Abstract
Background
Cryptococcosis is the third most common invasive fungal infection in solid organ transplant (SOT) recipients. There are no nationally representative data describing the incidence, risk factors, and outcomes of cryptococcosis after SOT.
Methods
We assembled a large cohort of adult SOT recipients using Classification of Diseases, Ninth Revision, Clinical Modification billing data from Healthcare Cost and Utilization Project State Inpatient Databases of Florida (2006–2012), New York (2006–2011), and California (2004–2010). Demographics, comorbidities, death, and cryptococcal infections coded during hospitalization were identified.
Results
A total of 42634 adults with SOT were identified during the study period. Cryptococcal disease was identified in 0.37% (n = 158), 44% of which had meningitis (n = 69). Median time to diagnosis of cryptococcosis was 464 days (range, 4–2393). The median time to onset of cryptococcosis was earlier for lung (191 days; range, 7.5–1816), heart (195 days; range, 4–1061), and liver (200 days; range, 4–1581) compared with kidney transplant recipients (616 days; range, 12–2393; P < .001, log rank test). Very early-onset disease (<30 days after transplantation) more frequently occurred in liver and lung transplant recipients. Lung transplant recipients had the highest risk of cryptococcosis (hazard ratio [HR], 2.10; 95% confidence interval [CI], 1.21–3.60). Cryptococcosis was associated with death (HR, 2.29; 95% CI, 1.68–3.11), after adjusting for age, type of SOT, and other comorbidities.
Conclusions
Cryptococcosis is rare after SOT, but it is associated with significantly increased risk of death. Lung transplant recipients are at highest risk for cryptococcosis among SOTs. Nonkidney transplants have earlier onset of cryptococcosis and higher risk of death compared with kidney transplant recipients.
Keywords: cryptococcosis, epidemiology, outcomes, solid organ transplant
Cryptococcosis is an important opportunistic fungal infection that causes significant mortality and morbidity among solid organ transplant (SOT) recipients [1–3]. Traditionally, cryptococcosis is most often associated with human immunodeficiency virus (HIV) infection; however, with the advent of effective antiretroviral therapy, the majority of cases in developed nations occur among non-HIV-infected patients, especially organ transplant recipients [4, 5]. Among 302 cases of cryptococcosis identified over a 14-year period from an academic medical center in the southern United States, 28% occurred in SOT recipients [4]. Results from the Transplant-Associated Infection Surveillance Network (TRANSNET), a consortium of 23 US transplant centers, showed that cryptococcosis is the third most common invasive fungal infection in SOT recipients, after invasive candidiasis and aspergillosis, comprising 8%–10% of all invasive fungal infections identified in SOT recipients [1, 6]. Overall incidence rates of cryptococcosis in cohorts of SOT recipients range from 0.2% to 4.1%, depending on the type of organ transplanted and the duration of follow-up, and has remained stable over the years [1, 3]. Mortality rates among all types of SOT recipients with cryptococcosis range from 14% to 19.6% and may approach 50% in recipients with meningitis [3, 6, 7].
Studies from academic medical centers contribute to our understanding of epidemiology of cryptococcosis and its outcomes in SOT recipients; however, they have limited generalizability. Cryptococcosis identified and managed in community hospitals after patients have transitioned away from transplant centers may have been missed, especially with the prolonged time to onset after transplant. There are no population-level data describing the epidemiology of cryptococcosis and cryptococcal meningitis posttransplantation. We assembled a large and more representative cohort of SOT recipients from multiple hospitals using the longitudinal Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project State Inpatient Databases (SID) to study the epidemiology of cryptococcosis and cryptococcal meningitis. The SID contain demographic and billing data that capture inpatient diagnoses and procedures through International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding. This approach allowed us to follow a large number of patients over a long period of time and identify cryptococcosis and cryptococcal meningitis regardless of whether patients were treated in the index transplant hospital or a different hospital.
METHODS
Study Design and Patient Population
We performed a retrospective cohort study of adults aged 18 years and older who underwent kidney, lung, liver, heart, pancreas, or intestine SOT, identified by ICD-9-CM procedure codes (55.69, 50.59, 37.51, 33.50, 33.51, 33.52, 33.6, 52.80, 52.86, 46.97) from 2004 to 2010 in California, 2006 to 2012 in Florida, and 2006 to 2011 in New York. These states and years were chosen based on the availability of patient-level encrypted identifiers to link admissions within and across hospitals over time and the population diversity of these states. We chose the cohort inception years to allow 1 year of prior data to identify comorbidities. Only the first transplant during the study period was included for all patients. We excluded patients who (1) lived in states other than the one where transplantation was performed, (2) were coded for cryptococcosis and cryptococcal meningitis within 1 year before transplantation, or (3) died on the day of transplant. This study was considered exempt by the Washington University School of Medicine Human Research Protection Office.
Demographic Data, Comorbidities, and Follow-up
Demographic characteristics of the study population were determined during the transplant admission. Comorbidities were identified using the Elixhauser classification and ICD-9-CM diagnosis codes within 1 year before and during the transplant hospitalization [8]. Comorbidities that were the primary reason leading to transplant were excluded (eg, renal failure in kidney transplant, liver disease in liver transplants). Transplant failure or rejection episodes were identified during readmissions but considered a potential risk factor for cryptococcosis only if it was identified before its onset. Inpatient readmissions were identified using the encrypted patient-level identifier. Dates of onset of cryptococcosis, cryptococcal meningitis, and transplant failure or rejections were estimated to be midpoint of the stay if they were coded during the initial transplant hospitalization. For events identified during readmissions, the date of admission was considered the date of onset. Prior transplantation was identified by a procedure code for SOT in the year before transplantation (only in the year reserved for comorbidities) or by a V-code to indicate prior transplant (V42.0, V42.7, V42.1, V42.6, V42.83, and V42.84). Transplant failure or rejection was not considered if it was coded during the transplant hospitalization in an individual with a history of SOT. Outcomes identified during the SOT hospitalization and during hospital readmissions were newly coded cryptococcosis (ICD-9-CM diagnosis code 117.5) and cryptococcal meningitis (ICD-9-CM diagnosis code 321.0). The ICD-9-CM diagnosis codes for septicemia, pneumonia, surgical site, and skin and soft tissue infection assigned during the admission coded for cryptococcosis were used to characterize the site of cryptococcal infection (codes listed in the Supplementary Material).
Statistical Analysis
Descriptive statistics were used to describe the demographic and clinical characteristics of the study population. Potential risk factors for cryptococcosis and inpatient death were analyzed using univariable and multivariable Cox proportional hazards models. To create a censor date, the transplant date was estimated as (1) the 15th day of the admission month for California and New York and (2) the midpoint of the discharge quarter for Florida (Florida SID does not provide an admission month). In the risk factor analysis for cryptococcosis, patients were censored at inpatient death or maximum follow-up. The proportional hazards assumption was evaluated for each variable using visual inspection of log-log survival curves and the correlation between Schoenfeld residuals and ranking of individual failure times [9]. Variables were specified by selecting clinically meaningful factors that could potentially be associated with cryptococcosis, and we sequentially eliminated variables with highest P values to arrive at a parsimonious model, followed by assessment of confounding to arrive at the final model. To evaluate whether the risk of death with and without cryptococcosis varied with time, we used a heavy-side function to assess differential risk within 1 year and after 1 year of transplant [10]. Statistical significance was set at P < .05. All analyses were performed using SAS Enterprise Guide 5.1 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
The final study population consisted of 42634 adult SOT recipients. A total of 63.7% had kidney transplantation, followed by 21.1% liver, 7.2% heart, and 5.2% lung transplants. Other or multiple organs were transplanted in 2.4% of the SOT patients (pancreas, intestine, or multiorgan). The mean age at the time of transplant was 51.7 years; 62.6% were males, 51.2% were white, 21.1% were Hispanic, 76.3% lived in large metropolitan areas, and 15.8% had Medicaid or self-pay listed as primary insurance. Commonly identified comorbidities were as follows: diabetes mellitus (37.5%), renal failure (12.0 %), and chronic lung disease (10.1%). A total of 1492 patients (3.5%) had history of a prior organ transplant(s). The median duration of follow-up after SOT was 1295 days (range, 1–3075 days).
Cryptococcal Infections
One hundred fifty-eight patients were coded for cryptococcosis (0.37%) after SOT. There was no significant difference in the incidence of cryptococcosis identified between the 3 states (0.35% in California, 0.37% in Florida, and 0.41% in New York; P = .661). Sixteen of 158 patients were coded for cryptococcosis during the index transplant hospitalization. A total of 69 (44%) patients were identified with meningitis. Of the remaining patients with nonmeningeal cryptococcosis, 41 (46.06%) were coded for pneumonia and 19 (21.34%) additional patients were coded for septicemia, skin and soft tissue, or surgical site infection. Eighty-nine (0.32%) patients with cryptococcosis were identified after kidney transplant, 40 (0.44%) after liver transplant, 15 (0.66%) after lung transplant, and 13 (0.42%) after heart transplantation. Twenty-six percent (38 of 158) of patients with cryptococcosis identified at readmission were treated at a hospital different from the index transplant surgery hospital. Eighty-three percent (57 of 69) of patients with cryptococcal meningitis had a procedure code for a lumbar puncture during the admission compared with 41% (36 of 89) of patients coded for cryptococcosis without meningitis (P < .001).
Lung transplant recipients had the highest risk of cryptococcosis compared with other organ transplant recipients, with a hazard ratio (HR) of 2.10 after adjusting for age and underlying comorbidities (Table 1). Increasing age, diabetes, and having Medicaid or no health insurance were also risk factors for cryptococcosis in the multivariable Cox model (Table 2).
Table 1.
Univariable Risk Factors for Cryptococcosis in Patients with and without Cryptococcosis among 42634 Solid Organ Transplant Recipients
| Risk Factor | No. (%) of Patients | HR (95% CI) | P Value | |
|---|---|---|---|---|
| With Cryptococcosis (n = 158) | Without Cryptococcosis (n = 42476) | |||
| Age | ||||
| 18–40 years | 16 (10.1) | 8456 (19.9) | 1 | |
| 41–50 years | 35 (22.2) | 8925 (21.0) | 2.07 (1.14–3.74) | .016 |
| 51–60 years | 50 (31.6) | 13119 (30.9) | 2.04 (1.16–3.58) | .013 |
| 60–70 years | 48 (30.4) | 9969 (23.5) | 2.67 (1.52–4.71) | .007 |
| >70 years | <11 | 2007 (4.7) | 2.50 (1.10–5.65) | .028 |
| Race | ||||
| White | 74 (46.8) | 20926 (49.3) | 1 | |
| Black | 17 (10.7) | 6363 (14.9) | 0.78 (0.46–1.34) | .359 |
| Hispanic | 42 (26.6) | 8981 (21.1) | 1.36 (0.93–1.98) | .114 |
| Asian or Pacific Islander | 13 (8.2) | 3364 (7.9) | 1.11 (0.62– 2.02) | .728 |
| Other or missing | 12 (7.6) | 2842 (6.7) | 1.31 (0.71–2.42) | .382 |
| Female sex | 54 (34.1) | 15882 (37.4) | 0.95 (0.89–1.01) | .596 |
| Type of Insurance | ||||
| Private/Medicare/Others | 133 (84.2) | 38489 (90.6) | 1 | |
| Medicaid/Self-pay | 25 (15.8) | 3987 (9.4) | 1.80 (1.17–2.75) | .007 |
| Income Quartile | ||||
| 0–25th | 73 (46.2) | 13081 (30.8) | 2.10 (1.30–3.33) | .002 |
| 26–50th | 35 (22.1) | 9367 (22.0) | 1.54 (0.91–2.58) | .105 |
| 51–75th | 26 (16.5) | 10115 (23.8) | 1.05 (0.60–1.83) | .857 |
| 76–100th | 24 (15.2) | 9913 (23.3) | 1 | |
| Type of Transplant | ||||
| Kidney | 89 (56.3) | 27129 (63.9) | 1 | |
| Liver | 40 (25.3) | 8970 (21.1) | 1.32 (0.91–1.92) | .142 |
| Lung | 15 (9.5) | 2223 (5.2) | 2.06 (1.19–3.57) | .009 |
| Heart | 13 (8.2) | 3059 (7.2) | 1.29 (0.72–2.30) | .397 |
| Others (multiorgan) | <11 | 1095 (2.6) | 0.27 (0.04–1.93) | .191 |
| Prior transplant failure/rejection | 85 (53.8) | 22475 (52.9) | 0.50 (0.16–1.59) | .244 |
| Other Comorbidities | ||||
| Diabetes mellitus | 86 (54.4) | 15898 (37.4) | 2.03 (1.48–2.78) | .001 |
| Renal failure | 16 (10.1) | 5110 (12.0) | 0.84 (0.52–1.41) | .512 |
| Chronic pulmonary disease | 19 (12.0) | 4308 (10.1) | 1.20 (0.74–1.93) | .421 |
| Congestive heart failure | 16 (10.1) | 4127 (9.7) | 1.02 (0.63–1.71) | .930 |
| Liver disease | 10 (6.3) | 2243 (5.3) | 1.22 (0.65–2.33) | .532 |
| Connective tissue disorder | <11 | 1923 (4.5) | 0.56 (0.20–1.51) | .252 |
| Prior transplantation | <11 | 1489 (3.5) | 0.51 (0.16–1.59) | .243 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 2.
Multivariable Cox Proportional Hazard Model of Risk Factors for Cryptococcosis
| Risk Factor | HR (95% CI) | P Value |
|---|---|---|
| Age | ||
| 18–40 years | 1.00 | |
| 41–50 years | 1.92 (1.06–3.48) | .030 |
| 51–60 years | 1.78 (1.01–3.13) | .046 |
| 60–70 years | 2.28 (1.22–3.86) | .007 |
| >70 years | 2.41 (1.03–4.95) | .041 |
| Type of Transplant | ||
| Kidney | 1.00 | |
| Liver | 1.16 (0.78–1.71) | .466 |
| Lung | 2.10 (1.21–3.60) | .009 |
| Heart | 1.15 (0.63–2.07) | .645 |
| Others (multiorgan) | 0.23 (0.03–1.64) | .142 |
| Diabetes mellitus | 1.95 (1.41–2.69) | <.001 |
| Medicaid/Self-pay | 1.99 (1.27–3.11) | .002 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
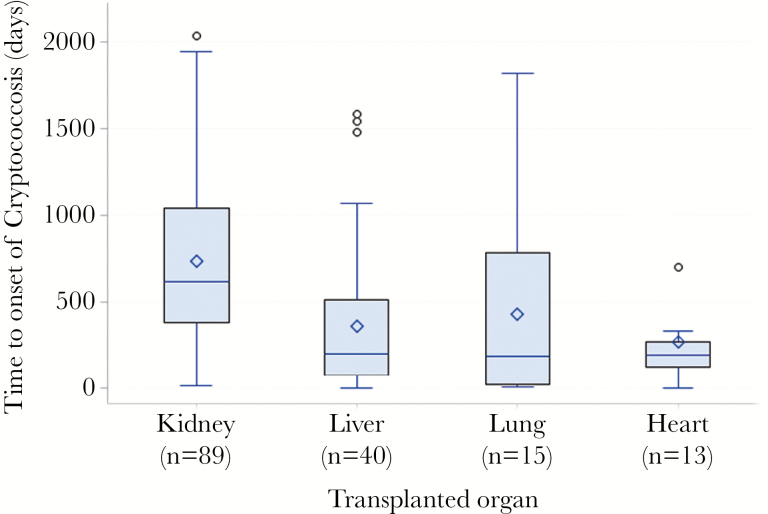
The median time to diagnosis of cryptococcosis from the date of transplant was 464 days (range, 4–2393). The median time to onset of disease was earlier after lung (191 days; range, 7.5–1816), heart (195 days; range, 4–1061), and liver (200 days; range, 4–1581) transplant than kidney transplant (616 days; range, 12–2393) (P < .001, Kruskal-Wallis test) (Figure 1).
Figure 1.
Time to onset of cryptococcosis in days, stratified by type of transplant. Boxes represent 25th–75th percentile values (interquartile range [IQR]), and horizontal lines represent the median and the diamonds the mean values. Error bars indicate the maximum and minimum observation below the upper and lower fences (1.5 IQR) and circles the maximum observations.
Very Early Versus Later Onset Cryptococcosis
Very early-onset disease (cryptococcosis occurring within 30 days after transplantation) developed in 16 (10.1%) of 158 patients (median 10.7 days; range, 4–23) after transplant, whereas later onset cryptococcosis more than 30 days after transplant occurred in the remaining 142 patients (median 515 days; range, 32–2393). The proportion of patients with very early-onset disease among liver or lung transplants was significantly higher than other transplants (P < .001, Fisher’s exact test). However, inpatient mortality did not differ between the patients with very early-onset versus later-onset cryptococcosis (P = 1.000, Fisher’s exact test).
Inpatient Mortality in Patients with Cryptococcosis
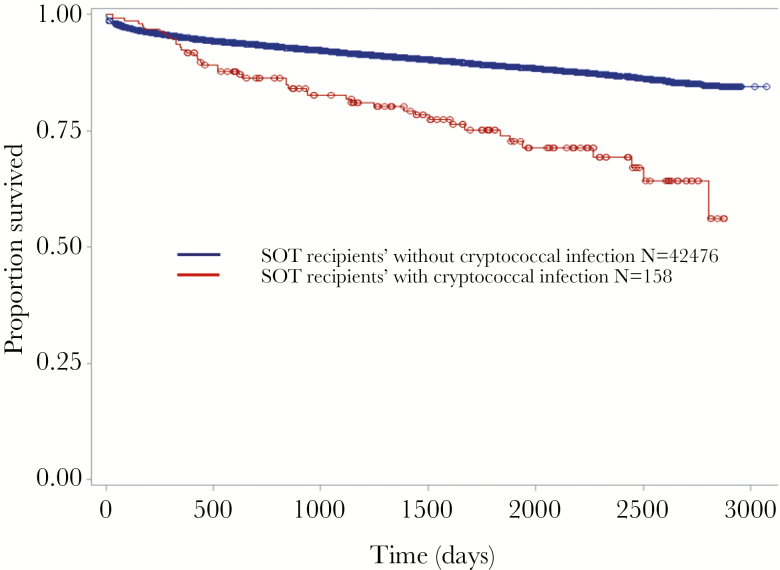
Forty-one of 158 (26.0%) patients with cryptococcosis died during the study period in-hospital versus 3879 of 42476 (9.1%) without cryptococcosis (HR, 2.57; P < .001) (Table 3 and Figure 2). Eighteen of 69 (26.1%) patients with cryptococcal meningitis died (HR 2.54, P < .001, compared with uninfected SOT recipients). In multivariable analysis, cryptococcosis was associated with a 2.3-fold increased risk of inpatient mortality (P < .001), adjusting for age, type of transplant, prior graft rejection or failure, and several comorbidities (Table 3). There was no increased mortality risk with cryptococcosis during the first year after transplant (HR, 1.39; P = .257); however, there was a 3.2-fold increased risk of mortality more than 1 year after transplant (P < .001) (Figure 2). The risk of death was highest for lung, followed by liver, heart, and other transplants compared with renal transplant. Increasing age, prior transplant failure or rejection, congestive heart failure, liver disease, having Medicaid or no health insurance, and several other comorbidities were independent risk factors for in-hospital death (Table 3). The median time to death from diagnosis of cryptococcosis was 87.5 days (range, 3–2792). The median time to death from transplant was 637 days (range, 32–2807) in patients with cryptococcosis and 359 days (range, 1–2858) in patients without cryptococcosis (P < .001, log rank test).
Table 3.
Cox Proportional Hazard Model of Risk Factors for Death in 42634 Solid Organ Transplant Recipients
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Risk Factor | HR (95% CI) | P | HR (95% CI) | P Value |
| Cryptococcosis | 2.57 (1.89–3.50) | <.001 | 2.29 (1.68–3.11) | <.001 |
| Cryptococcal Meningitis | 2.54 (1.60–4.04) | <.001 | ||
| Age | ||||
| 18–40 years | 1.00 | |||
| 41–50 years | 1.46 (1.30–1.65) | <.001 | 1.36 (1.19–1.53) | <.001 |
| 51–60 years | 2.01(1.81–2.24) | <.001 | 1.60 (1.43–1.80) | <.001 |
| 60–70 years | 2.77 (2.48–3.09) | <.001 | 2.29 (2.04–2.57) | <.001 |
| >70 years | 3.32 (2.87–3.85) | <.001 | 3.70 (3.14–4.30) | |
| Female sex | 0.95 (0.89–1.01) | .596 | ||
| Type of transplant | ||||
| Kidney | 1.00 | 1.00 | ||
| Liver | 2.67 (2.47–2.87) | <.001 | 2.47 (2.26–2.71) | <.001 |
| Lung | 4.86 (4.41–5.37) | <.001 | 3.68 (3.28–4.12) | <.001 |
| Heart | 2.47 (2.21–2.76) | <.001 | 2.32 (2.11–2.65) | <.001 |
| Others (multiorgan) | 3.35 (2.88–3.89) | <.001 | 2.21 (1.86–2.65) | <.001 |
| Type of Insurance > 70 years | ||||
| Medicaid/Self-pay | 1.46 (1.28–1.56) | <.001 | 1.24 (1.13–1.37) | <.001 |
| Prior transplant failure/rejection | 2.66 (2.46–2.86) | <.001 | 2.53 (2.38–2.77) | <.001 |
| Other comorbidities | ||||
| Diabetes mellitus | 1.27 (1.20–1.36) | <.001 | 1.14 (1.07–1.21) | .001 |
| Renal failure | 1.66 (1.53–1.80) | <.001 | ||
| Chronic pulmonary disease | 1.31 (1.20–1.44) | <.001 | ||
| Obesity | 1.04 (0.94–1.15) | .484 | ||
| Congestive heart failure | 1.68 (1.54–1.83) | <.001 | 1.58 (1.47–1.73) | <.001 |
| Liver disease | 1.41 (1.24–1.60) | <.001 | 1.22 (1.05–1.42) | .007 |
| Connective tissue disorder | 0.83 (0.70–0.98) | .020 | ||
| Solid tumors | 1.51 (1.12–2.04) | .006 | ||
| Hypertension | 0.52 (0.49–0.56) | <.001 | 0.87 (0.80–0.94) | <.001 |
| Hematological malignancies | 1.65 (1.11–2.48) | .013 | ||
| Cancer with metastasis | 1.95 (1.27–2.99) | .002 | ||
| Valvular heart disease | 1.52 (1.39–1.66) | <.001 | ||
| Depression | 1.38 (1.26–1.52) | <.001 | ||
| Pulmonary circulation disorder | 2.12 (1.95–2.31) | <.001 | ||
| Weight loss | 2.07 (1.90–2.27) | <.001 | 1.22 (1.11–1.34) | <.001 |
| Peripheral vascular disease | 1.34 (1.38–1.69) | <.001 | 1.46 (1.32–1.62) | <.001 |
| Electrolyte disorders | 1.51 (1.42–1.62) | <.001 | 1.18 (1.11–1.26) | <.001 |
| Psychoses | 1.48 (1.26–1.75) | <.001 | 1.21 (1.02–1.42) | .022 |
| Drug abuse | 1.57 (1.33–1.81) | <.001 | 1.20 (1.05–1.39) | .006 |
| Neurological disorders | 1.20 (1.06–1.36) | .002 | ||
| Prior transplantation | 1.23 (1.05–1.43) | .008 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 2.
Time to death in days, in a cohort of adult solid organ transplant (SOT) recipients, stratified by the presence or absence of cryptococcosis.
DISCUSSION
We found that cryptococcal infections were rare but associated with significant mortality in a large diverse population of SOT recipients from 3 US states. The overall incidence of cryptococcosis was 0.37%, similar to the 12-month incidence of 0.2% reported in the TRANSNET study [1]. Prior studies have found that 44%–60% of SOT recipients with cryptococcosis developed central nervous system (CNS) involvement [2, 11, 12]. We found that meningitis was coded in 44% of the SOT recipients with cryptococcosis. The Infectious Diseases Society of America guidelines recommend evaluating for meningitis by lumbar puncture in SOT recipients with positive cryptococcal antigen test and in all immunosuppressed patients with pulmonary cryptococcosis [13]. The proportion of patients coded for a lumbar puncture was significantly lower among the patients with cryptococcosis without meningitis compared with those patients coded for cryptococcal meningitis. The slightly higher proportion of patients without cryptococcal meningitis when compared with previous studies could be due to (1) an error in additional coding for meningitis or (2) an error in ascertainment, wherein an active search for CNS disease was not done in these patients [2, 11]. Among patients with nonmeningeal cryptococcosis, the most common site of involvement was pulmonary followed by septicemia and skin/soft tissue involvement, similar to the clinical presentations reported in prior cohorts of SOT recipients [2, 3].
The majority of cases of cryptococcosis are late infections and considered to represent reactivation of latent infection in the recipient [14]. The median time to cryptococcal disease from transplantation in our study (464 days) was consistent with the findings of the TRANSNET study (575 days) [1]. Very early- onset of cryptococcosis (within 1 month after transplant) is rare, and it was reported in 5% of 175 SOT recipients with cryptococcosis by Sun et al [15]. We found that 10% of SOT recipients were coded for very early-onset disease. Similar to the finding in the study by Sun et al [15], the majority of very early onset cryptococcal infection in our study occurred in liver and lung transplant recipients [15]. These very early-onset infections may reflect either undetected pretransplant or donor-derived infections. Albeit rare, this should prompt clinicians to consider cryptococcosis in the evaluation of very early-onset infections.
In this study, lung transplant recipients had the highest risk for cryptococcosis, followed by liver and heart transplant recipients. Kidney transplant recipients had the lowest risk. This has not been reported previously in the literature. The median time to onset of cryptococcosis after transplant was earlier for lung, heart, and liver (<1 year) compared with kidney transplant recipients (1.7 years). This was similar to the findings by Husain et al [16], where the median time to onset of cryptococcosis was 35 months for kidney and 25 months for heart, compared with 8.8 and 3 months for liver and lung transplant recipients. The higher risk and earlier onset of disease may reflect the greater intensity of immunosuppression with a higher risk of reactivation disease in the nonkidney transplant populations.
Twenty-six percent of patients with cryptococcosis died in-hospital versus 9.1% without disease over a median follow-up time of 3.5 years in this large cohort of SOT recipients. The 90-day mortality rate (13.3%) in our study was very similar to that reported in a multicenter study of 111 SOT recipients with cryptococcosis by Singh et al in the modern era of effective antifungal therapy (14%) [11]. In our multivariable model, cryptococcosis was significantly associated with over a 2-fold increase risk of death. Lung, liver, and heart transplant recipients were at higher risk for death compared with renal transplant recipients after adjusting for age, cryptococcosis, and other comorbidities. Insurance coverage affected risk of death with patients with Medicaid or no health insurance at higher mortality risk.
The strengths of this study are the large cohort size, the long duration of follow up that allowed capture of late occurring infections, and identification of cryptococcosis treated in a hospital other than the transplant center. Twenty-six percent of infections were diagnosed in a hospital other than the patient’s primary transplant center. Use of large administrative databases, such as the SID, adds to our understanding of outcomes of rare diseases among hospitalized patients and supplements detailed single- and multicenter studies. However, there are several limitations. The ICD-9-CM diagnosis codes used to identify cryptococcosis in this study have not been validated, although the code for cryptococcal meningitis has been used to describe the epidemiology of disease [17]. A validation study of ICD-9 diagnosis codes for several severe infections report positive predictive values >80% [18]. Because the study was limited to 3 states (California, Florida, and New York), there may have been regional biases introduced. Another limitation is that the data source used in this study contains only demographic and inpatient hospital ICD-9-CM billing data and does not have microbiology or other laboratory test results or data on antifungal or immune suppressive medications.
CONCLUSIONS
In summary, this is the first study that provides population-level information on the epidemiology of cryptococcosis after SOT in the current era. Cryptococcosis, although rare, was associated with significant mortality in this population. Lung, liver, and heart transplant recipients were at higher risk for cryptococcosis compared with kidney transplant recipients. Future research should focus on screening algorithms and consider prophylactic strategies, especially in liver and lung transplant recipients at risk of very early-onset disease.
Supplementary Material
Acknowledgments
Author contributions. The authors made the following contributions to the article: I. A. G. participated in research design, performed research and data analysis, and wrote the paper; C. A. Q. S. participated in research design, performed research and data analysis, and wrote the paper; W. G. P. participated in research design and wrote the paper; M. A. O. participated in research design, performed research and data analysis, and wrote the paper.
Financial support. This study used the services of the Center for Administrative Data Research, supported in part by Washington University Institute of Clinical and Translational Sciences Grant UL1 TR000448 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality, and Grant Number KM1CA156708 through the National Cancer Institute at the NIH.
Potential conflicts of interest. All authors: No reported conflicts.All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the transplant-associated infection surveillance network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 2. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One 2013; 8:e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis 2009; 48:1566–76. [DOI] [PubMed] [Google Scholar]
- 4. Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 2013; 124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 5. La Hoz RM, Pappas PG. Cryptococcal infections: changing epidemiology and implications for therapy. Drugs 2013; 73:495–504. [DOI] [PubMed] [Google Scholar]
- 6. Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis 2010; 12:220–9. [DOI] [PubMed] [Google Scholar]
- 7. Wu G, Vilchez RA, Eidelman B, et al. Cryptococcal meningitis: an analysis among 5521 consecutive organ transplant recipients. Transpl Infect Dis 2002; 4:183–8. [DOI] [PubMed] [Google Scholar]
- 8. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 9. Schoenfeld D. Partial residuals for the proportional hazards regression model on JSTOR. Biometrika 1982; 69:239–41. [Google Scholar]
- 10. Kleinbaum DG. Survival Analysis: A Self-Learning Text. New York: Springer; 1996. [Google Scholar]
- 11. Singh N, Alexander BD, Lortholary O, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis 2007; 195:756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis JA, Horn DL, Marr KA, Fishman JA. Central nervous system involvement in cryptococcal infection in individuals after solid organ transplantation or with AIDS. Transpl Infect Dis 2009; 11:432–7. [DOI] [PubMed] [Google Scholar]
- 13. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh N, Dromer F, Perfect JR, Lortholary O. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis 2008; 47:1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun HY, Alexander BD, Lortholary O, et al. Unrecognized pretransplant and donor‐derived cryptococcal disease in organ transplant recipients. Clin Infect Dis 2010; 51:1062–9. [DOI] [PubMed] [Google Scholar]
- 16. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis 2001; 7:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pyrgos V, Seitz AE, Steiner CA, et al. Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One 2013; 8:e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sickbert-Bennett EE, Weber DJ, Poole C, et al. Utility of international classification of diseases, ninth revision, clinical modification codes for communicable disease surveillance. Am J Epidemiol 2010; 172:1299–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.