Abstract
Background
Worldwide, indigenous populations appear to be at increased risk for invasive group A streptococcal (iGAS) infections. Although there is empirical evidence that the burden of iGAS disease is significant among remote First Nations communities in Northwestern Ontario, Canada, the epidemiology of iGAS infections in the area remains poorly characterized.
Methods
Individuals that met case definition for iGAS disease and whose laboratory specimens were processed by Meno Ya Win Health Centre in Sioux Lookout, Canada or who were reported to Thunder Bay District Health Unit, Canada were identified for the period 2009 to 2014. Case demographics, clinical severity, comorbidities, and risk factors were collected through chart review. Strain typing and antibiotic susceptibility were determined when possible. Basic descriptive statistics were calculated.
Results
Sixty-five cases of iGAS disease were identified, for an annualized incidence of 56.2 per 100 000. Primary bacteremia was present in 26.2% of cases, and cellulitis was identified in 55.4% of cases. The most common comorbidities identified were diabetes (38.5%) and skin conditions (38.5%). Prevalent risk factors included alcohol dependence (25%). Fourteen different emm types were identified among 42 isolates, with the most common being emm114 (17.4%), emm11 (15.2%), and emm118 (13.0%). Resistance to erythromycin and clindamycin was found in 24.6% of isolates.
Conclusions
Rural and remote First Nations communities in Northwestern Ontario experience iGAS infections at a rate 10 times the provincial and national average. Compared with other North American series, a lower proportion of isolates causing infection were of emm types included in candidate GAS vaccines.
Keywords: health equity, indigenous health, invasive group A Streptococcus
β-hemolytic group A Streptococcus (GAS) (also known as Streptococcus pyogenes) is a Gram-positive bacteria that causes a range of human illness and contributes to significant morbidity and mortality worldwide. Severe manifestations of invasive GAS include streptococcal toxic shock syndrome and necrotizing fasciitis [1].
High rates of invasive bacterial diseases, including invasive GAS (iGAS) disease, are frequently reported among indigenous populations in different countries, including Canada, the United States, Australia, and New Zealand [2–5]. A recent publication by the Public Health Agency of Canada from the International Circumpolar Surveillance system reported a rate of iGAS among indigenous peoples in Northern Canada approximately 2.5-fold higher than that of other Canadians [2]. No other peer-reviewed publications have characterized the incidence of iGAS among First Nations, Inuit or Metis (collectively referred to as the indigenous population of Canada).
Northwestern Ontario, Canada, is home to 26 remote on-reserve First Nations communities, comprising approximately 22 000 people (approximately 12% of registered First Nations in Ontario), who occupy a geographic area the size of France. Primary care, acute care, and public health services are fragmented and complicated by historical and political jurisdictional barriers. Sioux Lookout Meno Ya Win Health Centre (SLMHC) is the primary referral hospital for all 26 communities; however, critically ill patients may be transferred directly from their home community to tertiary centers in Thunder Bay, Ontario or Winnipeg, Manitoba. The First Nations and Inuit Health Branch of Health Canada is primarily responsible for surveillance and communicable disease control for on-reserve First Nations in Canada.
Recent publications have reported high rates of invasive bacterial diseases among remote on-reserve First Nations in Northwestern Ontario [6, 7]. We hypothesized that the burden of illness related to iGAS infections is particularly high in this region.
METHODS
All GAS-positive cultures processed by SLMHC between January 1, 2009 and December 31, 2014 were extracted from hospital electronic laboratory archives. Possible iGAS cases were identified if (1) the isolate was recovered from a normally sterile site (defined below), (2) the patient was admitted to hospital, or (3) the specimen was obtained in the operating room. Chart reviews were completed on all possible cases to determine whether patients met the Ontario Provincial Case Definition for iGAS [8]. The case definition included GAS isolated from normally sterile sites (blood, joint [excluding bursa], cerebrospinal, pleural, peritoneal, pericardial, and deep tissue/abscess specimens obtained during surgery) or GAS isolated from a nonsterile site with evidence of clinical severity (toxic shock syndrome, necrotizing fasciitis, pyomyositis, gangrene, meningitis, GAS pneumonia, or death directly attributed to iGAS).
In order to capture iGAS cases transferred directly from Sioux Lookout to Thunder Bay, cases reported to Thunder Bay District Health Unit (TBDHU) with a primary residence from one of the 26 communities were identified. As one of 36 public health units in Ontario, TBDHU has undertaken active surveillance for iGAS since an outbreak of emm59 iGAS in 2008 [9]. Duplicates of cases already identified through SLMHC were removed. Details on cases transferred directly to Winnipeg were unavailable.
Information collected by chart review included basic demographics, specimen details, clinical severity, comorbidities, risk factors, and antibiotic sensitivity profiles. Streptococcal toxic shock syndrome was defined as previously described [10]. History of skin condition included chronic dermatitis/wound causing breaks in skin integrity. Cases identified as nosocomial were excluded. In addition, the number of throat and wound swabs that yielded GAS and were submitted to SLMHC from the 26 communities was obtained for each year of the study. Crude incidence (removing duplicate isolates occurring within a 14-day period) and temporal trends of positive throat and wound swabs were assessed. The emm types of isolates from confirmed iGAS cases were determined through traditional Sanger sequencing using previously described primers and conditions [11].
Data were stored within Excel (Microsoft Office 2010; Microsoft, Redmond, WA) and analyzed using SPSS version 21 (IBM, Armank, NY). Basic descriptive statistics were completed with confidence intervals (CIs) where appropriate. Denominator data for the 26 communities were obtained from First Nations Inuit Health Branch, extracted from the Indian Registry System. Overall incidence was determined from an average of the crude rates each year and calculated per 100 000 population. Ethics approval was obtained through the SLMHC Research Review and Ethics Committee as well as the Research Ethics Board from the University of Toronto, Canada.
RESULTS
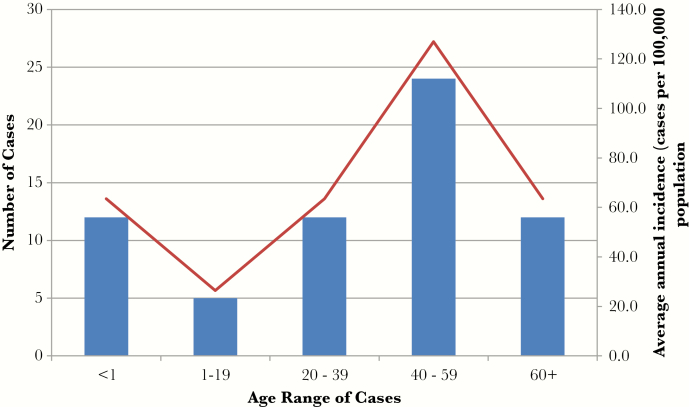
Overall, 6674 specimens processed between 2009 and 2014 yielded GAS, and 65 cases of iGAS disease were identified. The number of cases per year ranged from 9 to 18. The annualized incidence of iGAS was 56.2 per 100 000 (95% CI, 35.4–76.9). The mean age of cases was 40 years, with a range of <1 to 87 years. There was a bimodal distribution of age-specific incidence with peaks in the 0–19 and 40–59 age groups (Figure 1). Eleven cases (17%) occurred among infants less than 1 year of age.
Figure 1.
Incidence of invasive group A streptococcal cases in 26 rural and remote First Nations communities in Sioux Lookout area between 2009 and 2014 by age.
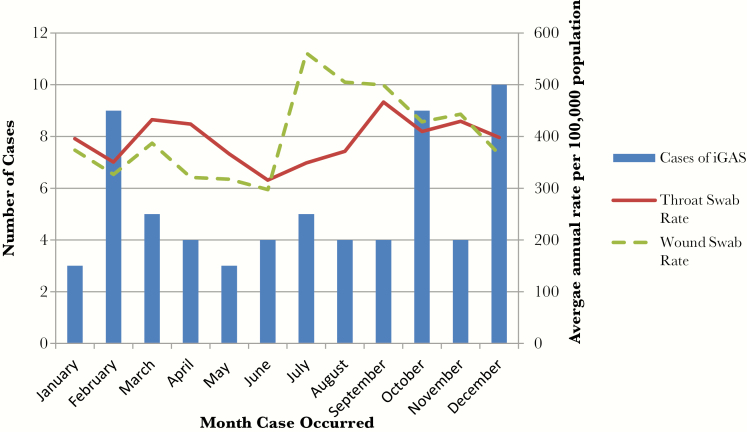
The majority of iGAS cases occurred during the months of October to March; however, there was no statistically significant seasonal trend (Figure 2). The annual proportion of wound swabs positive for GAS ranged from 40% to 48%. The average annual rate of wound swabs positive for GAS was 61 episodes per 1000 population. Rates increased between 2009 and 2013 and decreased in 2014. The average annual rate of throat swabs positive for GAS was 46.6 episodes per 1000 population. Rates increased between 2010 and 2014. Wound swabs positive for GAS were more prevalent during the summer months, whereas GAS-positive throat swabs did not demonstrate a seasonal trend. There was no detectable association between trends of iGAS incidence and either positive wound swab or positive throat swab rates.
Figure 2.
Temporal distribution of invasive group A streptococcal (iGAS) cases compared with group A streptococcal (GAS)-positive throat swabs and GAS-positive wound swabs submitted from 26 rural and remote First Nations communities in Northwestern Ontario between 2009 and 2014.
The 2 most common comorbidities identified were diabetes mellitus (38.5%) and skin conditions (38.5%) (Table 1). The most prominent other potential risk factor was alcohol dependence (25%). There was significant overlap in underlying conditions: among the 25 patients with underlying diabetes, 10 had skin conditions, 3 had alcohol dependence, and 2 had both skin conditions and alcohol dependence. Seven of 16 persons with alcohol dependence also had skin conditions, and 5 of 7 persons reporting intravenous drug use were also dependent on alcohol.
Table 1.
Characteristics of 65 iGAS Cases Identified From 26 Rural and Remote First Nations Communities in Northwestern Ontario Between 2009 and 2014
| Case Characteristics | Number (%) |
|---|---|
| Chronic underlying medical conditions | |
| Diabetes | 25 (38.5) |
| Skin conditions | 25 (38.5) |
| Coronary artery disease | 6 (9.2) |
| Active cancer | 3 (4.6) |
| Peritoneal dialysis* | 3 (4.6) |
| Hepatitis C | 3 (4.6) |
| Liver failure | 3 (4.6) |
| Connective tissue disorder | 3 (4.6) |
| Other potential risk factors | |
| Alcohol dependence | 16 (24.6) |
| Previous positive wound swab for GAS | 16 (24.6) |
| Regular use of nonsteroidal anti-inflammatory drug | 11 (16.9) |
| Injection drug use | 8 (12.3) |
| Other substance use | 6 (9.2) |
| Underhoused/homeless/living in shelter system | 5 (7.7) |
| Varicella within the previous month | 1 (1.5) |
Abbreviations: GAS, group A streptococcal; iGAS, invasive group A streptococcal.
*Patients must move from their rural or remote community to a larger center (primarily Thunder Bay) to receive hemodialysis. Only peritoneal dialysis is available at community level.
Overall, 49 (75.4%) cases had positive blood cultures; among the 13 with blood cultures that were negative or not done, 9 (13.8%) had positive deep tissue/abscess specimens (taken aseptically in the operating room), 5 (7.7%) had positive synovial fluid, and 1 (1.5%) had positive peritoneal fluid cultures. One case had a nonsterile specimen positive for GAS with evidence of clinical severity. Primary bacteremia was present in 17 (26.2%) cases (Table 2); 1 postpartum bacteremia was identified. Cellulitis was identified at the time of presentation in 36 (55.4%) cases, and septic arthritis was present in 6 (9.2%) cases. Streptococcal toxic shock syndrome and necrotizing fasciitis each occurred in 6 (9.2%) cases, and the crude 28-day case-fatality rate for all iGAS was 6.2%. No cases of nosocomial infection were identified.
Table 2.
Clinical Features of 65 iGAS Cases Identified From 26 Rural and Remote First Nations Communities in Northwestern Ontario Between 2009 and 2014
| Clinical Features | Number (%) |
|---|---|
| Culture source | |
| Blood | 49 (75.4) |
| Other sterile source | 15 (23.1) |
| Nonsterile source | 1 (1.5) |
| Clinical presentation | |
| Cellulitis | 36 (55.4) |
| Primary bacteremia | 17 (26.2) |
| Septic arthritis | 6 (9.2) |
| Pyomyositis | 2 (3.1) |
| Peritonitis | 2 (3.1) |
| Meningitis | 1 (1.5) |
| Pneumonia | 1 (1.5) |
| Disease severity | |
| Streptococcal toxic shock syndrome | 6 (9.2) |
| Necrotizing fasciitis | 6 (9.2) |
| Deceased | 4 (6.2) |
Abbreviations: iGAS, invasive group A streptococcal.
Antibiotic susceptibility profiles were available for 59 isolates. All isolates were sensitive to penicillin. Sixteen isolates (24.6%) were resistant to both erythromycin and clindamycin. Of the 65 iGAS cases, 46 isolates were available for emm typing. Among these, 14 different emm types were identified (Table 3). The most common emm types were emm114 (17.4%), emm11 (15.2%), emm118 (13.0%), emm68 (10.9%), and emm82 (10.9%). emm type variability over time was observed. Of the 16 isolates demonstrating resistance to erythromycin and clindamycin, 7 were identified as emm11 and 5 were identified as emm114. The cases associated with these isolates were from different communities and temporally unrelated.
Table 3.
emm types of 46 Cases of iGAS Identified From 26 Rural and Remote First Nations Communities in Northwestern Ontario Between 2009 and 2014
| emm types (N = 46) | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total No. |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 2 | 1 | 3 |
| 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| 11 | 0 | 0 | 0 | 1 | 3 | 3 | 7 |
| 53 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| 59 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| 68 | 0 | 0 | 0 | 1 | 1 | 3 | 5 |
| 80 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 82 | 0 | 0 | 2 | 3 | 0 | 0 | 5 |
| 83 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 87 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 101 | 0 | 1 | 1 | 0 | 1 | 0 | 3 |
| 114 | 1 | 3 | 2 | 1 | 1 | 0 | 8 |
| 115 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| 118 | 0 | 0 | 0 | 1 | 4 | 1 | 6 |
| Total | 1 | 5 | 5 | 9 | 16 | 10 | 46 |
Abbreviations: iGAS, invasive group A Streptococcus.
DISCUSSION
The incidence of iGAS in Canada increased significantly over the last decade (from 2.81 to 4.03 per 100 000 between 2000 and 2009) [12]. In 2013, the Canadian and Ontario rates for iGAS were 4.72 and 4.6 per 100 000 population, respectively [12, 13]. In contrast, the crude incidence rate of iGAS calculated in this study among 26 rural and remote First Nations communities in Northwestern Ontario between 2009 and 2014 was more than 10 times higher at a rate of 56.2 per 100 000 population. Although significantly higher than the incidence of iGAS among indigenous peoples in Northern Canada (2.25–20.44 per 100 000 population) [2], it is comparable to indigenous populations in Australia and New Zealand [3, 4, 14, 15].
High prevalence of substance use has been thought to be a contributing factor to overall higher rates of iGAS in Northwestern Ontario [9]. The high burden of substance use among indigenous peoples in Canada is recognized nationally as a legacy of historical assimilation policies, multigenerational trauma, and systemic racism [16]. The prevalence of alcohol dependence among iGAS cases in this study (25%) was consistent with iGAS epidemiology for both indigenous and non-indigenous populations in Northern Australia [17]. Prescription drug abuse, particularly injection drug use (IDU), is a significant issue facing many of the communities included in this study [18, 19]. However, only 12.3% of cases in this study were identified as having a history of IDU. Although it is likely underreported, this proportion is consistent with iGAS epidemiology reported in non-indigenous populations [20–22].
Diabetes and skin conditions were found to be common comorbidities among iGAS cases in this review. The prevalence of diabetes among First Nations in Canada is disproportionately high compared with the non-indigenous population [23]. Given the increased risk of iGAS associated with underlying diabetes, this contributes to increased risk for First Nation communities [24].
In addition, high prevalence of skin conditions such as eczema and impetigo has been observed in many remote First Nations communities [25]. It has been hypothesized in Northern Australia that a major risk factor for GAS bacteremia in Aboriginal people is exposure to an overall high burden of GAS infections, primarily skin infections such as impetigo and pyoderma [3, 26]. These populations also experience high prevalence of nonsuppurative sequelae to GAS infection including acute rheumatic fever and poststreptococcal glomerulonephritis [27, 28]. Although largely eliminated from Canada, rheumatic fever in First Nations communities in Northwestern Ontario has been documented at rates consistent with Northern Australia (21.3 and 26 per 100 000, respectively) [29]. In Australia, the burden of GAS associated with skin and soft-tissue infections has been primarily related to inadequate and overcrowded housing [30, 31]. Disparities in the social determinants of health, including inadequate and overcrowded housing, are well documented public health issues facing First Nations communities in Northwestern Ontario [32–34].
The proportion of iGAS isolates that demonstrated resistance to erythromycin (24.6%) and clindamycin (24.6%) was higher than reported in other regions in Canada. A recent analysis of iGAS cases from Peel and Toronto regions in Ontario demonstrated an increase in erythromycin resistance from 2.2% in 1992–1995 to 19.5% in 2008–2010 and then to 7.5% in 2013 [35]. A 2011 publication from the Canadian province of British Columbia reported resistance in all GAS isolates in 2011 to be 14.5% and 11.9% for erythromycin and clindamycin, respectively [36].
LIMITATIONS
Data from Winnipeg could not be accessed; therefore, our data likely underestimates iGAS infection acquired in some communities. Given the small population size and reported case numbers, rates should be interpreted with caution. Risk factors were self-identified in patient charts and may therefore be underestimated. Community population statistics from the Indian Registry System relies on individuals to register for “Indian status” and may thus underrepresent the true population of communities, which would result in an overestimation of iGAS rates.
CONCLUSIONS
More than 200 different GAS emm types have been reported worldwide [37]. The most common emm types identified in the present investigation belong to the so-called skin (eg, emm83, emm101) and generalist (eg, emm68, emm82, emm87, emm114) emm types with only a few strains belonging to emm types with tropism for throat (eg, emm1) [38]. This is similar to the epidemiology of iGAS cases in nearby Thunder Bay region but differs from the remainder of Ontario [9, 13]. This variation in emm type distribution has important vaccine implications. A 30-valent M-protein vaccine for GAS began clinical trials in Canada and the United States in September 2015. Although this new vaccine is reported to account for greater than 90% and 78% of invasive disease serotypes in the United States and Europe, respectively, only 70% of the emm types identified in this population are covered. The diversity of strains and rapid serotype replacement observed in Northwestern Ontario may mean that the vaccine will offer reduced protection in a population that experiences a disproportionate burden of severe disease [39, 40].
Acknowledgments
We thank Leah Vanderploeg and other public health nurses at Thunder Bay District Health Unit for conducting chart reviews and confirming case details.
Financial support. No funding was received for this study.
Potential conflicts of interest. All authors: No reported conflicts.All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 2000; 13:470–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li YA, Martin I, Tsang R, et al. Invasive bacterial diseases in Northern Canada, 2006–2013. Can Commun Dis Rep 2016; 42:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyd R, Patel M, Currie BJ, et al. High burden of invasive group a streptococcal disease in the Northern Territory of Australia. Epidemiol Infect 2016; 144:1018–27. [DOI] [PubMed] [Google Scholar]
- 4. Whitehead BD, Smith HV, Nourse C. Invasive group a streptococcal disease in children in Queensland. Epidemiol Infect 2011; 139:623–8. [DOI] [PubMed] [Google Scholar]
- 5. Rudolph K, Bruce MG, Bruden D, et al. Epidemiology of invasive group A streptococcal disease in Alaska, 2001 to 2013. J Clin Microbiol 2016; 54:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirlew M, Rea S, Schroeter A, et al. Invasive CA-MRSA in northwestern Ontario: a 2-year prospective study. Can J Rural Med 2014; 19:99–102. [PubMed] [Google Scholar]
- 7. Kelly L, Tsang RS, Morgan A, et al. Invasive disease caused by Haemophilus influenzae type A in Northern Ontario First Nations communities. J Med Microbiol 2011; 60(Pt 3):384–90. [DOI] [PubMed] [Google Scholar]
- 8. Ontario Ministry of Health and Long-Term Care. Infectious Diseases Protocol, 2013. Appendix B: Provincial Case Definitions. Group A streptococcal disease, invasive. Available at: http://www.health.gov.on.ca/en/pro/programs/publichealth/oph_standards/docs/gas_cd.pdf. Accessed 22 May 2014. [Google Scholar]
- 9. Athey TB, Teatero S, Sieswerda LE, et al. High incidence of invasive group a Streptococcus disease caused by strains of uncommon emm types in Thunder Bay, Ontario, Canada. J Clin Microbiol 2016; 54:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breiman RF, Davis JP, Facklam RR, et al. Defining the group A streptococcal toxic shock syndrome: rationale and consensus definitions. JAMA 1993; 269:390–91. [PubMed] [Google Scholar]
- 11. Beall B, Gherardi G, Lovgren M, et al. emm and sof gene sequence variation in relation to serological typing of opacity-factor-positive group A streptococci. Microbiology 2000; 146:1195–209. [DOI] [PubMed] [Google Scholar]
- 12. Public Health Agency of Canada. Notifiable diseases on-line: invasive group A streptococcal disease. Available at: http://dsol-smed.phac-aspc.gc.ca/dsol-smed/ndis/charts.php?c=pl Accessed April 21, 2016. [Google Scholar]
- 13. Ontario Agency for Health Protection and Promotion (Public Health Ontario). Reportable Disease Trends in Ontario, 2013. Toronto, ON: Queen’s Printer for Ontario; 2015. [Google Scholar]
- 14. Harris P, Siew DA, Proud M, et al. Bacteraemia caused by beta-haemolytic streptococci in North Queensland: changing trends over a 14-year period. Clin Microbiol Infect 2011; 17:1216–22. [DOI] [PubMed] [Google Scholar]
- 15. Safar A, Lennon D, Stewart J, et al. Invasive group A streptococcal infection and vaccine implications, Auckland, New Zealand. Emerg Infect Dis 2011; 17:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chansonneuve D. Addictive Behaviours Among Aboriginal People in Canada. Aboriginal Healing Foundation; 2007. Available at: http://www.ahf.ca/downloads/addictive-behaviours.pdf Accessed 21 April 2016. [Google Scholar]
- 17. Gear RJ, Carter JC, Carapetis JR, et al. Changes in the clinical and epidemiological features of group a streptococcal bacteraemia in Australia’s Northern Territory. Trop Med Int Health 2015; 20:40–7. [DOI] [PubMed] [Google Scholar]
- 18. Kelly L, Guilfoyle J, Dooley J, et al. Incidence of narcotic abuse during pregnancy in northwestern Ontario: three-year prospective cohort study. Can Fam Physician 2014; 60:e493–8. [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon J, Dooley J, Balfour-Boehm J, et al. The evolving nature of narcotic use in Northwestern Ontario. Can J Rural Med 2014; 19:158–60. [PubMed] [Google Scholar]
- 20. Lamagni TL, Neal S, Keshishian C, et al. Epidemic of severe Streptococcus pyogenes infections in injecting drug users in the UK, 2003-2004. Clin Microbiol Infect 2008; 14:1002–9. [DOI] [PubMed] [Google Scholar]
- 21. Zachariadou L, Stathi A, Tassios PT, et al. Differences in the epidemiology between paediatric and adult invasive Streptococcus pyogenes infections. Epidemiol Infect 2014; 142:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin J, Murchan S, O’Flanagan D, Fitzpartrick F. Invasive group A streptococcal disease in Ireland, 2004 to 2010. Euro Surveill 2011; 16:pii: 19988. [PubMed] [Google Scholar]
- 23. Public Health Agency of Canada. Diabetes in Canada: Facts and Figures from a Public Health Perspective. Ottawa: Available at: http://www.phac-aspc.gc.ca/cd-mc/diabetes-diabete/index-eng.php Accessed 21 April 2016. [Google Scholar]
- 24. Langley G, Hao Y, Pondo T, et al. The impact of obesity and diabetes on the risk of disease and death due to invasive group A Streptococcus infections in adults. Clin Infect Dis 2016; 62:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. First Nations Information Governance Centre. First Nations Regional Health Survey (RHS) 2008/10: National report on adults, youth and children living in First Nations communities. Ottawa, ON: FNIGC; Available at: http://fnigc.ca/sites/default/files/First_Nations_Regional_Health_Survey_2008-10_National_Report.pdf Accessed 1 Apr 2015. [Google Scholar]
- 26. Carapetis JR, Walker AM, Hibble M, et al. Clinical and epidemiological features of group A streptococcal bacteraemia in a region with hyperendemic superficial streptococcal infection. Epidemiol Infect 1999; 122:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDonald MI, Towers RJ, Andrews RM, et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis 2006; 43:683–9. [DOI] [PubMed] [Google Scholar]
- 28. Marshall CS, Cheng AC, Markey PG, et al. Acute post-streptococcal glomerulonephritis in the Northern Territory of Australia: a review of 16 years data and comparison with the literature. Am J Trop Med Hyg 2011; 85:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gordon J, Kirlew M, Schreiber Y, et al. Acute rheumatic fever in First Nations communities in northwestern Ontario: Social determinants of health “bite the heart”. Can Fam Physician 2015; 61:881–6. [PMC free article] [PubMed] [Google Scholar]
- 30. Steer AC, Carapetis JR, Nolan TM, Shann F. Systematic review of rheumatic heart disease prevalence in children in developing countries: the role of environmental factors. J Paediatr Child Health 2002; 38:229–34. [DOI] [PubMed] [Google Scholar]
- 31. Jaine R, Baker M, Venugopal K. Acute rheumatic fever associated with household crowding in a developed country. Pediatr Infect Dis J 2011; 30:315–9. [DOI] [PubMed] [Google Scholar]
- 32. Statistics Canada. 2006 Census: Aboriginal Peoples in Canada in 2006: Inuit, Métis and First Nations, 2006 Census: First Nations People. Ottawa, ON: Statistics Canada; Available at: http://www12.statcan.ca/census-recensement/2006/as-sa/97-558/pdf/97-558-XIE2006001.pdf. Accessed 6 February 2017. [Google Scholar]
- 33. First Nations Information Governance Centre. First Nations Regional Health Survey (RHS) phase 2 (2008/10) Ontario region final report. Ontario region report on the adult, youth and children living in First Nations communities. Toronto, ON: Chiefs of Ontario; Available at: http://fnigc.ca/sites/default/files/docs/first_nations_regional_health_survey_rhs_phase_2_08-10_ontario_region_final_report_12nov01v8.pdf. Accessed 6 February 2017. [Google Scholar]
- 34. Assembly of First Nations. Fact Sheet – First Nations Housing On-Reserve. June 2013. Available at: http://www.afn.ca/uploads/files/housing/factsheet-housing.pdf Accessed 21 April 2016. [Google Scholar]
- 35. Kandel C, Daneman N, Gold W, et al. Invasive Group A Streptococcal Infections in Ontario, Canada: 1992–2013. Poster Abstract: IDWeek 2015; Thursday October 8, 2015. Available at: https://idsa.confex.com/idsa/2015/webprogram/Paper51217.html. [Google Scholar]
- 36. British Columbia Centre for Disease Control. Antibiotic Resistance Trends in the Province of British Columbia. 2011. Available at: http://www.bccdc.ca/resource-gallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/Epid/AntimicrobialResistanceTrendsinBC_2011.pdf Accessed 21 April 2016. [Google Scholar]
- 37. Steer AC, Law I, Matatolu L, et al. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis 2009; 9:611–6. [DOI] [PubMed] [Google Scholar]
- 38. Bessen DE, Lizano S. Tissue tropisms in group A streptococcal infections. Future Microbiol 2010; 5:623–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 2011; 29:8175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richardson LJ, Towers RJ, Cheng AC, et al. Diversity of emm sequence types in group A beta-haemolytic streptococci in two remote Northern Territory Indigenous communities: implications for vaccine development. Vaccine 2010; 28:5301–5. [DOI] [PubMed] [Google Scholar]