ABSTRACT
Experiments examining the social dynamics of bacterial quorum sensing (QS) have focused on mutants which do not respond to signals and the role of QS-regulated exoproducts as public goods. The potential for QS signal molecules to themselves be social public goods has received much less attention. Here, we analyze how signal-deficient (lasI) mutants of the opportunistic pathogen Pseudomonas aeruginosa interact with wild-type cells in an environment where QS is required for growth. We show that when growth requires a “private” intracellular metabolic mechanism activated by the presence of QS signal, lasI mutants act as social cheats and outcompete signal-producing wild-type bacteria in mixed cultures, because they can exploit the signals produced by wild-type cells. However, reducing the ability of signal molecules to diffuse through the growth medium results in signal molecules becoming less accessible to mutants, leading to reduced cheating. Our results indicate that QS signal molecules can be considered social public goods in a way that has been previously described for other exoproducts but that spatial structuring of populations reduces exploitation by noncooperative signal cheats.
KEYWORDS: Pseudomonas aeruginosa, evolution, infectious disease, microbial ecology, opportunistic infections, quorum sensing, virulence
IMPORTANCE
Bacteria communicate via signaling molecules to regulate the expression of a whole range of genes. This process, termed quorum sensing (QS), moderates bacterial metabolism under many environmental conditions, from soil and water (where QS-regulated genes influence nutrient cycling) to animal hosts (where QS-regulated genes determine pathogen virulence). Understanding the ecology of QS could therefore yield vital clues to how we might modify bacterial behavior for environmental or clinical gains. Here, we demonstrate that QS signals act as shareable public goods. This means that their evolution, and therefore population-level responses to interference with QS, will be constrained by population structure. Further, we show that environmental structure (constraints on signal diffusion) alters the accessibility of QS signals and demonstrates that we need to consider population and environmental structure to help us further our understanding of QS signaling systems.
INTRODUCTION
Bacterial quorum sensing (QS) is a cell-to-cell signaling mechanism that coordinates a range of behaviors at the population level (1, 2). QS facilitates density-dependent production of extracellular molecules, including nutrient-scavenging enzymes and virulence factors. These molecules have been termed “public goods” because their benefits can be shared by all cells in the local population (3–6). Because these QS-regulated exoproducts are metabolically costly for cells to produce, QS can also be exploited by noncooperating “cheats”: cells that do not respond to QS signals and so pay no costs but which exploit wild-type populations because they benefit from the public goods produced by wild-type neighboring cells (4–7). Experiments into the social dynamics of QS have traditionally focused on these “signal-blind” mutants, and a number of studies have shown that such mutants can arise in laboratory cultures and during infections (8–14). Under various laboratory conditions and in in vivo infection models, they have been shown to act as social cheats (6, 15–17).
However, little attention has been paid to whether QS signals themselves can act as exploitable public goods, despite there being a metabolic cost associated with the production of QS signals (even in the absence of downstream responses [18, 19]). Previous experiments have shown that signal-negative mutants can act as cheats, but these were conducted under conditions where QS-dependent exoproducts enhance growth: these studies therefore do not separate the fitness effects of producing from those of responding to signal (5, 6). Here, we analyze how signal-negative (lasI) mutants of the opportunistic pathogen Pseudomonas aeruginosa socially interact with wild-type cells in an environment where growth requires the cells to have a functional QS system but where the fitness benefits of QS are “private” to individual cells. P. aeruginosa regulates the production of many virulence factors through two N-acylhomoserine lactone (AHL)-based QS systems. These systems, termed the las and rhl systems, produce and respond to the signals N-(3-oxododecanoyl)-l-homoserine lactone (3O-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), respectively (1, 20). We conducted our experiments in a growth medium containing adenosine as a carbon source. Adenosine is deaminated to form inosine, which is degraded inside the cell by a nucleoside hydrolase (Nuh) to hypoxanthine plus ribose; hypoxanthine is then metabolized to produce glyoxylate plus urea (21). QS is crucial for growth in this medium because the las system (through the regulator LasR) positively regulates Nuh. Because Nuh acts intracellularly, any loss of fitness due to mutation of the signal gene lasI will be directly due to the lack of signal—not to any downstream effect on the production of extracellular enzymes. We demonstrate that, when provided with adenosine as a carbon source, lasI mutants act as cheats: they grow poorly in monoculture but have a higher relative fitness than the signal-producing wild type in mixed cultures. In contrast, lasR mutants, which cannot regulate Nuh in the presence of signal, do not gain any fitness benefits in mixed culture with wild-type cells.
In contrast to experiments performed in well-mixed liquid medium in test tubes, interactions between bacterial cells in natural environments (including infections) are affected by spatial assortment and structuring (22–24). This affects how behaviors evolve (25–29). We tested how simple spatial structuring, through the addition of agar to the growth medium, alters QS signal diffusion and the social dynamics of wild-type and lasI mutant cells. Consistent with work on other bacterial public goods (28), we demonstrate that the ability of lasI mutants to cheat is significantly reduced in structured populations. These results have implications for understanding how and why bacterial signaling evolves and the likely evolutionary fate of different types of QS mutants under varied environmental conditions (30).
RESULTS
las mutants grow poorly in an environment where QS is required for growth.
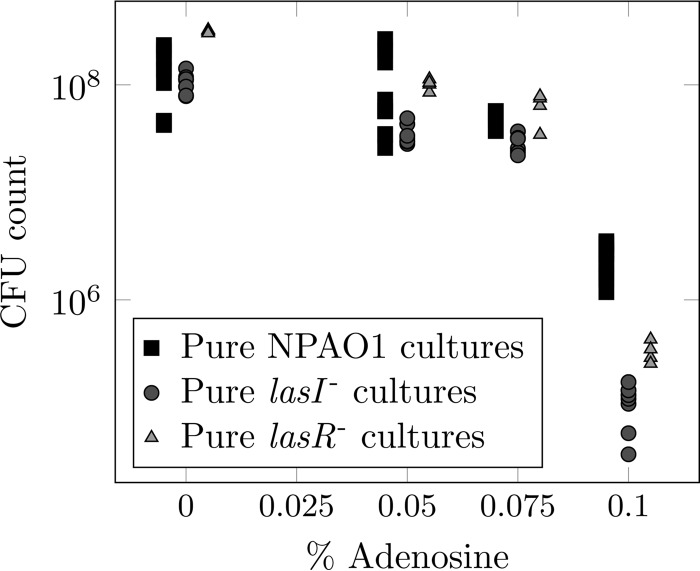
Previous work has shown that las mutants grow poorly in medium where QS is required for growth (5, 7, 31). We first confirmed that both lasI and lasR mutants were reduced in fitness in the specific medium that we chose for our experiments. We grew PAO1 and each mutant in a minimal medium base containing 0.1% (wt/vol) carbon source. The carbon source was composed of Casamino Acids (CAA; available for use by all cells, regardless of genotype) and adenosine (which can be metabolized only when QS is functional in cells), in various ratios. As the relative amount of adenosine increased and cells were increasingly dependent on QS, the total cell density after 48 h was reduced, and this effect was more pronounced in lasI mutant monocultures than in wild-type monocultures (Fig. 1) (when all the available carbon was supplied as adenosine, wild-type cultures grew to 4.6% of the density that they attained when all carbon was supplied as CAA; for las mutants, this value was 0.1 to 0.2%). When all the available carbon was supplied as adenosine, lasI mutant monocultures grew to approximately 7% of the density of wild-type monocultures.
FIG 1 .
Population density (CFU) after 48 h of growth in quorum sensing medium with various ratios of Casamino Acids to adenosine supplied to a total concentration of 0.1% (wt/vol) carbon source. Squares, individual wild-type cultures; circles, lasI mutant cultures; triangles, lasR mutant cultures.
Signal-negative lasI mutants act as social cheats in adenosine-based growth medium, but signal-blind lasR mutants do not.
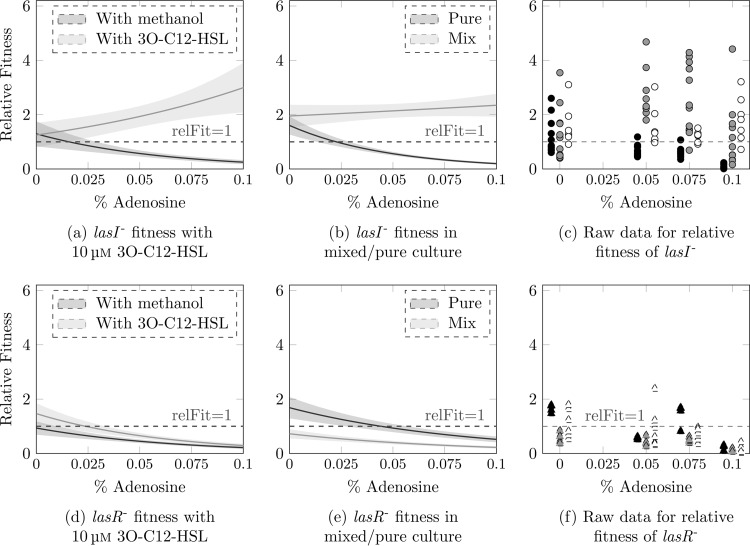
We next tested whether adding purified 3O-C12-HSL, or coculture with signal-producing wild-type bacteria, could restore the growth of lasI mutants. We calculated the fitness of lasI mutants relative to the wild type (i) in pure culture with or without exogenous 3O-C12-HSL and (ii) in 1:1 coculture with wild-type PAO1. Experiments were conducted in quorum sensing medium with various ratios of CAA and adenosine as described above, and cultures were grown for 48 h. A relative fitness of 1 signifies similar growth of mutant and wild-type bacteria, while values of <1 reflect relatively poorer growth of the mutant and values of >1 reflect better growth of the mutant. Figure 2 shows raw data and fitted models describing how the relative fitness of lasI (Fig. 2a, b, and c) and lasR (Fig. 2d, e, and f) mutants is affected by culture conditions.
FIG 2 .
Results of fitting generalized linear models to relative fitness data from experiments with lasI (a and b) and lasR (d and e) mutants. Lines show fitted models; shaded areas denote standard deviations. Relative fitness is compared between pure cultures of each mutant with added 3O-C12-HSL or with a solvent-only control (a and d) and between pure cultures and mixed culture with wild-type bacteria (b and e). Raw data for these experiments are shown in panels c and f (black symbols, fitness in pure culture [solvent-only control]; white symbols, fitness in pure culture supplemented with 10 µM 3O-C12-HSL; gray symbols, fitness in a 1:1 mixture with wild type).
Pure lasI mutant cultures became progressively less fit than the wild type as access to carbon depended more on quorum sensing (negative correlation between relative fitness and percent adenosine: coefficient, −21.6; P < 0.001). However, when 10 µM exogenous 3O-C12-HSL was supplied, lasI mutants surpassed the wild type in growth (positive correlation between relative fitness and percent adenosine: coefficient, 8.74, P = 0.003) (Fig. 2a and c) This result is consistent with previous work demonstrating a cost to 3O-C12 production (18). As predicted, this ability of lasI mutants to use exogenous signal, combined with the cost of signal production to the wild type, means that lasI mutants grown in coculture with wild-type bacteria act as social cheats: the average relative fitness was consistently >1 and did not decline as percent adenosine increased (coefficient, 1.8; P = 0.88) (Fig. 2b and c). There was, however, a slight drop in relative fitness when all carbon was supplied as adenosine (Fig. 2c). This is most likely attributable to the wild-type bacteria growing more slowly and taking longer to fully switch on QS responses. Both wild-type growth (Fig. 1) and the pool of available signal (see Fig. S2 in the supplemental material) are reduced under this condition, leaving less opportunity for exploitation by cheats.
Post hoc comparisons confirmed that lasI mutant relative fitness was significantly increased in mixed populations versus pure cultures in all media containing adenosine (t tests, P < 0.01). When all carbon was supplied as CAA and signal was not required, there was no significant effect of pure versus mixed culture on fitness (P > 0.95) Taken together with the fact that mixed cultures grew to a lower density than the wild-type cultures (Fig. S3), these results indicate that lasI mutants have an increased fitness when grown in the presence of wild-type bacteria under conditions requiring social interaction, while in turn decreasing wild-type fitness. Theory predicts that when population growth is strongly dependent on cooperation, cheats should be under negative frequency-dependent selection (they should have a greater fitness advantage when rarer [26, 32]). Consistent with this prediction, the relative fitness of the lasI mutant in intermediate adenosine/CAA ratios was negatively correlated with its initial frequency in the population (Fig. S4).
To ensure that the effect described above was due to the social dynamics of signal production, and not to the well-documented social dynamics of downstream exoproducts, this experiment was repeated using a lasR mutant. lasR mutants are unable to respond to 3O-C12-HSL and should therefore not be able to derive fitness benefits from exogenous signal in our setup. We found the same negative correlations between percent adenosine and CFU (Fig. 1) and percent adenosine and fitness relative to the wild type (Fig. 2d and f) (coefficient, −12.8; P = 0.001) as with the lasI mutant for lasR mutant monocultures. Crucially, lasR mutant relative fitness was not rescued by adding 3O-C12-HSL or by coculture with the wild type (coefficient, −10.7; P < 0.001) (Fig. 2d, e, and f), i.e., these mutants could not exploit wild-type bacteria.
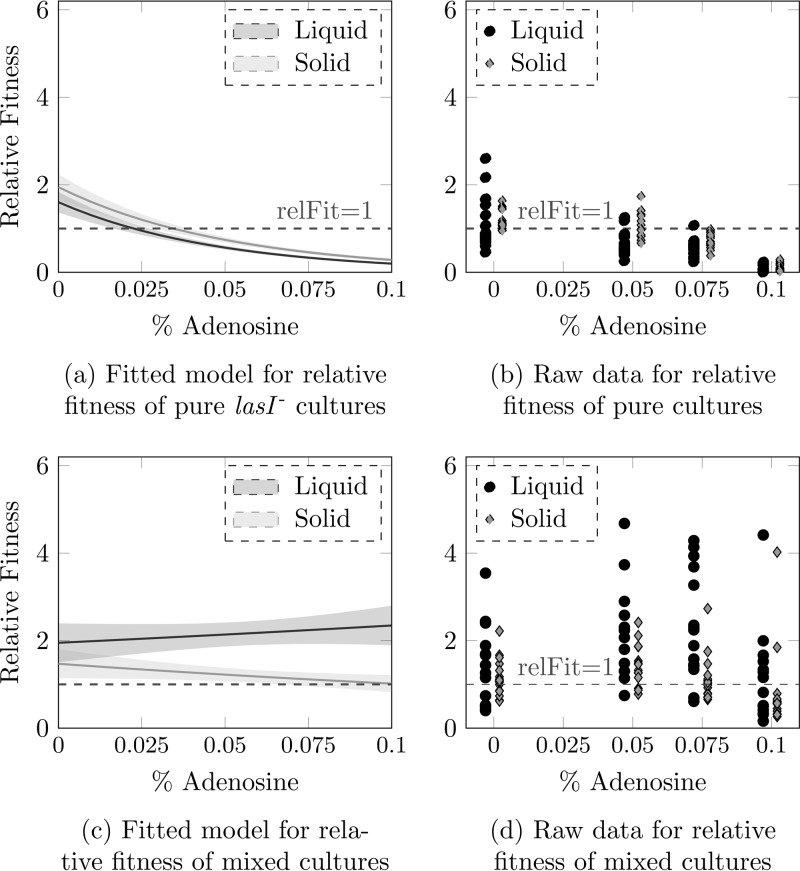
Slowing signal diffusion reduces lasI mutant cheating.
As a last step, we tested whether impeding the diffusion of signal molecules would make the lasI mutant less effective as a cheat. Reduced diffusion was achieved by adding agar to solidify the growth medium (Fig. S1). lasI mutant monocultures showed comparable declines in fitness in liquid and solid media (Fig. 3a and b) (analysis of variance [ANOVA], liquid/solid F1,124 = 6.13, P = 0.01; adenosine F1,123 = 117.58, P < 0.001; interaction F1,122 = 0.1658, P = 0.68). In mixed cultures, the relative fitness of the lasI mutant was positively correlated with the percentage of carbon available as adenosine in liquid cultures, as expected under cheating, but when the medium was solidified, lasI mutant relative fitness actually showed a modest negative correlation with percent adenosine (Fig. 3c and d) (ANOVA, liquid/solid F1,123 = 11.1324, P = 0.001; adenosine F1,124 = 0.0236, P = 0.88; interaction F1,122 = 0.9760, P = 0.33). This demonstrates that there is less opportunity for cheating in an environment where signal molecules cannot diffuse freely.
FIG 3 .
Comparison of lasI mutant relative fitness in pure culture (a and b) or mixed culture with the wild type (c and d). Panels a and c show the results of fitting generalized linear models to relative fitness data from experiments in liquid versus agar-supplemented medium. Lines show fitted models; shaded areas denote standard deviations. Raw data are shown in panels b and d (black circles, liquid medium; gray diamonds, agar-supplemented medium).
Supplementing growth medium with agar retards diffusion of 3O-C12-HSL. (a) Expression of a 3O-C12-HSL-dependent luminescent reporter construct is delayed and reduced when signal-negative reporter bacteria are separated from a reservoir or purified signal by an agar barrier. Lines and shading show means and standard deviations for 15 replicates. (b) The experimental setup, as described in Materials and Methods, is easily visualized by replicating the experiment on a larger scale and adding red food coloring in place of bacterial signal. Download FIG S1, PDF file, 2.8 MB (2.9MB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Measurements of 3O-C12-HSL concentration in wild-type supernatant after 48 h. (a) The total concentration of 3O-C12-HSL declines with increasing adenosine. (b) After dividing the signal concentration by the CFU of the producing culture, one observes the reverse trend—concentration of 3O-C12-HSL per CFU increases with adenosine level and reaches its maximum in pure adenosine. Download FIG S2, PDF file, 0.1 MB (110.7KB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Population density (CFU) after 48 h of growth in quorum sensing medium, Blue circles, individual wild-type cultures; yellow circles, lasI mutant and wild-type mixed cultures; red circles, lasI cultures. Download FIG S3, PDF file, 0.05 MB (50.1KB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative fitness of lasI mutants after 48-h growth in medium containing intermediate levels of adenosine is negatively correlated with their initial frequency in the population (coefficient, −0.69; P < 0.001). Panel a shows the result of fitting a linear model to relative fitness data from experiments with different starting frequencies of lasI in 0.05% and 0.075% adenosine. Lines show fitted models; shaded areas denote standard deviations. Raw data for these experiments are shown in panel b (black circles, 0.05% adenosine; gray diamonds, 0.075% adenosine). Download FIG S4, PDF file, 0.1 MB (68.6KB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
While there has been some discussion of QS signals as public goods (e.g., reference 33), most published work on the social evolution of QS focuses on signal-blind mutants and the benefits of cheating on the production of QS-regulated exoproducts (5, 7, 14, 34). Here, we provide the first direct evidence that, in addition to regulating the production of public goods, QS signal molecules are themselves capable of acting as public goods. Social cheating in the context of QS can therefore take multiple forms, depending on the environmental circumstances in which bacteria find themselves. Previous research had shown that (i) signal-blind mutants can be cheats when growth depends on the production of QS-dependent extracellular enzymes and (ii) cheating by signal-blind mutants can be constrained when some “private” QS-controlled processes contribute to growth (31). Following recent confirmation that QS signals are costly to make (18), we now add two new perspectives to the social evolution of QS: (iii) that signal-negative mutants can be cheats when growth depends entirely on private goods, regardless of any downstream effects on exoenzyme production, and (iv) that this signal cheating can occur only when the environment permits sufficient diffusion of signal molecules.
A lasI mutant grew poorly in monoculture, but growth could be rescued either by adding purified 3O-C12-HSL signal or by coculture with wild-type, signal-producing bacteria. In cocultures, the fitness of lasI mutants relative to the wild type increased as we forced the bacteria to rely more on adenosine for carbon. As the population became more reliant on QS, lasI mutants gained a greater fitness payoff from exploiting costly, diffusible signal produced by the wild type. lasR mutants did not gain a similar advantage from coculture with the wild type in adenosine medium. These signal-blind mutants can take up signals but cannot respond to them and so cannot switch on expression of the nuh hydrolase required for growth on adenosine (21, 31). The contrasting results for the two different QS mutants confirm that, in this environment, the QS signal itself acts as a public good.
Adding agar to the growth medium lowered both the diffusion of signal molecules and the relative fitness of cheats in mixed culture. Thus, adding simple spatial structuring into our system had a significant impact on the ability of signal-negative mutants to cheat on the wild type. There was no effect of structuring on relative fitness in pure cultures: even though agar enhanced the overall growth rate of the bacteria, the basic costs and benefits of signaling remained the same in liquid and solid media. This final observation is consistent with work on other bacterial public goods (28). We thus predict that the evolution of QS signaling strategies will be influenced by population genetic and spatial structure and that signal-negative cheats might rise to appreciable frequencies only in environments where signals diffuse freely. For example, the thick, adhesive mucus and bacterial biofilm polymers that block the airways of cystic fibrosis patients with chronic lung infection may partially protect producers from cheating by signal-negative mutants (24). Spatial dynamics play a huge role in the real-life ecology of environmentally and clinically important microbial ecosystems and are therefore of considerable interest to microbiologists investigating the roles of bacteria in processes as diverse as geochemical cycling, soil health, fouling, and infection (35–37).
Our work opens up new avenues for exploring how, when, and why bacterial signaling evolves in different environments and why we find a variety of QS mutant genotypes and phenotypes in natural environments (8–14, 38, 39). P. aeruginosa clones carrying mutations in the lasI (3O-C12-HSL) and rhll (C4-HSL) QS signals have been isolated from chronically infected cystic fibrosis patients and mechanically ventilated hospital patients (8–11). Given what we are now learning about the evolution of traits such as QS and how spatial structure changes the evolutionary dynamics, we suggest that there is a need to carefully consider the experimental design of in vitro experiments to increase their relevance to actual infections (40). To be forewarned is to be forearmed: a more accurate understanding of microbial ecology and evolution, gained from more realistic lab experiments, will be a vital weapon in the fight against infection.
MATERIALS AND METHODS
Bacterial strains.
The strains used were the wild-type Pseudomonas aeruginosa laboratory strain PAO1 and isogenic mutants created via insertion of a gentamicin resistance gene in the QS genes lasI (PAO1 lasI::Gm; referred to as lasI mutant [41]) and lasR (PAO1 lasR::Gm; referred to as lasR mutant [16]). To test 3O-C12-HSL diffusion in different media, we used a reporter strain of the lasI mutant. This contains a chromosomal luxCDABE fusion to the promoter of the lasB gene, which encodes the QS-dependent protease LasB (PAO1 plasB::lux [41]).
Growth conditions.
Quorum sensing medium (QSM) was modified from previous studies (5, 34, 42). QSM consisted of OS minimal medium [7.01 g Na2HPO4, 6.8 g KH2PO4, 1.19 g MgSO4⋅7H2O, 1 g (NH4)2SO4, 88 mg CaCl2⋅2H2O, 2 mg FeSO4⋅7H2O, 0.2 mg (NH4)6Mo7O24⋅4H2O, 1 ml Hutner’s “Metals 44,” per liter] (43) supplemented with 0.1% (wt/vol) carbon sources as a mix of Casamino Acids (CAA) and adenosine. The medium was filter sterilized. The exact ratio of CAA and adenosine was varied as detailed in Results. Liquid culture experiments were conducted in 24-well plates with a volume of 2 ml of medium. Cultures were incubated overnight in LB medium at 37°C on an orbital shaker and standardized to an optical density at 600 nm (OD600) of 0.8 to 0.9; 2 µl of pure or mixed inoculum was added to each experimental culture. The starting frequency of the mutant was determined by diluting and plating the starter cultures to determine the number of CFU of each genotype. Experimental cultures in QSM were incubated at 37°C with orbital shaking for 24 h or 48 h. After that time, cultures were diluted and replica plated on LB and LB plus gentamicin (25 μg/ml) agar to enumerate the CFU of PAO1 and lasI or lasR mutants in mixed culture. Experiments in solid media were conducted in QSM plus 2% (wt/vol) agar in 1-ml volumes in 48-well plates. Inoculation and culture conditions were otherwise identical to experiments in liquid medium. To break up agar prior to dilution and plating, the solid 1-ml agar cubes were retrieved from the plate and divided into thirds with a sterile metal spatula; each third was placed in a screw-cap tube containing 500 μl phosphate-buffered saline and 6 metal beads (Cambio) and homogenized using a FastPrep-24 5G bead beater (MP Biomedicals).
To test whether the lasI mutant was under negative frequency-dependent selection in medium where cheating could occur in the above-described experiments, mixed populations containing lasI mutants at initial frequencies of 1%, 10%, 40%, and 60%, as well as pure wild-type and lasI mutant cultures, were inoculated into 2 ml liquid QSM containing 0.05% (wt/vol) adenosine plus 0.05% (wt/vol) CAA and 2 ml liquid QSM containing 0.075% (wt/vol) adenosine plus 0.025% (wt/vol) CAA in 24-well plates for 5-fold replication. Cultures were grown for 48 h on an orbital shaker and replica plated as described above.
Measures of signal concentration.
To measure the concentration of QS signal (N-3-oxododecanoyl-l-homoserine lactone [3O-C12-HSL]) present in 48-h cultures, 100 µl of each culture supernatant was mixed with 100 µl of a log-phase culture of a luminescent Escherichia coli bioreporter (pSB1075 [44]) in the wells of a 96-well plate. This mixture was incubated for 4 h in a Tecan multimode plate reader, and luminescence and OD600 were recorded at 15-min intervals. To estimate the 3O-C12-HSL concentration, the luminescence of experimental samples was compared with a calibration curve constructed using QSM supplemented with known concentrations of purified 3O-C12-HSL.
Assaying the effect of agar on QS signal diffusion.
Agar has been successfully used to retard the diffusion of other bacterial exoproducts (28). To verify that agar affects 3O-C12-HSL diffusion in QSM and to determine the optimal agar concentration to use in further experiments, we devised a “sandwich experiment” in which a population of bacteria that switch on a luminescent reporter gene in response to QS signal but which cannot themselves produce signal were separated from a reservoir of purified signal by a layer of agar-supplemented medium. By measuring the time to expression of the luminescent reporter, we can assess the extent to which the agar barrier delays diffusion of the signal from the reservoir to the reporter population. An 0.1-ml amount of LB supplemented with 0.5% (wt/vol) agar and containing 0.5 µM purified 3O-C12-HSL was added to the wells of a 48-well plate and allowed to solidify. A second layer of 0.8 ml LB supplemented with 1, 2, 3, or 4% (wt/vol) agar was then added on top of the signal-containing layer. Each agar concentration was replicated in 6 wells. This layer was allowed to solidify, and a final layer of LB containing 0.5% (wt/vol) agar and the reporter PAO1 lasI mutant plasB::lux (overnight culture at OD600 of 0.2) was added. The plate was incubated in a Tecan multimode reader for 8 h, and luminescence was read at 10-min intervals. As shown in Fig. S1 in the supplemental material, an increasing agar concentration progressively delayed and reduced expression of luminescence. In order to check if higher luminescence was due to increased bacterial numbers, bacteria were retrieved and CFU were counted by plating. Median CFU was similar when 1% or 2% agar was used (approximately 1.4 × 107) but decreased by 30% when more agar was added (to approximately 1 × 107). It was difficult to determine whether this was due to agar retarding growth at high concentrations or simply due to the increased difficulty of thoroughly homogenizing medium rich in agar. One percent agar was therefore chosen for use in further experiments.
Statistical analysis.
Relative fitness of mutants, v, was calculated as x2(1 − x1)/x1(1 − x2), where x1 is the starting frequency of the mutant and x2 is the end frequency. It follows from the definition that a relative fitness of <1 signifies a decrease in mutant frequency, while a relative fitness of >1 signifies an increase in mutant frequency. To calculate relative fitness of the mutant in pure culture, mutant and wild-type monocultures were randomly paired. Statistical analysis of the results was conducted in R 3.2.3 (45) using generalized linear models assuming an underlying gamma distribution, with adenosine treated as a continuous variable and block and treatment (liquid-solid medium) fitted as factors. Raw data for all analyses reported are supplied in the supplemental material (Data Set S1).
Data for all analyses reported in the text (Mundetal_rawdata.xlsx). Download DATA SET S1, XLSX file, 0.02 MB (23.6KB, xlsx) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
A.M. was sponsored by the Erasmus+ Program. This work was supported by a Human Frontier Science Program Young Investigators grant to S.P.D. (RGY0081/2012) and a NERC grant to S.P.D. (NE/J007064/1), as well as by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
We thank Alex Truman for supplying purified 3O-C12-HSL; Sheyda Azimi and James Gurney for helpful comments on the experimental work; and Burkhard Hense, who recently sadly passed away, for useful discussion.
Footnotes
Citation Mund A, Diggle SP, Harrison F. 2017. The fitness of Pseudomonas aeruginosa quorum sensing signal cheats is influenced by the diffusivity of the environment. mBio 8:e00353-17. https://doi.org/10.1128/mBio.00353-17.
REFERENCES
- 1.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. 2013. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol 67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford ST, Bassler BL. 2012. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West SA, Griffin AS, Gardner A, Diggle SP. 2006. Social evolution theory for microorganisms. Nat Rev Microbiol 4:597–607. doi: 10.1038/nrmicro1461. [DOI] [PubMed] [Google Scholar]
- 4.Brown SP, Johnstone RA. 2001. Cooperation in the dark: signalling and collective action in quorum-sensing bacteria. Proc Biol Sci 268:961–965. doi: 10.1098/rspb.2001.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diggle SP, Griffin AS, Campbell GS, West SA. 2007. Cooperation and conflict in quorum-sensing bacterial populations. Nature 450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 6.Rumbaugh KP, Diggle SP, Watters CM, Ross-Gillespie A, Griffin AS, West SA. 2009. Quorum sensing and the social evolution of bacterial virulence. Curr Biol 19:341–345. doi: 10.1016/j.cub.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 7.Sandoz KM, Mitzimberg SM, Schuster M. 2007. Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci U S A 104:15876–15881. doi: 10.1073/pnas.0705653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilder CN, Allada G, Schuster M. 2009. Instantaneous within-patient diversity of Pseudomonas aeruginosa quorum-sensing populations from cystic fibrosis lung infections. Infect Immun 77:5631–5639. doi: 10.1128/IAI.00755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjarnsholt T, Jensen PØ, Jakobsen TH, Phipps R, Nielsen AK, Rybtke MT, Tolker-Nielsen T, Givskov M, Høiby N, Ciofu O, Scandinavian Cystic Fibrosis Study Consortium . 2010. Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS One 5:e10115. doi: 10.1371/journal.pone.0010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dénervaud V, TuQuoc P, Blanc D, Favre-Bonté S, Krishnapillai V, Reimmann C, Haas D, van Delden C. 2004. Characterization of cell-to-cell signaling-deficient Pseudomonas aeruginosa strains colonizing intubated patients. J Clin Microbiol 42:554–562. doi: 10.1128/JCM.42.2.554-562.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köhler T, Buckling A, Van Delden C. 2009. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc Natl Acad Sci U S A 106:6339–6344. doi: 10.1073/pnas.0811741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaber JA, Carty NL, McDonald NA, Graham ED, Cheluvappa R, Griswold JA, Hamood AN. 2004. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J Med Microbiol 53:841–853. doi: 10.1099/jmm.0.45617-0. [DOI] [PubMed] [Google Scholar]
- 14.West SA, Winzer K, Gardner A, Diggle SP. 2012. Quorum sensing and the confusion about diffusion. Trends Microbiol 20:586–594. doi: 10.1016/j.tim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Wilder CN, Diggle SP, Schuster M. 2011. Cooperation and cheating in Pseudomonas aeruginosa: the roles of the las, rhl and pqs quorum-sensing systems. ISME J 5:1332–1343. doi: 10.1038/ismej.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popat R, Crusz SA, Messina M, Williams P, West SA, Diggle SP. 2012. Quorum-sensing and cheating in bacterial biofilms. Proc Biol Sci 279:4765–4771. doi: 10.1098/rspb.2012.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollitt EJG, West SA, Crusz SA, Burton-Chellew MN, Diggle SP. 2014. Cooperation, quorum sensing, and evolution of virulence in Staphylococcus aureus. Infect Immun 82:1045–1051. doi: 10.1128/IAI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruparell A, Dubern JF, Ortori CA, Harrison F, Halliday NM, Emtage A, Ashawesh MM, Laughton CA, Diggle SP, Williams P, Barrett DA, Hardie KR. 2016. The fitness burden imposed by synthesizing quorum sensing signals. Nature Sci Rep 6:33101. doi: 10.1038/srep33101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller L, Surette MG. 2006. Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol 4:249–258. doi: 10.1038/nrmicro1383. [DOI] [PubMed] [Google Scholar]
- 20.Williams P, Cámara M. 2009. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Heurlier K, Dénervaud V, Haas D. 2006. Impact of quorum sensing on fitness of Pseudomonas aeruginosa. Int J Med Microbiol 296:93–102. doi: 10.1016/j.ijmm.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF. 2008. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci U S A 105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazli M, Bjarnsholt T, Kirketerp-Møller K, Jørgensen B, Andersen AS, Krogfelt KA, Givskov M, Tolker-Nielsen T. 2009. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J Clin Microbiol 47:4084–4089. doi: 10.1128/JCM.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decho AW. 2014. Localization of quorum sensing by extracellular polymeric substances (EPS): considerations of in situ signaling, p 105–121. In Hagen SJ (ed), The physical basis of bacterial quorum communication. Springer, New York, NY. [Google Scholar]
- 25.Gerdt JP, Blackwell HE. 2014. Competition studies confirm two major barriers that can preclude the spread of resistance to quorum-sensing inhibitors in bacteria. ACS Chem Biol 9:2291–2299. doi: 10.1021/cb5004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton WD. 1964. The genetical evolution of social behaviour I and II. J Theor Biol 7:1–16. [DOI] [PubMed] [Google Scholar]
- 27.Taylor PD. 1992. Altruism in viscous populations—an inclusive fitness model. Evol Ecol 6:352–356. doi: 10.1007/BF02270971. [DOI] [Google Scholar]
- 28.Kümmerli R, Griffin AS, West SA, Buckling A, Harrison F. 2009. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc Biol Sci 276:3531–3538. doi: 10.1098/rspb.2009.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allison SD. 2005. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol Lett 8:626–635. doi: 10.1111/j.1461-0248.2005.00756.x. [DOI] [Google Scholar]
- 30.Harrison F. 2013. Bacterial cooperation in the wild and in the clinic: are pathogen social behaviours relevant outside the laboratory? Bioessays 35:108–112. doi: 10.1002/bies.201200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dandekar AA, Chugani S, Greenberg EP. 2012. Bacterial quorum sensing and metabolic incentives to cooperate. Science 338:264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross-Gillespie A, Gardner A, West SA, Griffin AS. 2007. Frequency dependence and cooperation: theory and a test with bacteria. Am Nat 170:331–342. doi: 10.1086/519860. [DOI] [PubMed] [Google Scholar]
- 33.Smith RP, Boudreau L, You L. 2014. Engineering cell-to-cell communication to explore fundamental questions in ecology and evolution, p 620–676. In Hagen SJ (ed), The physical basis of bacterial quorum communication. Springer, New York, NY. [Google Scholar]
- 34.Darch SE, West SA, Winzer K, Diggle SP. 2012. Density-dependent fitness benefits in quorum-sensing bacterial populations. Proc Natl Acad Sci U S A 109:8259–8263. doi: 10.1073/pnas.1118131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadell CD, Drescher K, Foster KR. 2016. Spatial structure, cooperation and competition in biofilms. Nat Rev Microbiol 14:589–600. doi: 10.1038/nrmicro.2016.84. [DOI] [PubMed] [Google Scholar]
- 36.Tolker-Nielsen T, Molin S. 2000. Spatial organization of microbial biofilm communities. Microb Ecol 40:75–84. [DOI] [PubMed] [Google Scholar]
- 37.Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, Jensen PØ, Høiby N. 2013. The in vivo biofilm. Trends Microbiol 21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Popat R, Pollitt EJG, Harrison F, Naghra H, Hong KW, Chan KG, Griffin AS, Williams P, Brown SP, West SA, Diggle SP. 2015. Conflict of interest and signal interference lead to the breakdown of honest signaling. Evolution 69:2371–2383. doi: 10.1111/evo.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feltner JB, Wolter DJ, Pope CE, Groleau MC, Smalley NE, Greenberg EP, Mayer-Hamblett N, Burns J, Déziel E, Hoffman LR, Dandekar AA. 2016. lasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio 7:e01513-16. doi: 10.1128/mBio.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts AEL, Kragh KN, Bjarnsholt T, Diggle SP. 2015. The limitations of in vitro experimentation in understanding biofilms and chronic infection. J Mol Biol 427:3646–3661. doi: 10.1016/j.jmb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Jadhav GP, Chhabra SR, Telford G, Hooi DSW, Righetti K, Williams P, Kellam B, Pritchard DI, Fischer PM. 2011. Immunosuppressive but non-LasR-inducing analogues of the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-l-homoserine lactone. J Med Chem 54:3348–3359. doi: 10.1021/jm2001019. [DOI] [PubMed] [Google Scholar]
- 42.Heurlier K, Dénervaud V, Haenni M, Guy L, Krishnapillai V, Haas D. 2005. Quorum-sensing-negative (lasR) mutants of Pseudomonas aeruginosa avoid cell lysis and death. J Bacteriol 187:4875–4883. doi: 10.1128/JB.187.14.4875-4883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ornston LN, Stanier RY. 1966. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem 241:3776–3786. [PubMed] [Google Scholar]
- 44.Winson MK, Swift S, Fish L, Throup JP, Jørgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GSAB. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating n-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett 163:185–192. doi: 10.1111/j.1574-6968.1998.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 45.R Core Development Team 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementing growth medium with agar retards diffusion of 3O-C12-HSL. (a) Expression of a 3O-C12-HSL-dependent luminescent reporter construct is delayed and reduced when signal-negative reporter bacteria are separated from a reservoir or purified signal by an agar barrier. Lines and shading show means and standard deviations for 15 replicates. (b) The experimental setup, as described in Materials and Methods, is easily visualized by replicating the experiment on a larger scale and adding red food coloring in place of bacterial signal. Download FIG S1, PDF file, 2.8 MB (2.9MB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Measurements of 3O-C12-HSL concentration in wild-type supernatant after 48 h. (a) The total concentration of 3O-C12-HSL declines with increasing adenosine. (b) After dividing the signal concentration by the CFU of the producing culture, one observes the reverse trend—concentration of 3O-C12-HSL per CFU increases with adenosine level and reaches its maximum in pure adenosine. Download FIG S2, PDF file, 0.1 MB (110.7KB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Population density (CFU) after 48 h of growth in quorum sensing medium, Blue circles, individual wild-type cultures; yellow circles, lasI mutant and wild-type mixed cultures; red circles, lasI cultures. Download FIG S3, PDF file, 0.05 MB (50.1KB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative fitness of lasI mutants after 48-h growth in medium containing intermediate levels of adenosine is negatively correlated with their initial frequency in the population (coefficient, −0.69; P < 0.001). Panel a shows the result of fitting a linear model to relative fitness data from experiments with different starting frequencies of lasI in 0.05% and 0.075% adenosine. Lines show fitted models; shaded areas denote standard deviations. Raw data for these experiments are shown in panel b (black circles, 0.05% adenosine; gray diamonds, 0.075% adenosine). Download FIG S4, PDF file, 0.1 MB (68.6KB, pdf) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data for all analyses reported in the text (Mundetal_rawdata.xlsx). Download DATA SET S1, XLSX file, 0.02 MB (23.6KB, xlsx) .
Copyright © 2017 Mund et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.