Abstract
Cutaneous diphtheria is uncommon in Europe. In this study, we report a case of imported cutaneous infection due to a non-toxigenic but tox gene-bearing (NTTB) strain of Corynebacterium diphtheriae. The NTTB strains are recognized as emerging pathogens across Europe, and physicians and bacteriologists should be aware of the circulation of these strains.
Keywords: Corynebacterium diphtheria, cutaneous diphtheria, infectious disease, MALDI-TOF MS, tox gene
Infections caused by toxigenic strains of Corynebacterium diphtheriae have become uncommon in Europe as a result of widespread vaccination. Cutaneous diphtheria is endemic in tropical countries but uncommon in Europe. However, sporadic cases still occur, predominantly imported from areas of high endemicity. We report a case of cutaneous infection due to C diphtheriae biotype mitis in a young man returning from Senegal to France.
CASE REPORT
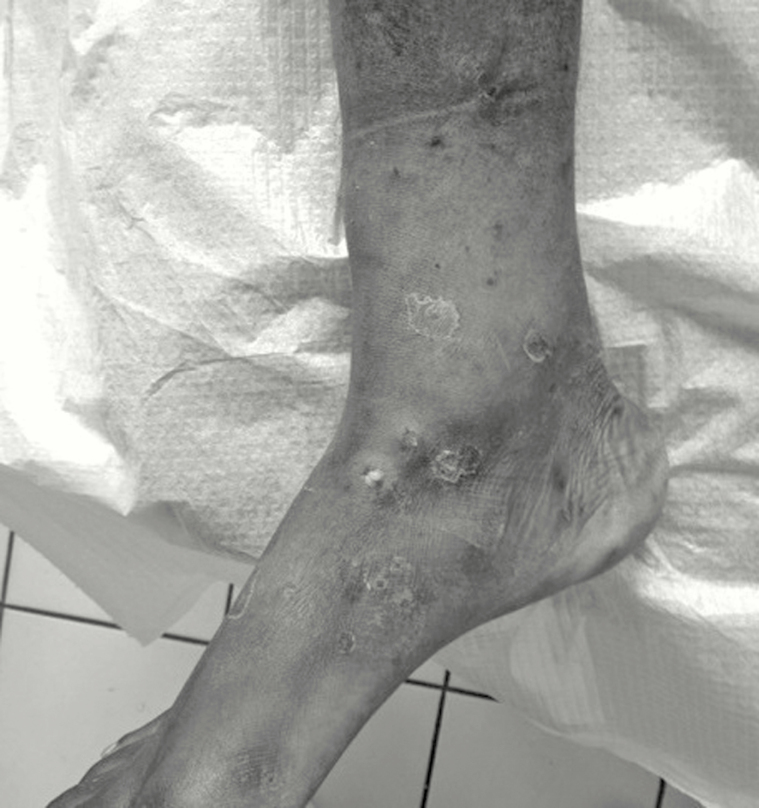
On September 22, 2015, a 21-year-old man with an unremarkable medical history, returning from Senegal with multiple infected mosquito bites on legs and arms, was admitted to our emergency department. Cutaneous lesions had developed several days after walking barefoot in wetlands in Senegal. Skin examination showed maculopapular ulcerative and crusted lesions on legs (Figure 1) and arms. He received amoxicillin/clavulanate (3 grams per day for 14 days) and was discharged.
Figure 1.
Skin ulceration visible on legs of a patient with cutaneous diphtheria.
Leg lesion swabs were cultured on blood agar and chocolate agar (bioMérieux, Marcy-l’Etoile, France). After 24 hours of incubation, abundant growth of small non-hemolytic and β-hemolytic colonies was observed on both media. Rapid identification using matrix-assisted laser desorption-ionization time-of-flight mass spectrometry ([MALDI-TOF MS] Microflex LT; Bruker Daltonics) was performed directly on both colony types. Staphylococcus aureus and Streptococcus pyogenes were identified in the β-hemolytic colonies. In the non-hemolytic colonies, C diphtheriae was identified with a score value of 2.243. This isolate was sent to the National Reference Center for Corynebacteria of the diphtheriae Complex ([NRC-Cd] Institut Pasteur, Paris, France). Three days later, the patient was hospitalized in the infectious diseases department without alteration of his general condition, fever, pharyngitis, pseudomembranous angina, cervical adenitis, or clinical evidence of toxemia but with persistent superficial skin ulcerations on both legs. C-reactive protein was 9 mg/L, and leukocyte count was 6200/mm3; human immunodeficiency virus status was seronegative, and there was no laboratory evidence of immunosuppression.
On September 25, NRC-Cd confirmed the identification of C diphtheriae biovar mitis and polymerase chain reaction analysis revealed the presence of the tox gene. The same day, the patient received diphtheria antitoxin because of his unclear vaccination status. Late examination of his vaccination record showed an up-to-date vaccination against diphtheria, as well as his close contacts. A delayed titration of antibodies against diphtheria toxin ([DT] before antitoxin administration) revealed a protective level of 3.28 IU/mL, consistent with full serologic protection. The clinical course was favorable with rapid and complete skin ulcer resolution 7 days after beginning antibiotics.
Cultures of an oropharyngeal swab obtained at readmission were negative. Tracing of close contacts of the patient was initiated on admission and 1 physician was identified. He received oral antibiotic prophylaxis. Cultures of his throat swabs were negative.
One week later, Elek’s test performed by the NRC-Cd did not reveal toxin secretion. The strain was resistant to fosfomycin, penicillin, and trimethoprim (minimum inhibitory concentrations, >1024, 0.125, and >32 mg/L, respectively). Results of molecular typing by multilocus sequence typing (MLST) showed that the strain belonged to ST387. This ST is novel, and no other isolate with this ST has been recorded so far in the MLST database (http://pubmlst.org/cdiphtheriae/).
DISCUSSION
Cutaneous diphtheria is uncommon in France but not rare in tropical regions [1]. The disease is characterized by shallow skin ulcers that are usually chronic, often after insect bites or minor traumas [2]. The incubation period, on average 2 to 4 days for respiratory tract diphtheria, is not well defined for cutaneous infection [3]. Consistent with previous reports, C diphtheriae was found in our patient in association with S aureus and S pyogenes [4]. Reacher et al [5] found 28% of C diphtheriae isolates to be associated with β-hemolytic streptococci. Cutaneous diphtheria in Europe is mainly travel-related [3, 5].
Cutaneous diphtheria is usually due to toxigenic C diphtheriae producing the tox gene-encoded DT, an ADP-ribosyltransferase of 58.35 kDa [1]. The tox gene is carried by a corynebacteriophage and regulated by the chromosome-encoded regulator DtxR (DT repressor). Integration of tox-carrying bacteriophages into the bacterial genome (lyzogenization) can convert non-toxigenic strains into toxigenic and virulent strains [6].
Non-toxigenic C diphtheriae is increasingly recognized as an emerging pathogen across Europe [7, 8]. In France, between 2000 and 2010, 44 non-toxigenic isolates were sent to the NRC-Cd. Of the C diphtheriae isolates sent to the NRC-Cd, 90% do not carry the tox gene [8]. Current French guidelines recommend to considering non-toxigenic C diphtheriae as a potential pathogen and to treat infected patients [8]. Infections caused by non-toxigenic C diphtheriae are not preventable by vaccination, and pathogenic mechanisms remain unclear [2]. Lowe et al [4] published a 10-year review of 33 cases of cutaneous diphtheria in Canada where all C diphtheriae isolates, from wounds, were non-toxigenic. Non-toxigenic C diphtheriae usually completely lack the tox gene. However, as with the C diphtheriae isolate from our studied patient, some rare non-toxigenic strains do bear the tox gene but do not express it and are called “non-toxigenic tox gene-bearing” (NTTB) [6]. Groman et al [9] found that 14 of 43 non-toxigenic US isolates carried at least part of the toxin gene (as verified with gene probes). In the United Kingdom, Zakikhany et al [6] identified 5 (4.6%) NTTB isolates, from 4 humans and 1 cat, among 108 non-toxigenic C diphtheriae biovar mitis strains. Molecular analysis of NTTB isolates suggested 2 mechanisms causing the blockage of tox gene expression, a single base deletion resulting in a frameshift or the insertion of an insertion sequence (IS) element in the gene [6]. Moreover, non-toxigenic strains tend to replace toxigenic strains with widespread vaccination [10]. Simultaneous presence of toxigenic and non-toxigenic bacteria within a same population has even been described by Simmons et al [11]. Because toxigenic and non-toxigenic colonies are indistinguishable in terms of morphology on agar plates, microbiologists should test several colonies (from the primary plate) for toxin production before reporting that a strain is non-toxigenic. In the present case, the possibility of a double population cannot be excluded. The NTTB C diphtheriae constitute a tox gene reservoir conferring a theoretical risk of re-emerging toxin expression through spontaneous reversion into toxigenic strains or through homologous recombination between different corynebacteriophages [6]. Considering the high pathogenicity and transmissibility of C diphtheriae, prompt identification of the pathogen, including NTTB isolates, is of utmost importance. The MALDI-TOF MS was shown to be an accurate and rapid procedure for identification of corynebacteria [12]. Identification of C diphtheriae using MALDI-TOF MS should alert the microbiologist and trigger further search for the tox gene. Here, detection of C diphtheriae allowed rapid initiation of effective antibiotic therapy and protective measures for healthcare workers and other close contacts.
CONCLUSIONS
Non-toxigenic strains of C diphtheriae are now recognized as emerging pathogens across Europe [7]. In this study, we report a case of cutaneous infection due to an NTTB C diphtheriae strain acquired in Senegal. Because of public health implications associated with toxigenic C diphtheriae, rapid and reliable identification of Corynebacterium species and the research of tox gene are mandatory. The MALDI-TOF MS can be applied successfully to identify C diphtheriae. This case highlights the importance of support from reference laboratories, which can provide rapidly microbiological results that contribute to prevent the further spread of the pathogen.
Acknowledgments
We thank E. Collatz for English-language editing assistance.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Efstratiou A, Engler KH, Dawes CS, Sesardic D. Comparison of phenotypic and genotypic methods for detection of diphtheria toxin among isolates of pathogenic corynebacteria. J Clin Microbiol 1998; 36:3173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Benoist AC, White JM, Efstratiou A, et al. Imported cutaneous diphtheria, United Kingdom. Emerg Infect Dis 2004; 10:511–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jakovljev A, Steinbakk M, Mengshoel A, et al. Imported toxigenic cutaneous diphtheria in a young male returning from Mozambique to Norway, March 2014. Euro Surveill 2014; 19:pii:20835. [DOI] [PubMed] [Google Scholar]
- 4. Lowe CF, Bernard KA, Romney MG. Cutaneous diphtheria in the urban poor population of Vancouver, British Columbia, Canada: a 10-year review. J Clin Microbiol 2011; 49:2664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reacher M, Ramsay M, White J, et al. Nontoxigenic Corynebacterium diphtheriae: an emerging pathogen in England and Wales? Emerg Infect Dis 2000; 6:640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zakikhany K, Neal S, Efstratiou A. Emergence and molecular characterisation of non-toxigenic tox gene-bearing Corynebacterium diphtheriae biovar mitis in the United Kingdom, 2003–2012. Euro Surveill 2014; 19:pii:20819. [DOI] [PubMed] [Google Scholar]
- 7. Funke G, Altwegg M, Frommelt L, von Graevenitz A. Emergence of related nontoxigenic Corynebacterium diphtheriae biotype mitis strains in Western Europe. Emerg Infect Dis 1999; 5:477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belchior E, Bonmarin I, Caumes E, et al. Rapport du Haut Conseil de Sante Publique: Conduite à Tenir Lors de l’Apparition d’un Cas de Diphtérie. 2011. Available at: http://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspr20110304_conduitediphterie.pdf.
- 9. Groman N, Cianciotto N, Bjorn M, Rabin M. Detection and expression of DNA homologous to the tox gene in nontoxinogenic isolates of Corynebacterium diphtheriae. Infect Immun 1983; 42:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mokrousov I. Corynebacterium diphtheriae: genome diversity, population structure and genotyping perspectives. Infect Genet Evol 2009; 9:1–15. [DOI] [PubMed] [Google Scholar]
- 11. Simmons LE, Abbott JD, Macaulay ME, et al. Diphtheria carriers in Manchester: simultaneous infection with toxigenic and non-toxigenic mitis strains. Lancet 1980; 1:304–5. [DOI] [PubMed] [Google Scholar]
- 12. Farfour E, Leto J, Barritault M, et al. Evaluation of the Andromas matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of aerobically growing Gram-positive bacilli. J Clin Microbiol 2012; 50:2702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]