for prescribing antibiotics [10].
UNITED STATES FOOD AND DRUG ADMINISTRATION SKIN INFECTION CATEGORIZATION
In 1998, the US Food and Drug Administration (FDA) proposed a system that categorized SSTIs as either “uncomplicated” (superficial infections that can usually be treated by antimicrobial therapy or surgical incision alone) or “complicated” (involving deeper tissues or requiring substantial surgical intervention), with the later including DFI. In 2013, the FDA adopted a new Guidance for Industry [11] document for the development of drugs for treating a newly proposed category of infection called “acute bacterial skin and skin structure infections” (ABSSSI). This document, which defines ABSSSI as a bacterial infection of the skin with a lesion size area of ≥75 cm2 (based on the area of redness, edema, or induration), includes only 3 types of infection: cellulitis/erysipelas, wound infection, and major cutaneous abscess. The Guidance also specified types of infection that it did not address, including, “less serious skin infections, such as impetigo and minor cutaneous abscess, as well as infections needing more complex treatment regimens, such as infections resulting from animal or human bites, necrotizing fasciitis, DFI, decubitus ulcer infection, myonecrosis, and ecthyma gangrenosum.” The Guidance invites sponsors interested in developing drugs to treat these excluded infections to discuss plans with the FDA. These recent changes now leave no pathway for a new antibiotic to receive approval for any of these other common SSTIs without going through what would likely be a prolonged and costly negotiation process with the FDA.
CLASSIFICATION OF DIABETIC FOOT INFECTIONS
A recent large study of enrollees in a US health plan found that among more than 2.2 million episodes of SSTIs evaluated from 2005 to 2010, 10% occurred in persons with diabetes [12]. Because infections of the foot are now among the most common serious complication of diabetes [13], and they are often associated with comorbidities such as peripheral neuropathy and arterial disease, specialized classification schemes were developed for DFI. The classification, devised in 2004 by the Infectious Diseases Society of America (IDSA) guidelines committee for DFIs, and adopted by the expert panel on infection of the International Working Group on the Diabetic Foot (IWGDF) [14, 15], both defines when a wound is infected and classifies the infection’s clinical severity. The scheme has achieved widespread acceptance internationally and has been validated in numerous studies [16–22].
Most DFIs develop in an ulceration of the skin, usually related to an unperceived injury to a neuropathic (and therefore insensate) foot. In patients with or without diabetes, many other injuries can cause wounds that become infected, eg, mechanical trauma, prolonged pressure, venous or arterial insufficiency, chemical or thermal burns, animal or human bites, surgery, or primary dermatologic disorders. Bacterial pathogens, after infecting the epidermis, may translocate to deeper structures through the lymphatics or by direct extension. The presence of a rich capillary network beneath the dermal papillae plays a key role in localizing infection as well as in producing an acute inflammatory response [4].
HOW SIMILAR ARE DIABETIC FOOT INFECTIONS AND SKIN AND SOFT TISSUE INFECTIONS?
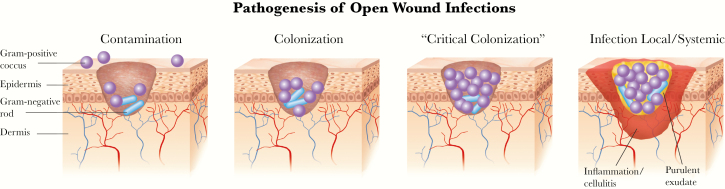
As discussed, almost all SSTIs follow a break in the protective skin envelope that allows microorganisms to first contaminate (defined as the transient presence of organisms that are not actively replicating), then colonize (replicating but not causing a host response or tissue damage), and if unchecked, eventually infect (initiating a host response, manifest as classic signs or symptoms of inflammation) the subepidermal tissues (Figure 1). Some wound experts believe there is an intermediate stage between colonization and infection, often called “critical colonization,” defined as organisms present in numbers (usually >105 bacteria/gram of tissue) sufficient to at least hinder wound healing if not to produce overt evidence of infection [23]. However, there is no consensus on how to define this state or whether it even exists [24].
Figure 1.
The typical evolution of a superficial wound infection. The growth of microorganisms in a wound and the host response determine how far along in this spectrum the process goes.
Infecting organisms in DFI and other types of SSTI are usually derived from those that colonize the surrounding normal skin, which may represent resident or transient flora. Some pathogens are endogenous, shed from sites of colonization, such as the anterior nares or perineum, and deposited in the wound by direct inoculation. Others are exogenous, introduced as a consequence of percutaneous trauma (accidental or surgical), or during care for the wound. The likelihood that infection will occur in a colonized wound is (1) directly related to the inoculum size of the contaminating organism and its intrinsic virulence and (2) inversely related to the host’s defense capabilities. Some organisms such as β-hemolytic streptococci and Staphylococcus aureus are inherently virulent in wounds, whereas others mainly become pathogens when they are present in combinations, inoculate damaged skin, or colonize a wound in an immunocompromised host. The presence of local ischemia, necrotic tissue, or a foreign body increases both the likelihood that a wound will become infected and the severity of the infection.
Studies have shown that the pathogens causing DFIs and those causing other types of SSTIs are largely the same, in both North America and Europe [25]. These pathogens usually consist of aerobic Gram-positive cocci (especially S aureus and, less frequently, streptococci), but specific exposures, such as water-related trauma or bite wounds, are associated with less common pathogens (eg, Enterobacteriaceae, Pseudomonas aeruginosa, obligate anaerobes) [17]. Likewise, the antibiotic susceptibility patterns for the causative pathogens are similar in both types of infections. A higher rate of SSTIs caused by methicillin-resistant S aureus has been noted in the United States, but rates in some parts of Europe and elsewhere have been increasing. More recent studies of DFIs, particularly those conducted in Asia and Africa, have reported that Gram-negative organisms (particularly P aeruginosa) are predominant in wound infections. In all locations, strains resistant to widely prescribed antibiotic agents are becoming more common [26], with a greater likelihood of highly resistant Gram-negative aerobes (eg, those with extended-spectrum β-lactamases or carbapenemases) [27].
Although DFIs occur in a particular host substrate, characterized by variable degrees of metabolic derangements and poorly understood immunodeficiencies, they share almost all other pathophysiological, microbiological, clinical, diagnostic, and therapeutic features of other types of SSTIs. What is characteristic (although not unique) to DFIs is that almost all affected patients have peripheral neuropathy. Although the presence of neuropathy predisposes to developing the wounds that become infected and may delay infection coming to the attention of the patient, it probably does not affect either infection severity or the approach to treatment. However, the presence of peripheral arterial disease, also found in most patients with a DFI, is almost certainly associated with worse outcomes for the infection [28, 29]. Skin and soft tissue infections in patients with, compared with without, diabetes are 5 times more likely to be associated with complications and 4 times more likely to result in hospitalization [12].
LIMITATIONS OF CURRENT CLASSIFICATIONS
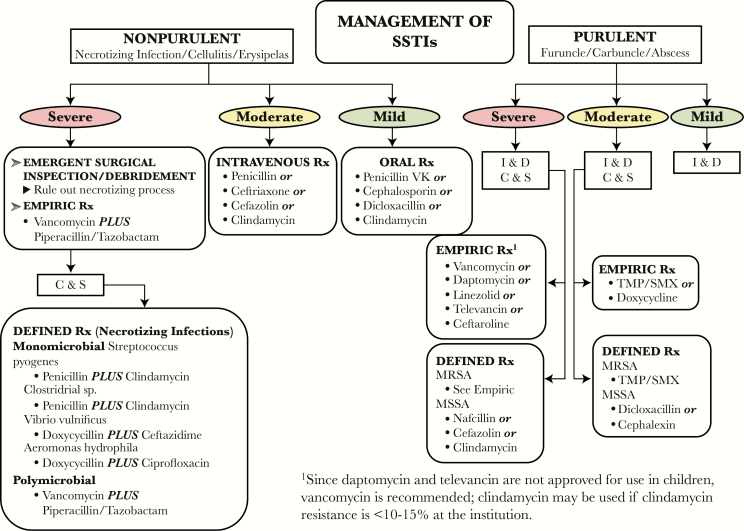
Several authorities, including clinical researchers, professional societies, and regulatory bodies, have promoted schemes to describe the various types of SSTIs in diabetic and nondiabetic patients. Perhaps the most frequently cited classification of SSTI is the Practice Guidelines of the IDSA [30], which generally divides infections by skin extension (uncomplicated, complicated), rate of progression (acute versus chronic), and tissue necrosis (necrotizing versus nonnecrotizing). Unfortunately, the scheme is of limited utility for wound infections, because the main management scheme (Figure 2) does not include wounds as part of the nosology. To the extent that the IDSA SSTI guideline addresses wound infection at all, the recommendations are directed solely to postsurgical wounds. The narrow scope of these recommendations limits their relevance with respect to other types of wound infections.
Figure 2.
Infectious Diseases Society of America classification of skin and soft tissue infections (SSTIs), which does not explicitly include wound infections [4]. C & S, culture and sensitivity; I & D, incision and drainage; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S aureus; Rx, treatment; TMP/SMX, trimethoprim-sulfamethoxazole.
Various severity-of-illness systems have been proposed for SSTIs, but none has been widely adopted [7]. An expert panel led by Eron proposed a SSTI classification scheme in 2003 [31] that divided infections into 4 classes based on the patient’s presentations: (1) afebrile and healthy; (2) febrile and ill-appearing but no unstable comorbidities; (3) toxic appearance, or at least 1 unstable comorbidity, or a limb-threatening infection; and (4) sepsis syndrome or life-threatening infection, eg, necrotizing fasciitis. Ki and Rotstein [32] argued that Eron’s scheme was “overly simplified” and the descriptions of clinical presentations were ambiguous. Therefore, they proposed a rather complicated scheme that grades infection severity (mild or moderate/severe) based on the presence of comorbidities, systemic signs of sepsis, the wound’s location (specifically involvement of the head or hand), the size of the lesion, and the presence of findings suggesting severe or necrotizing infection [32]. Likewise, the United Kingdom’s Department of Health and Social Services’ Clinical Resource Efficiency Support Team (CREST) published a guideline on the management of cellulitis that was largely based on the Eron scheme [33]. The CREST system, which does not appear to be used outside the United Kingdom, suffers from ambiguous definitions for clinical presentation and a lack of objective measurements of infections severity. Noting the lack of a validated severity grading system to predict clinical outcome in SSTIs, Marwick et al [34] aimed to retrospectively apply a modification of the Eron/CREST classification to a cohort of hospitalized SSTI patients. They found that patients with more severe infection had worse outcomes; furthermore, most patients with the least severe infections were “over-treated” (with unnecessarily broad-spectrum antibiotic therapy), whereas those with more severe infections were usually inadequately treated [34]. More recently, Hashem et al [7] combined the CREST scheme with the Standardized Early Warning Score ([SEWS] a predictor of clinical deterioration) to create mutually exclusive severity classes for SSTIs. They assessed the appropriateness of antibiotic therapy using the 4 proposed classes based on the SEWS, along with the presence of “sepsis” and comorbidities, in a retrospective, hypothesis-generating evaluation of hospitalized patients with SSTIs [7]. Similar to Marwick et al [34], they found that the majority of patients with the least severe infections were overtreated, whereas almost half of those with the most severe infections were undertreated [7]. Of note, they specifically excluded patients with DFIs from enrollment in this study. Each of these few reported studies was retrospective and only enrolled hospitalized patients. Our review of the literature disclosed no other clinically useful published guidelines for the diagnosis and management of wound infections and similar superficial SSTIs.
A PROPOSAL FOR MODIFIED SKIN AND SOFT TISSUE INFECTIONS CLASSIFICATION SCHEME
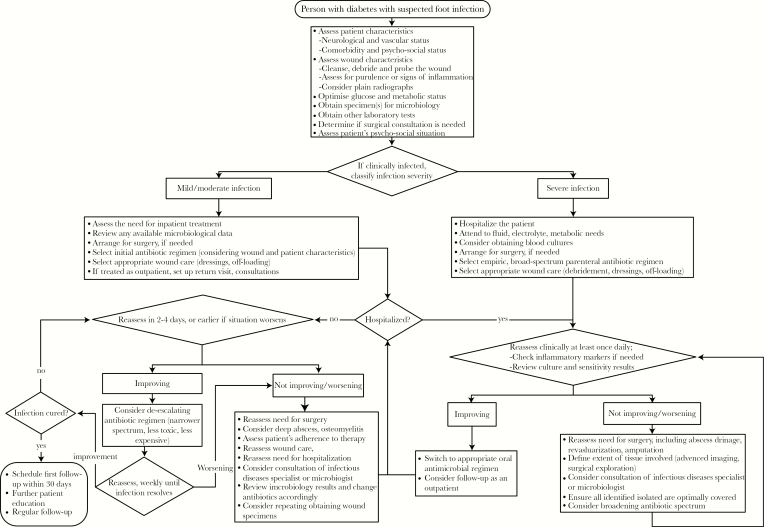
As noted above, evidence supports that for DFI patients, the IDSA/IWGDF DFI classification scheme is useful for predicting the need for and duration of hospitalization, the likelihood of undergoing lower extremity amputation, and other adverse outcomes. We believe that the algorithm developed by the IWGDF for an infected DFU (Figure 3) is the most suitable published tool currently available for guiding clinicians in managing patients with such infections [3]. This scheme has the advantages of being straightforward and intuitive, evidenced-based, practical, clinically relevant, and potentially applicable to nondiabetic superficial skin infections. With this in mind, we developed a modified version of the IWGDF approach to the infected wound that is based on the belief that almost all items in the algorithm are common across both populations. This diagnostic scheme is largely compatible with the previously mentioned expert panel classifications, as well as the current IDSA guidelines (Table 1).
Figure 3.
Approach to the patient with diabetes and a suspected foot infection [3].
Table 1.
Concordance of IDSA Classification Schemes for Severity of SSTIs (COCLASSTI) and Infected DFUs (Adapted From 3 and 4)a
| Skin and Soft Tissue Infections | Infected Diabetic Foot Ulcers | |||||
|---|---|---|---|---|---|---|
| Category | Clinical Features | Current Management | IDSA Infection Severity | Clinical Features | Current Management | Amenable to Topical Therapy? |
| Class 1 | Superficial skin infections • Impetigo • Ecthyma • Superficial, limited wound infections |
Drainage (if required) and oral antibiotics in the outpatient setting. Occasionally topical antibiotics | Mild | Local infection involving only the skin and the subcutaneous tissue (without involvement of deeper tissues and without systemic signs as described below). If erythema, must be >0.5 cm to ≤2 cm around the ulcer. Exclude other causes of an inflammatory response of the skin (eg, trauma, gout, fracture). | Usually treated with oral antibiotics in the outpatient setting | Yes |
| Class 2A | Systemically well Erysipelas & cellulitis Purulent skin and soft infections • Abscess • Furuncle, carbuncle Traumatic wounds • Surgical site infections • Animal bites • Other trauma (eg, pressure, thermal, pun cture, crush) |
Oral or intravenous (often outpatient) antibiotic therapy; may require short period of hospital observation | Moderate - Class A | Local infection (as described above), but with erythema extending >2 cm from rim of ulcer | May be treated with oral, or initial parenteral with rapid switch to oral, antibiotics | Potentially, but as adjunctive to systemic antibiotic therapy |
| Class 2B | Systemically unwell, but no systemic inflammatory response syndrome (SIRS) Erysipelas & cellulitis Purulent skin and soft infections • Abscess • Furuncle carbuncle Traumatic wounds • Surgical site infections • Animal bites Other trauma (eg, pressure, thermal, puncture, crush) |
Oral or outpatient parenteral antibiotic therapy; may require short period of hospital observation | Moderate - Class B | Local infection (as described above) involving structures deeper than skin and subcutaneous tissues (eg, abscess, osteomyelitis, septic arthritis, fasciitis), but with no evidence of systemic inflammatory response syndrome (as described below) | May be treated with oral or initial parenteral antibiotics | No |
| Class 3 | Sepsis syndrome and life-threatening infection Necrotizing infections of skin and soft tissues • Necrotizing fasciitis • Gas gangrene • Pyomyositis |
Likely to require admission to intensive care unit, urgent surgical assessment, and treatment with parenteral antibiotics | Severe | Local infection (as described above) with evidence of SIRS, as manifested by ≥2 of the following: • Temperature >38°C or <36°C • Heart rate >90 beats/ min • Respiratory rate >20 breaths/min or PaCO2 <32 mm Hg • White blood cell count >12 000 or <4000 cells/μL or ≥10% immature (band) forms |
Treat, at least initially with parenteral antibiotic(s) | No |
Abbreviations: DFU, diabetic foot ulcer; IDSA, Infectious Disease Society of America; SIRS, systemic inflammatory response syndrome.
aNote that in the original publications, the rows in boldface type are not separated into “A” and “B”, as shown here. Infection defined as presence of at least 2 of the following items: (1) local swelling or induration; (2) erythema; (3) local tenderness or pain; (4) local warmth; (5) purulent discharge (thick, opaque to white or sanguineous secretion).
Beginning with the SSTI portion of our classification scheme, we have divided the various types of infections discussed in the IDSA SSTI guideline by severity into 3 classes. Class 1 includes several types of superficial skin infections that have in common that they are usually treated in the outpatient setting, typically with oral (and occasionally topical) antibiotic agents. Class 2 infections are either more extensive or affect deeper tissues (or both) and always require systematic antibiotic therapy. This class is divided into 2 categories: 2A is for patients who present systemically well with an infection that is a pyoderma or caused by some form of trauma; 2B is for patients who are systemically unwell in some way but do not meet the criteria for systemic inflammatory response syndrome (SIRS) [35]. Class 2B patients may require initial treatment in the inpatient setting, perhaps with parenteral antibiotic therapy. Class 3 infections manifest either with SIRS, or are potentially limb- or life-threatening, and often require urgent surgical consultation.
We think the IDSA DFI classification scheme also might benefit from a slight modification. The main problem with the current system is that the “moderate” infection category is quite broad and heterogeneous. To address this issue, and to make the system better parallel our proposal for SSTIs, we suggest dividing the moderate designation into (1) Class A for local infections that are more “horizontally” extensive with erythema extending >2 cm from the rim of a wound and (2) Class B for infections that are more “vertically” extensive, extending below the subcutaneous tissue.
THE ROLE OF TOPICAL ANTIMICROBIAL THERAPY
The management of all SSTIs, including infected DFUs, largely consists of any needed surgical debridement, properly selected antimicrobial therapy, and appropriate wound care. For moderate and severe infections, initial antibiotic therapy must be systemic, often parenteral to start, with a change to an oral agent when the infection is responding and the patient is stable. Mildly infected open wounds are often treated with oral antibiotic therapy but afford the possibility of topical antimicrobial therapy. Like mildly infected DFU, superficial SSTIs (eg, those related to venous stasis ulcers, low-grade pressure ulcers, abrasions, limited surgical wounds, inflammatory skin disorders [eg, eczema], or limited thermal burns) may be appropriately treated by topical administration of an agent with an appropriate (usually broad) antibacterial spectrum. Because these wounds are not usually ischemic, and most do not require special pressure off-loading, the clinical success with topical antimicrobial therapy, as determined by resolution of clinical signs and symptoms of infection, will likely be at least as good as that seen with infected DFUs.
Delivering an antimicrobial directly into a wound has many potential advantages [36]. It may achieve high levels of the agent at the infected site, even with an ischemic wound, while avoiding systemic exposure and its attendant risks to various organs. Locally applied, compared with systemic, antibiotic therapy usually requires smaller doses, thereby reducing the risk of inducing antimicrobial resistance. Finally, topical therapy may allow treatment with agents not currently available (or safe) for systemic therapy. These features all fit nicely with the tenets of antimicrobial stewardship for wounds [14]. Thus, a topical agent with the right spectrum of activity might be effective, either as primary therapy for a mild infection or as adjunctive therapy to systemic treatment for moderate or severe DFI.
Although topical agents have long been used for treating superficial skin infections such as impetigo, there are relatively few well designed studies of topical treatment of infected wounds [36]. One large randomized controlled trial of a topical antimicrobial peptide, pexiganan, demonstrated that it was similarly effective to oral antibiotic therapy for treating infected DFUs [37]. Furthermore, some of the infecting bacteria isolated from the wounds of patients in the ofloxacin treatment group developed resistance to that drug, whereas patients treated with pexiganan did not develop resistance to pexiganan. Based on these data, the IDSA DFI guidelines suggest that topical antimicrobial therapy may be appropriate for mildly infected open wounds with minimal cellulitis [2]. It is likely that other superficial SSTIs may be amenable to topical antimicrobial therapy.
DISCUSSION
An essential component of evaluating a patient with an infected wound is determining the severity of the infection. This assessment dictates the key decisions about treatment, including the site (outpatient versus inpatient), the need for (and urgency of) any surgical interventions, the route of antimicrobial therapy (topical, oral, parenteral), the spectrum of empiric antibiotic therapy (narrow or broad), and the need for any adjunctive treatments (eg, hyperbaric oxygen, corticosteroids, antitoxins). What most clinicians want is guidance that is clear and concise and will aid in selecting appropriate management. As noted, there is currently no widely accepted or evidence-based classification for infected wounds. The current IDSA guidelines for SSTIs have useful schemes for some types of infections (eg, nonpurulent vs purulent, and surgical site) but not for most of the various kinds of wounds (eg, traumatic, burns, pressure) commonly seen in the outpatient and inpatient settings [4]. Other proposed classifications are mostly directed at the severe end of the spectrum of SSTIs, adding an assessment of patient comorbidities, but do not clarify issues related to the size and depth of the wound. The IDSA guideline classification scheme for DFIs [2] is easy to perform and predicts important outcomes. It has been widely implemented throughout the world for both clinical decision making and research purposes for more than a decade. We believe that it is also likely to perform well for classifying wounds in patients without diabetes, especially with our suggested slight change for moderate infections. Therefore, we suggest that with only minor modifications, the management approach to infections in both of these patient populations can be harmonized into the proposed new COCLASSTI scheme, as shown in Table 1.
CONCLUSIONS
Of note, because of the limited published data on topical antimicrobial therapy, neither the SSTI nor the DFI guideline has much discussion of this route of treatment. Because the ability to treat a SSTI with topical antimicrobial therapy is a potentially important distinction in a classification scheme, we have introduced this novel dimension by adding a column to Table 1 addressing the potential for such future treatments. We think this scheme, and our discussion of the issues, may be helpful in managing the common and often serious problem of wound infections. It could also serve as a useful tool in the development of new therapeutic agents for SSTIs. Because the pathogenesis, bacterial etiology, clinical presentation, and management of diabetic wound infections are essentially the same as those of superficial, localized SSTIs (Table 2), the pathway to testing and approving new antimicrobials to treat these infections should logically be similar. We look forward to comments on these proposals from our colleagues who care for patients with SSTI.
Table 2.
Clinically Relevant Common Features of DFIs and Superficial, Localized SSTI
| Initiating event | Disruption of the protective skin barrier by any of several mechanisms |
| Pathophysiology | Microorganisms first colonize and, if unchecked, spread to infect the contiguous subepidermal tissues |
| Bacterial pathogens - Predominant - Occasional* |
Staphylococcus aureus (MSSA, MRSA) and Streptococcus species (usually group B); coagulase-negative staphylococci; Enterococcus; Enterobacteriaceae; obligate anaerobes (especially in bite wounds) |
| Clinical presentation/ diagnosis | • Purulent secretions • Erythema • Swelling or induration • Warmth • Pain or tenderness |
| Management | • Obtain appropriate material for culture (usually infected tissue); and • Consider the need for surgical intervention (especially debridement, incision and drainage); and • Initiate antimicrobial therapy |
| Antibiotic approach | • Initial therapy is usually empiric, based on the likeliest pathogens and their probable antibiotic susceptibility patterns in a specific geographic/clinical location: o Usually oral administration; initial parenteral for some moderate and all severe infections o Potential for topical treatment for infected superficial, open wounds |
| Duration of antibiotic therapy | Usually 7–14 days |
| Therapeutic goal | Resolution of clinical signs and symptoms used to diagnose the presence of infection |
Abbreviations: DFIs, diabetic foot infections; SSTI, skin and soft tissue infections; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S aureus.
*Usually seen in patients who have recently received antibiotic therapy, who have a long-standing wound, who reside in or have frequent exposure to healthcare settings, or who have specific epidemiological exposures.
Acknowledgments
We thank Robert J. DeLuccia for initiating and encouraging discussion on this topic as well as providing constructive suggestions for our consideration.
Financial support. This work was supported by Dipexium Pharmaceuticals, Inc (New York, NY).
Potential conflicts of interest. B. A. L. and M. H. S. report personal fees from Dipexium Pharmaceuticals Ltd, outside the submitted work. W. S. J. reports the following: personal fees from Dipexium, during the conduct of the study; personal fees from Merck and personal fees from Medicines Company, outside the submitted work.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis 2016; 29:109–15. [DOI] [PubMed] [Google Scholar]
- 2. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54:e132–73. [DOI] [PubMed] [Google Scholar]
- 3. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016; 32:45–74. [DOI] [PubMed] [Google Scholar]
- 4. Stevens D. Infections of the skin, muscles, and soft tissues. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: Mc-Graw Hill Professional Publishing; 2015: Chapter 156. [Google Scholar]
- 5. Cross L. The classification and management of skin and soft tissue infections. Int Emerg Nurs 2013; 21:84–8. [DOI] [PubMed] [Google Scholar]
- 6. Miller LG, Daum RS, Creech CB, et al. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med 2015; 372:1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashem NG, Hidayat L, Berkowitz L, Venugopalan V. Management of skin and soft-tissue infections at a community teaching hospital using a severity-of-illness tool. J Antimicrob Chemother 2016; 71:3268–75. [DOI] [PubMed] [Google Scholar]
- 8. Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–91. [DOI] [PubMed] [Google Scholar]
- 9. Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ansari F, Erntell M, Goossens H, Davey P. The European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis 2009; 49:1496–504. [DOI] [PubMed] [Google Scholar]
- 11. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment. Available at: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm071185.pdf Accessed 22 September 2016. [Google Scholar]
- 12. Suaya JA, Eisenberg DF, Fang C, Miller LG. Skin and soft tissue infections and associated complications among commercially insured patients aged 0-64 years with and without diabetes in the U.S. PLoS One 2013; 8:e60057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293:217–28. [DOI] [PubMed] [Google Scholar]
- 14. Lipsky BA, Dryden M, Gottrup F, et al. Antimicrobial stewardship in wound care: a position paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J Antimicrob Chemother 2016; 71:3026–35. [DOI] [PubMed] [Google Scholar]
- 15. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016; 32:45–74. [DOI] [PubMed] [Google Scholar]
- 16. Lavery LA, Armstrong DG, Murdoch DP, et al. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis 2007; 44:562–5. [DOI] [PubMed] [Google Scholar]
- 17. Kish TD, Chang MH, Fung HB. Treatment of skin and soft tissue infections in the elderly: A review. Am J Geriatr Pharmacother 2010; 8:485–513. [DOI] [PubMed] [Google Scholar]
- 18. Dhatariya K. Admission avoidance using intramuscular antibiotics for the treatment of borderline foot infections in people with diabetes in a tertiary care foot clinic. BMJ Qual Improv Rep 2013; 2:pii: u201211.w729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson SW, Drew RH, May DB. How long to treat with antibiotics following amputation in patients with diabetic foot infections? Are the 2012 IDSA DFI guidelines reasonable? J Clin Pharm Ther 2013; 38:85–8. [DOI] [PubMed] [Google Scholar]
- 20. Wukich D, Hobizal KB, Brooks MM. Severity of diabetic foot infection and rate of limb salvage. Foot Ankle Int 2013; 34:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wukich D, Hobizal K, Raspovic K, Rosario BL. SIRS is valid in distinguishing severe from moderate diabetic foot infections. Diabetes Care 2013; 36:3706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chuan F, Tang K, Jiang P, et al. Reliability and validity of the perfusion, extent, depth, infection and sensation (PEDIS) classification system and score in patients with diabetic foot ulcer. PLoS One 2015; 10:e0124739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woo KY, Alam T, Marin J. Topical antimicrobial toolkit for wound infection. Surg Technol Int 2014; 25:45–52. [PubMed] [Google Scholar]
- 24. Abbass M, Uçkay I, Lipsky BA. Diabetic foot infection, antibiotics are to cure infection, not to heal wounds. Expert Opin Clinical Pharmacother 2015; 16:821–8. [DOI] [PubMed] [Google Scholar]
- 25. Nurjadi D, Friedrich-Jänicke B, Schäfer J, et al. Skin and soft tissue infections in intercontinental travellers and the import of multi-resistant Staphylococcus aureus to Europe. Clin Microbiol Infect 2015; 2:567.e1–10. [DOI] [PubMed] [Google Scholar]
- 26. Eckmann C, Lawson W, Nathwani D, et al. Antibiotic treatment patterns across Europe in patients with complicated skin and soft-tissue infections due to methicillin-resistant Staphylococcus aureus: a plea for implementation of early switch and early discharge criteria. Int J Antimicrob Agents 2014; 44:56–64. [DOI] [PubMed] [Google Scholar]
- 27. Tascini C, Lipsky BA, Iacopi E, et al. KPC-producing Klebsiella pneumoniae rectal colonization is a risk factor for mortality in patients with diabetic foot infections. Clin Microbiol Infect 2015; 21:790.e1–3. [DOI] [PubMed] [Google Scholar]
- 28. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008; 51:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edmonds M. Double trouble: infection and ischemia in the diabetic foot. Int J Low Extrem Wounds 2009; 8:62–3. [DOI] [PubMed] [Google Scholar]
- 30. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis 2014; 59:147–59. [DOI] [PubMed] [Google Scholar]
- 31. Eron LJ, Lipsky BA, Low DE, et al. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother 2003; 52:i3–17. [DOI] [PubMed] [Google Scholar]
- 32. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. CREST (Clinical Resource Efficiency Support Team). CREST Guidelines on the Management of Cellulitis in Adults. DHSS Northern Ireland; Available at: http://www.acutemed.co.uk/docs/Cellulitis%20guidelines,%20CREST,%2005.pdf Accessed 3 October 2016. [Google Scholar]
- 34. Marwick C, Broomhall J, McCowan C, et al. Severity assessment of skin and soft tissue infections: cohort study of management and outcomes for hospitalized patients. J Antimicrob Chemother 2011; 66:387–97. [DOI] [PubMed] [Google Scholar]
- 35. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009; 49:1541–9. [DOI] [PubMed] [Google Scholar]
- 37. Lipsky BA, Holroyd KJ, Zasloff M. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 2008; 47:1537–45. [DOI] [PubMed] [Google Scholar]