Abstract
A 63-year-old woman on rituximab maintenance for follicular lymphoma presented with headaches, vomiting, and fever, and was diagnosed with eastern equine encephalomyelitis by cerebrospinal fluid polymerase chain reaction. Eastern equine encephalomyelitis immunoglobulin (Ig)G/IgM remained negative due to rituximab treatment, and magnetic resonance imaging showed minimal abnormalities, making this a diagnostically challenging case. Despite therapy with intravenous Ig, the patient rapidly declined and died on hospital day 12. Autopsy revealed perivascular and parenchymal chronic inflammation, with an absence of B lymphocytes, and virally infected neurons throughout the central nervous system.
Keywords: arbovirus, eastern equine encephalitis, immunosuppressed, neuroinvasive, rituximab
CASE REPORT
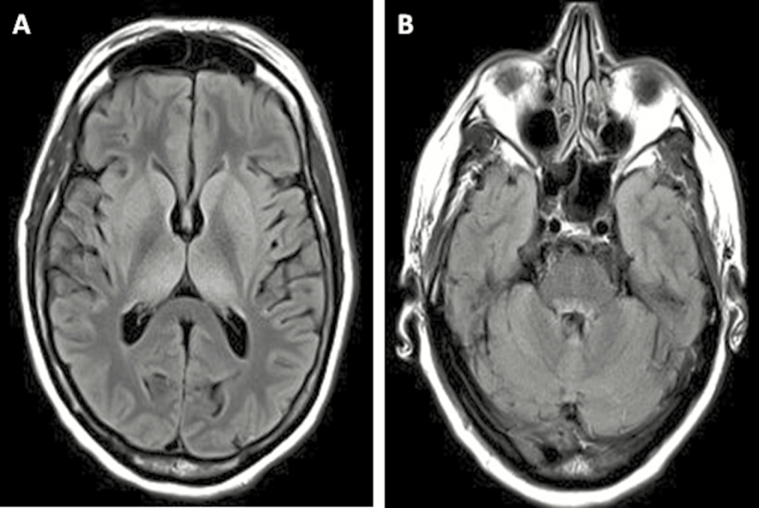
A 63-year-old woman from Massachusetts with significant mosquito exposure due to lake proximity presented in September with 4 days of headaches, vomiting, and fever. The patient’s history was significant for follicular lymphoma, treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) plus intrathecal methotrexate, with maintenance rituximab (most recent dose 3 months before presentation). On physical exam, the patient was febrile up to 105.3°F. Neurologic exam revealed mild resistance to neck flexion, marked inattention, bilateral upper extremity tremulousness, and normal reflexes. Magnetic resonance imaging (MRI) showed subtle T2/fluid-attenuated inversion recovery (FLAIR) bilateral thalamic and basal ganglia hyperintensities (Figure 1). Cerebrospinal fluid (CSF) analysis demonstrated 530 white blood cells (63% neutrophils, 17% lymphocytes), 106.9 mg/dL protein, and CSF/serum glucose ratio 0.7.
Figure 1.
Brain magnetic resonance imaging (MRI) findings are depicted. Axial T2/fluid-attenuated inversion recovery MRI showing subtle hyperintensities in the basal ganglia and thalamus bilaterally (A) and in ventral pons (B) on hospital day 4.
Initial infectious workup, including CSF testing for eastern equine encephalitis virus (EEEV) antibodies and West Nile virus (WNV) antibodies and nucleic acid, was negative. Blood lymphocyte panel indicated an absence of B lymphocytes consistent with rituximab treatment. Due to suspicion that the patient would not produce a detectable antibody response, CSF was retested for EEEV by qualitative reverse-transcription polymerase chain reaction (PCR) targeting the structural polyprotein coding sequence, performed at the William A. Hinton State Laboratory Institute, Department of Public Health (Boston, MA), which returned positive. The patient was empirically treated with vancomycin, ceftriaxone, ampicillin, acyclovir, and AmBisome, levetiracetam for temporal electrographic seizures, and 3 days of intravenous solumedrol followed by 4 days of intravenous immunoglobulins (IVIGs). She eventually required intubation for decreased oxygen saturation, experienced continued decline on examination, and died on hospital day 12.
At autopsy, gross examination revealed an edematous brain with Duret hemorrhages involving the tegmentum of the midbrain and upper pons. Microscopic examination showed collections of perivascular and parenchymal chronic inflammatory cells throughout the central nervous system and leptomeninges, comprised of CD3-positive T lymphocytes in the absence of B lymphocytes with minimal acute inflammation (Figure 2A–C). Activated microglia were present throughout. Patchy edema was observed with parenchymal vacuolation. Pyknotic, hypereosinophilic neurons were widespread. Arteriolosclerosis, but no evidence of arteritis or thrombosis, was identified. There was no evidence of involvement by hematologic malignancy; viral inclusions were not detected. Immunohistochemistry for EEEV was performed as previously described using eastern equine encephalomyelitis immune ascites fluid (V-515-701-562; American Type Culture Collection, Manassas, VA) [1]. Infected neurons were present throughout the brain in a patchy, perivascular pattern, particularly in ischemic areas of the cortex and thalamus, as well as the anterior horn of the spinal cord (Figure 2D–F). Specificity for EEEV was confirmed in the prior study by lack of staining of control brains with other forms of viral and nonviral encephalitis [1].
Figure 2.
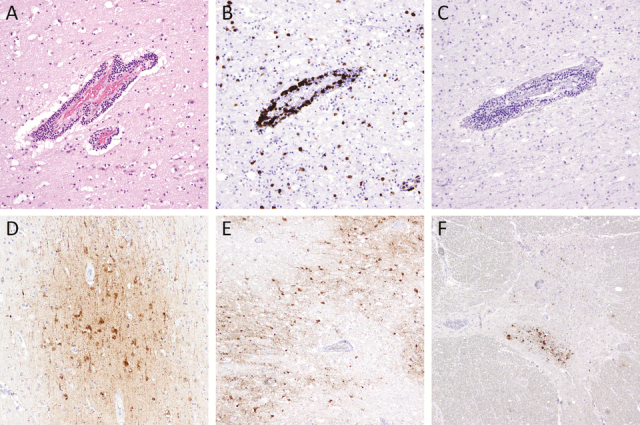
Immunohistochemistry depicting inflammatory reaction and eastern equine encephalitis virus (EEEV) in the central nervous system are depicted. Perivascular and parenchymal chronic inflammatory infiltrates in a section of frontal cortex (A), consisting predominantly of CD3+ T lymphocytes (B) with no CD79a+ B lymphocytes (C). The EEEV is shown in neuronal cytoplasm in cortex (D), thalamus (E), and anterior horn cells in the thoracic spinal cord (F). Original magnification, ×40 (E and F), ×100 (D), and ×200 (A–C); hematoxylin and eosin stain (A); immunostains CD3 (B), CD79 (C), and EEEV (D–F).
DISCUSSION
Eastern equine encephalitis virus is a single-stranded ribonucleic acid virus (member of the Alphavirus genus in the Togaviridae family), transmitted by mosquitoes, that causes a severe, often fatal, neurological illness [2]. An average of 6–8 cases per year are reported in the United States, predominantly in the Atlantic and Gulf coasts and Great Lakes (8 cases reported in 2014) [3]. After a 4- to 10-day incubation period, a systemic illness occurs followed by encephalitis characterized by fever, headaches, vomiting, convulsions, and coma. Cerebrospinal fluid often shows neutrophilic pleocytosis and elevated protein levels. Diagnosis is typically made by detection of immunoglobulin (Ig)M and neutralizing antibodies from serum or CSF, and occasionally by PCR. No vaccine is currently available, and treatment is supportive with case reports of successful recovery after IVIG [4–6].
Our remarkable case of EEE highlights the importance of considering host factors in pre-mortem diagnostic testing. Typical MRI findings in more traditional cases of EEE include T2/FLAIR hyperintensities in the basal ganglia, thalamus, and cerebral cortex; less commonly in the brainstem [1, 7]. In our case, the combination of nonspecific imaging findings was initially attributed to a toxic/metabolic process. The medical uncertainty was further exacerbated by negative serologies, which led to the implementation of ineffective treatments. Common histopathologic findings of EEE include diffuse meningoencephalitis with acute and chronic perivascular and parenchymal inflammatory infiltrates, neuronal destruction, necrosis, gliosis, and vasculitis, involving the basal ganglia, thalamus, and cortex [7, 8]. Involvement of the spinal cord is rare, and it may be indicative of more severe disease. Although neutrophils were present in CSF samples, a prominent neutrophilic component was not observed in post-mortem brain tissue. The lack of this hallmark histologic feature may have further hindered making the diagnosis of EEEV had the pre-mortem testing remained negative, highlighting the need to account for the temporal sequence of events in interpreting diagnostic testing results.
Rituximab is a monoclonal anti-CD20 antibody that causes B-cell death, and it is increasingly used in the treatment of hematologic malignancies and autoimmune diseases. A range of severe infections have been associated with rituximab treatment including reactivation of hepatitis B virus, reactivation of JC virus leading to progressive multifocal leukoencephalopathy, and enterovirus encephalitis [9]. No prior cases of EEE have been reported in patients treated with rituximab; however, several fatal cases of WNV meningoencephalitis have been described in the literature [10–13]. Similar to our patient, diagnosis of WNV was made by PCR in the setting of negative IgG/IgM serology due to a lack of antibody response, and no B cells were identified by immunohistochemical staining of autopsy brain tissue. In these rare cases, a high index of suspicion is required to make the correct diagnosis.
CONCLUSIONS
Eastern equine encephalitis virus is a neuroinvasive arboviral infection that requires a high degree of suspicion for efficient diagnosis and treatment. We report the first case of EEE in a patient on rituximab, which required PCR testing for diagnosis due to failure to produce detectable antibodies. This case illustrates the importance of considering host factors in iatrogenically immunocompromised patients.
Acknowledgment
Financial support. SSM is supported by NIH T32 NIAG000222.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Silverman MA, Misasi J, Smole S, et al. Eastern equine encephalitis in children, Massachusetts and New Hampshire, USA, 1970–2010. Emerg Infect Dis 2013; 19:194–201; quiz 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greenlee JE. The equine encephalitides. Handb Clin Neurol 2014; 123:417–32. [DOI] [PubMed] [Google Scholar]
- 3. Lindsey NP, Lehman JA, Staples JE, Fischer M. West Nile virus and other nationally notifiable arboviral diseases—United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:929–34. [DOI] [PubMed] [Google Scholar]
- 4. Mukerji SS, Lam AD, Wilson MR. Eastern equine encephalitis treated with intravenous immunoglobulins. Neurohospitalist 2016; 6:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golomb MR, Durand ML, Schaefer PW, et al. A case of immunotherapy-responsive eastern equine encephalitis with diffusion-weighted imaging. Neurology 2001; 56:420–1. [DOI] [PubMed] [Google Scholar]
- 6. Wendell LC, Potter NS, Roth JL, et al. Successful management of severe neuroinvasive eastern equine encephalitis. Neurocrit Care 2013; 19:111–5. [DOI] [PubMed] [Google Scholar]
- 7. Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of eastern equine encephalitis. N Engl J Med 1997; 336:1867–74. [DOI] [PubMed] [Google Scholar]
- 8. Farber S, Hill A, Connerly ML, Dingle JH. Encephalitis in infants and children caused by the virus of the eastern variety of equine encephalitis. JAMA 1940; 114:1725–31. [Google Scholar]
- 9. Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol 2010; 47:187–98. [DOI] [PubMed] [Google Scholar]
- 10. Levi ME, Quan D, Ho JT, et al. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplant 2010; 24:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Slater B, Rudd R, et al. First isolation of West Nile virus from a patient with encephalitis in the United States. Emerg Infect Dis 2002; 8:1367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morjaria S, Arguello E, Taur Y, et al. West Nile virus central nervous system infection in patients treated with rituximab: implications for diagnosis and prognosis, with a review of literature. Open Forum Infect Dis 2015; 2:ofv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mawhorter SD, Sierk A, Staugaitis SM, et al. Fatal West Nile virus infection after rituximab/fludarabine–induced remission for non-Hodgkin’s lymphoma. Clin Lymphoma Myeloma 2005; 6:248–50. [DOI] [PubMed] [Google Scholar]