Abstract
Background
Although the incidence of Clostridium difficile infection (CDI) is increasing, available CDI treatment options are limited in terms of sustained response after treatment. This phase 3 trial assessed the efficacy and safety of surotomycin, a novel bactericidal cyclic lipopeptide, versus oral vancomycin in subjects with CDI.
Methods
In this randomized, double-blind, active-controlled, multicenter, international trial, subjects with CDI confirmed by a positive toxin result were randomized to receive surotomycin (250 mg twice daily) or vancomycin (125 mg 4 times daily) orally for 10 days. The primary endpoints were clinical response at end of treatment and evaluation of surotomycin safety. The key secondary endpoints were clinical response over time and sustained clinical response through a 30- to 40-day follow-up period. Clostridium difficile infection recurrence during follow-up and time to diarrhea resolution were also analyzed.
Results
In total, 570 subjects were randomized and had confirmed CDI; 290 subjects received surotomycin and 280 subjects received vancomycin. Surotomycin clinical cure rates at end of treatment (surotomycin/vancomycin: 79.0%/83.6%; difference of −4.6%; 95% confidence interval, −11.0 to 1.9]), clinical response over time (stratified log-rank test, P = .832), and sustained clinical response at end of trial (Day 40–50) (60.6%/61.4%; difference of −0.8%; 95% CI, −8.8 to 7.1) in the microbiological modified intent to treat population did not meet noninferiority or superiority criteria versus vancomycin. Both treatments were generally well tolerated.
Conclusions
Surotomycin failed to meet the criteria for noninferiority versus vancomycin for the primary and key secondary endpoints in this trial.
Keywords: antibiotic, Clostridium difficile infection, surotomycin, vancomycin
Clostridium difficile infection (CDI) remains a leading cause of hospital-acquired diarrhea [1, 2]. The incidence of CDI has increased over the last decade in the United States from 4.5 to 8.2 incidents per 1000 hospital discharges [1], and epidemic strains, such as the North American pulsed-field type 1/restriction endonuclease analysis type B1/ribotype 027 (BI/NAP1/027) strain, have emerged [3, 4]. Furthermore, in a large US study, CDI recurrence occurred in ~14%–21% of patients after treatment of the initial CDI episode [5]. As a consequence of the incidence and impact of CDI, there remains a significant unmet need for additional CDI therapies, particularly those able to decrease posttherapy recurrence rates and increase sustained response.
Surotomycin ([SUR] CB-183,315; MK-4261) is a novel cyclic lipopeptide in development for the treatment of CDI. In phase 1 clinical trials, SUR ≤1000 mg twice daily (BID) had only modest disruptive effects on healthy gut microbiota, sparing the natural barrier to C difficile colonization [6]. In phase 2 clinical trials, CDI cure rates were similar for SUR 125 mg BID, 250 mg BID, and oral vancomycin (VAN) 125 mg 4 times daily (QID). Clostridium difficile infection recurrence rates were significantly lower after SUR 250 mg BID compared with VAN 125 mg QID [7]. The main objectives of this phase 3 clinical trial were to demonstrate the safety and efficacy of SUR 250 mg BID versus VAN 125 mg QID in adults with CDI.
MATERIAL AND METHODS
Trial Design
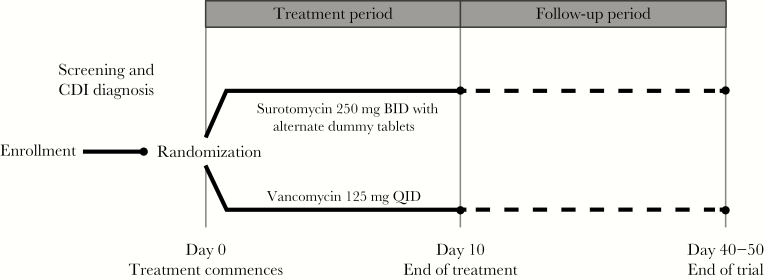
This randomized, double-blind, active-controlled trial, consisting of 2 treatment arms (protocol MK-4261-005; ClinicalTrials.gov identifier NCT01597505), was conducted between July 28, 2012 and March 20, 2015 in accordance with the Declaration of Helsinki, Good Clinical Practice Guidelines, and the approval of local institutional review boards. All subjects provided written informed consent. The trial consisted of a 10-day treatment period (Day 1–10) and follow-up visits 2 days after end of treatment ([EOT] Day 10–13), Day 24 ± 3 days, and Day 40–50. During the treatment period, subjects received oral SUR 250 mg BID with alternate dummy tablets or the active comparator, oral VAN 125 mg QID. The primary endpoint of the trial was clinical response at EOT. Key secondary endpoints were clinical response over time and sustained clinical response 30–40 days post-EOT.
Trial Population
Subjects were enrolled at 115 sites in North America (51 sites), Europe (62 sites), and the Middle East (2 sites). Eligible subjects were ≥18 and <90 years of age, had diarrhea with a minimum of 3 unformed bowel movements (UBMs), or >200 mL volume of stool (ie, for those with a collection device) over a 24-hour period before randomization/treatment administration. Furthermore, subject stool samples must have been C difficile toxin-positive within 48 hours of treatment administration, as identified by enzyme immunoassay (EIA), polymerase chain reaction (PCR), or cell culture cytotoxin neutralization assay. Subjects with toxic megacolon and/or small bowel ileus or those who had received a fecal transplant or treatment for the current episode of CDI were excluded. Other exclusion criteria included subjects with >2 episodes of CDI within 90 days of trial therapy, a history of inflammatory bowel disease (eg, ulcerative colitis, Crohn’s disease, or microscopic colitis), a positive stool culture for other enteropathogens, life-threatening illness at the time of enrollment as measured by a score of 4 using a modified Horn’s index [8], or a life expectancy of <8 weeks.
Randomization and Treatment
Subjects were randomly assigned to treatment arms (1:1; Figure 1) based on a centralized computer-generated randomization schedule and stratified by age (<75 or ≥75 years of age) and number of prior CDI episodes (0 or ≥1) in the last 90 days. Subjects, trial staff, and the sponsor remained blinded until the database was locked. An interactive voice/web response system was used for allocation of identification numbers and treatment kits.
Figure 1.
Trial design. BID, twice daily; CDI, Clostridium difficile infection; QID, 4 times daily.
Either SUR 250 mg BID or VAN 125 mg QID was administered according to the randomized allocation. In the SUR treatment arm, additional placebo dummy treatments were included to match the QID dosing regimen of VAN. Commercially available oral VAN tablets were backfilled with microcrystalline cellulose as a filler to prevent rattling and maintain the blind. During the 10-day treatment period, a single capsule was administered at breakfast, lunch, dinner, and bedtime.
Clinical Outcomes
All subjects were evaluated for clinical response 2 days after the last dose of study drug, and all subjects continued to be assessed throughout the course of the study (ie, through the late follow-up visit, on Day 40–50). A favorable clinical response (cure) was defined as resolution of diarrhea (ie, ≤2 loose stools per 24 hours for 2 consecutive days) and no need for additional CDI treatment after the trial treatment period. If the treatment did not achieve a favorable clinical response, the outcome was deemed a failure. After initial cure, sustained clinical response was identified if there was no recurrence of CDI before the final follow-up visit (Day 40–50). Recurrence was identified by a minimum of 3 UBMs over a 24-hour period (or >200 mL stool volume over 24 hours—for those with a collection device) and positive detection of toxigenic C difficile from a stool sample.
Microbiology
Baseline and recurrent diarrhea stool samples were analyzed onsite for the detection of C difficile toxin. Stool samples were also analyzed for the presence of B1/NAP1/027-positive epidemic strains of C difficile.
Pharmacokinetic Evaluations
A selection of sites participated in an intensive pharmacokinetic (PK) substudy. At these sites, intensive blood and stool sampling occurred on Day 5–7 over a dosing interval. Plasma and fecal SUR and VAN concentrations were determined by a validated liquid chromatography with tandem mass spectrometry method (Tandem Laboratories, West Trenton, NJ and Salt Lake City, UT).
Safety Assessments
Adverse events (AEs), defined by the protocol as nonserious, were collected from first dose of trial treatment until 7 days after the last dose. Serious AEs (SAEs) were collected from the first dose until 30 days after the last dose. Vital signs (eg, heart rate, blood pressure, and temperature), physical examination, electrocardiogram, and laboratory safety tests were measured throughout the trial. The safety population consisted of all randomized subjects who received any amount of study drug.
Data Analysis and Statistics
The 95%–95% fixed margin approach was used to justify the noninferiority based on a meta-analysis of the medical literature. The selected clinically relevant noninferiority margin of 10% was demonstrated to preserve 51.7% of the active control effect. Assuming an 83% clinical response rate for both treatment arms, 258 subjects per arm would have at least 85% power to demonstrate SUR noninferiority versus VAN based on the 10% noninferiority margin and at a 1-sided significance interval of 0.025. Given that an expected 85% of randomized subjects would meet criteria to be included in the microbiological modified intent-to-treat population ([mMITT] all randomized subjects with a confirmed diagnosis of CDI), a total of 304 subjects per arm were enrolled.
Noninferiority and superiority tests were conducted for clinical outcomes in the mMITT population. Two-sided 95% confidence intervals (CIs) were calculated for favorable clinical outcomes associated with the treatments. Noninferiority was identified when the difference between SUR and VAN arms 95% CI was ≥ −10% and superior when the difference was >0%. Analyses were stratified by age (<75 or ≥75 years of age) and previous episodes of CDI in the last 90 days (0 or ≥1). The Kaplan-Meier method with a stratified log-rank P value was used to determine clinical response over time. All statistical tests were 2-sided and conducted at the 0.05 significance level. Descriptive statistics were provided for continuous data. The PK analysis population consisted of subjects who provided at least 3 serial plasma samples postdosing on Day 5–7. Individual plasma PK parameters were determined by noncompartmental analysis using WinNonlin® (version 6.3 or higher; Pharsight Corporation, Mountain View, CA).
RESULTS
Trial Population
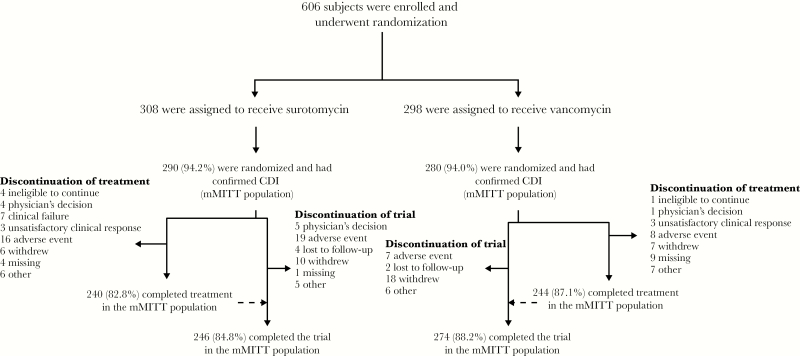
A total of 606 subjects were enrolled and randomized, 570 of whom had confirmed CDI (ie, 290 in the SUR arm and 280 in the VAN arm) and were included in the mMITT population (Figure 2). The baseline and demographic characteristics of the mMITT population are shown in Table 1. The characteristics of the treatment groups were similar. Study drug completion rates in the mMITT population were 82.8% and 87.1% for SUR and VAN, respectively.
Figure 2.
Subject flow diagram. CDI, Clostridium difficile infection; mMITT, microbiological modified intent to treat.
Table 1.
Trial Population Demographic and Baseline Characteristicsa
| Characteristic | Treatment Arm | Total (N = 570) | |
|---|---|---|---|
| Surotomycin (N = 290) | Vancomycin (N = 280) | ||
| Female, n (%) | 117 (40.3) | 114 (40.7) | 231 (40.5) |
| Race, n (%) | |||
| Black or African American | 15 (5.2) | 22 (7.9) | 37 (6.5) |
| White | 260 (89.7) | 248 (88.6) | 508 (89.1) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 18 (6.2) | 9 (3.2) | 27 (4.7) |
| Not Hispanic or Latino | 266 (91.7) | 264 (94.3) | 530 (93.0) |
| Age at first dose (years) | |||
| Mean (SD) | 61.1 (17.6) | 61.5 (18.4) | 61.3 (18.0) |
| Median (range) | 64.0 (18‒89) | 64.5 (18‒89) | 64.0 (18‒89) |
| <75 years, n (%) | 211 (72.8) | 200 (71.4) | 411 (72.1) |
| Body mass index (kg/m2) | |||
| Mean (SD) | 26.4 (7.2) | 27.5 (6.7) | 26.9 (7.0) |
| Subject’s hospitalization status at baseline, n (%) | |||
| Inpatient | 178 (61.4) | 181 (64.6) | 359 (63.0) |
| Outpatient | 108 (37.2) | 90 (32.1) | 198 (34.7) |
| ICU status at baseline, n (%) | |||
| Yes | 11 (3.8) | 8 (2.9) | 19 (3.3) |
| CDI Epidemiologic Classification, n (%) | |||
| 1 ‒ HCF-onset, HCF-associated CDI | 92 (31.7) | 91 (32.5) | 183 (32.1) |
| 2 ‒ Community-onset, HCF-associated CDI | 53 (18.3) | 53 (18.9) | 106 (18.6) |
| 3 ‒ Community-associated CDI | 121 (41.7) | 103 (36.8) | 224 (39.3) |
| 4 ‒ Indeterminate disease | 24 (8.3) | 33 (11.8) | 57 (10.0) |
| Severe disease, n (%) | |||
| ESCMID Comprehensive Criteria | 215 (74.1) | 209 (74.6) | 424 (74.4) |
| ESCMID Abbreviated Criteria | 1 (0.3) | 0 | 1 (0.2) |
| IDSA Criteria | 9 (3.1) | 15 (5.4) | 24 (4.2) |
| UBM and WBC Criteria | 95 (32.9) | 96 (34.3) | 191 (33.6) |
| Horn’s Index | 47 (16.4) | 45 (16.3) | 92 (16.4) |
| ≥1 previous episode of CDI | 49 (17.1) | 51 (18.5) | 100 (17.8) |
| BI/NAP1/027 strain-positive | 59 (23.1) | 67 (27.2) | 126 (25.1) |
| Mean number of UBMs at baseline (SD) | 7.1 (4.6) | 6.6 (4.3) | 6.9 (4.5) |
Abbreviations: CDI, Clostridium difficile infection; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; HCF, healthcare facility; ICU, intensive care unit; IDSA, Infectious Diseases Society of America; SD, standard deviation; UBM, unformed bowel movements; WBC, white blood cells.
Baseline values were taken as the last nonmissing result before first administration of the study drug. Therefore, some numbers do not represent the total number of initially enrolled subjects.
Efficacy
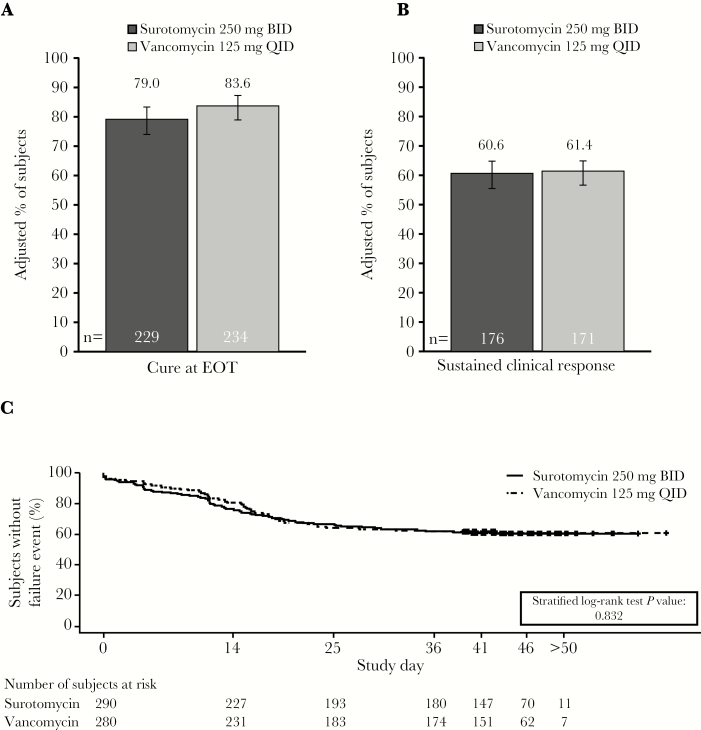
Clinical cure at EOT (Day 1–13) was observed in 79.0% (229 of 290) of subjects after SUR treatment and 83.6% (234 of 280) of subjects after VAN. The primary noninferiority endpoint, clinical cure at EOT, was not met. The difference between clinical response rates (SUR–VAN) was −4.6% (95% CI, −11.0 to 1.9) (Figure 3A). Neither of the 2 key secondary endpoints were met; SUR did not demonstrate superiority over VAN for the sustained clinical response at the end of the trial (SUR 60.6% vs VAN 61.4%; difference: −0.8%; 95% CI, −8.8 to 7.1) (Figure 3B) or for clinical response over time (defined as clinical response through EOT and the sustained clinical response from EOT to Day 40; stratified log-rank test P = .832) (Figure 3C).
Figure 3.
Clinical outcomes according to treatment (microbiological modified intent to treat population). (A) Clinical cure rate at EOT (Day 10), (B) sustained clinical response at the end of the trial (Day 40–50), and (C) Kaplan-Meier analysis of clinical response over time. The estimated adjusted proportions were weighted averages across all strata, constructed using Mehrotra-Railkar continuity-corrected minimum-risk stratum weights. Sustained clinical response was defined as a subject deemed a cure at EOT who did not have recurrence, did not die, and was not lost to follow-up. BID, twice daily; EOT, end of treatment; QID, 4 times daily.
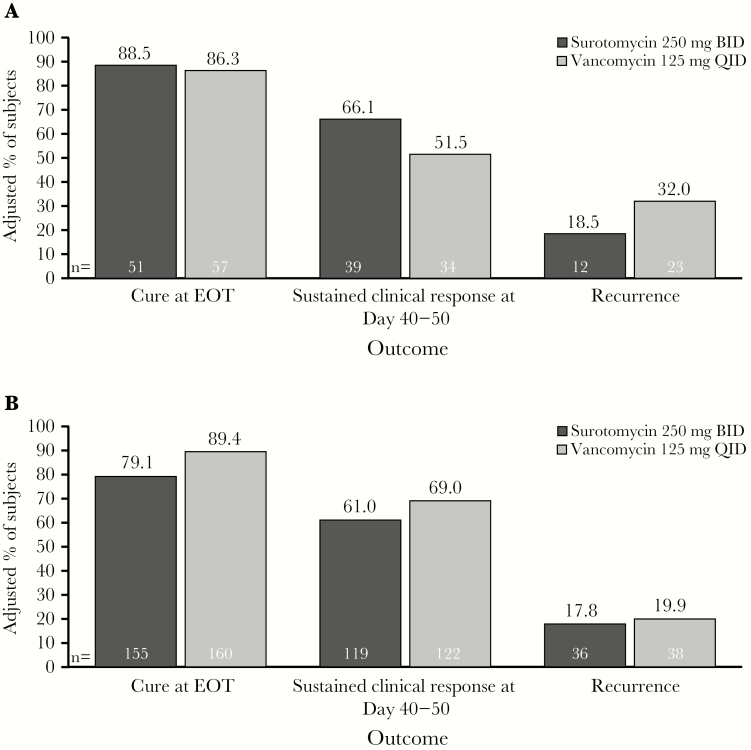
Recurrence (ie, subjects who were cured and had a CDI recurrence or were lost to follow-up, died, or had their last follow-up contact before Day 40) occurred in 53 subjects (17.7%) in the SUR arm and in 63 subjects (21.2%) in the VAN arm (difference −3.5%; 95% CI, −10.0 to 3.0). A Kaplan-Meier analysis demonstrated that time to resolution of diarrhea (ie, ≤2 UBM per 24-hour period) was similar between groups (SUR vs VAN; P = .431). Although the Kaplan-Meier analysis of time to diarrhea reappearance (ie, ≥3 UBM per 24-hour period) in subjects who had a clinical response with SUR versus those with VAN initially supported SUR superiority (P = .011), when adjusted for multiplicity, this efficacy endpoint was not met. In subjects infected with the C difficile BI/NAP1/027 strain at baseline, the cure rate and sustained clinical response rate were numerically higher and recurrence rates were lower with SUR versus VAN (Figure 4A). However, none of these comparisons were statistically significant. In subjects infected at baseline with a non-BI/NAP1/027 strain, cure at the EOT and sustained clinical response were lower and recurrence was higher with SUR compared with VAN, and the upper limit of the 95% CI was <0 (Figure 4B). Response rates at EOT were numerically lower for both treatments in the population identified as toxin positive by EIA (cure rates: SUR, 77.2% [95% CI, 69.1%–83.8%]; VAN, 79.8% [95% CI, 71.3%–86.3%]) versus PCR (cure rates: SUR, 80.2% [95% CI, 73.4%–85.6%]; VAN, 86.0% [95% CI, 80.0%–90.4%]).
Figure 4.
Clinical responses in subjects with infections deemed to be caused by (A) Clostridium difficile BI/NAP1/027 and (B) non-BI/NAP1/027 C difficile strains at baseline (microbiological modified intent to treat population). The estimated adjusted proportions were weighted averages across all strata, constructed using Mehrotra-Railkar continuity-corrected minimum-risk stratum weights. BID, twice daily; EOT, end of treatment; QID, 4 times daily.
Pharmacokinetics of Surotomycin
A total of 18 subjects receiving SUR from selected clinical sites were included in the PK substudy population. The median (range) fecal concentration of SUR was 1216 µg/g (range, 0–3780 µg/g). Most subjects had SUR fecal concentrations greater than the minimum inhibitory concentration of 1 µg/mL at which the growth of 90% of the C difficile is inhibited, suggesting that adequate therapeutic fecal SUR concentrations were achieved [9]. Plasma PK parameters of SUR (n = 18 subjects) and VAN (n = 6) were also calculated and showed rapid SUR absorption with median time to maximum concentration of 1 hour postdose with detectable plasma concentrations until ~12 hours after dosing (Supplementary Table 1).
Safety
Treatment-emergent AEs (TEAEs) were experienced by 148 (48.5%) of SUR-treated subjects and 158 (55.2%) of VAN-treated subjects. The proportion of subjects with at least 1 AE, study drug-related AEs, or at least 1 SAE was generally similar between treatments (Table 2). All except 2 SAEs (perforated jejunal ulcer and cardiac arrest with ventricular fibrillation; both in the SUR group) were considered unrelated to the study drug. The percentage of subjects with AEs leading to discontinuation or death was numerically higher in the SUR arm compared with VAN; however, none of the deaths were considered treatment-related. The majority of TEAEs in both treatment arms (SUR, 75%; VAN, 86%) were mild or moderate in intensity.
Table 2.
Overall Summary of Treatment-Emergent AEs (All Randomized Subjects, Irrespective of CDI Confirmation Status)a
| Category | Surotomycin (N = 305) n (%) |
Vancomycin (N = 286) n (%) |
|---|---|---|
| Subjects with at least 1 AE | 148 (48.5) | 158 (55.2) |
| AEs by maximum severityb | ||
| Mild | 68 (22.3) | 84 (29.4) |
| Moderate | 43 (14.1) | 52 (18.2) |
| Severe | 37 (12.1) | 22 (7.7) |
| AEs by strongest relationship to study drugc | ||
| Not related | 124 (40.7) | 132 (46.2) |
| Related | 24 (7.9) | 26 (9.1) |
| At least 1 SAE | 44 (14.4) | 37 (12.9) |
| At least 1 treatment-related SAE | 2 (0.7) | 0 |
| AE causing discontinuation of study drug | 17 (5.6) | 8 (2.8) |
| Treatment-related AE causing discontinuation of study drug | 4 (1.3) | 0 |
| Death | 18 (5.9) | 9 (3.1) |
| Treatment-related death | 0 | 0 |
Abbreviations: AE, adverse events; CDI, Clostridium difficile infection; SAE, serious AEs.
A treatment-emergent AE was defined as any AE occurring from the first dose of study drug through the last study evaluation that was new in onset or was a pre-existing condition that was aggravated in severity or frequency.
Subjects were counted only once with the most severe event. AEs with a missing severity were analyzed as severe.
Subjects were counted only once with the strongest relationship to study drug. AEs with missing relationship were analyzed as related.
The highest frequency of AE (System Organ Class) was in gastrointestinal disorders followed by infections and infestations, the majority of which were urinary tract infections (Table 3). The most common TEAEs (Preferred Term) that were deemed drug-related by the investigator were nausea (SUR, 2.0%; VAN, 1.7%), vomiting (SUR, 1.3%; VAN, 0%), and increased alanine aminotransferase (ALT) (SUR, 1.0%; VAN, 2.1%).
Table 3.
Summary of TEAEs by MedDRA System Organ Class (Incidence ≥5%), Preferred Term (Incidence ≥2%), and Relationship to Study Drug (All Randomized Subjects, Irrespective of CDI Confirmation Status)a
| System Organ Class Preferred Term | Surotomycin (N = 305) |
Vancomycin (N = 286) |
||
|---|---|---|---|---|
| Related n (%) | Not Related n (%) | Related n (%) | Not Related n (%) | |
| Subjects with at least 1 TEAE | 24 (7.9) | 124 (40.7) | 26 (9.1) | 132 (46.2) |
| Cardiac disorders | 1 (0.3) | 17 (5.6) | 0 | 11 (3.8) |
| Gastrointestinal disorders | 13 (4.3) | 59 (19.3) | 12 (4.2) | 52 (18.2) |
| Abdominal pain | 2 (0.7) | 14 (4.6) | 3 (1.0) | 3 (1.0) |
| Constipation | 0 | 6 (2.0) | 1 (0.3) | 3 (1.0) |
| Diarrhea | 0 | 10 (3.3) | 0 | 14 (4.9) |
| Nausea | 6 (2.0) | 14 (4.6) | 5 (1.7) | 17 (5.9) |
| Vomiting | 4 (1.3) | 6 (2.0) | 0 | 10 (3.5) |
| General disorders and administration-site conditions | 1 (0.3) | 24 (7.9) | 1 (0.3) | 24 (8.4) |
| Edema peripheral | 0 | 4 (1.3) | 0 | 7 (2.4) |
| Infections and infestations | 0 | 45 (14.8) | 1 (0.3) | 40 (14.0) |
| Urinary tract infection | 0 | 15 (4.9) | 0 | 7 (2.4) |
| Investigations | 5 (1.6) | 18 (5.9) | 10 (3.5) | 19 (6.6) |
| Alanine aminotransferase increased | 3 (1.0) | 4 (1.3) | 6 (2.1) | 5 (1.7) |
| Metabolism and nutrition disorders | 1 (0.3) | 21 (6.9) | 2 (0.7) | 20 (7.0) |
| Dehydration | 0 | 4 (1.3) | 0 | 6 (2.1) |
| Hypokalemia | 0 | 5 (1.6) | 0 | 7 (2.4) |
| Musculoskeletal and connective tissue disorders | 0 | 8 (2.6) | 2 (0.7) | 17 (5.9) |
| Arthralgia | 0 | 0 | 0 | 6 (2.1) |
| Nervous system disorders | 1 (0.3) | 21 (6.9) | 4 (1.4) | 26 (9.1) |
| Headache | 1 (0.3) | 11 (3.6) | 1 (0.3) | 17 (5.9) |
Abbreviations: CDI, Clostridium difficile infection; MedDRA, Medical Dictionary for Regulatory Activities; TEAEs; treatment-emergent adverse events.
AEs with the relationship to study drug missing were considered to be related in this table. Subjects who experienced more than 1 event were counted only once per System Organ Class and Preferred Term using the greatest relationship to study drug. System Organ Classes and Preferred Terms were based on MedDRA dictionary, version 15.0.
Nine subjects experienced protocol-specified Closely Monitored Events: 1 subject in the SUR arm with creatine phosphokinase (CPK) levels >1000 U/L post-baseline; 1 subject in the VAN arm with ALT or aspartate aminotransferase (AST) >8 x upper limit of normal (ULN); 2 subjects in each treatment arm with ALT or AST >3 × ULN and total bilirubin >2 × ULN; and 5 subjects (SUR arm: 2, VAN arm: 3, of which 1 subject in each group had concurrent total bilirubin >2 × ULN, as described above) demonstrated ALT or AST >3 × ULN with concurrent fatigue, nausea, vomiting, right upper-quadrant pain or tenderness, fever, rash, or eosinophilia. No individual or population trends in ALT, AST, total bilirubin, or CPK levels were noted. None of the 4 subjects with ALT or AST >3 × ULN and total bilirubin >2 × ULN were deemed related to the study drug or were consistent with fulfilling Hy’s Law [10].
There were no clinically relevant trends in mean change from baseline for vital signs or hematology or chemistry parameters in either treatment group. The proportion of subjects with QTcF or QTcB (Fridericia’s or Bazett’s corrected QT interval, respectively) values ≥500 ms during the trial was comparable across groups (QTcF: SUR 3.0%, VAN 3.4%; QTcB: SUR 5.9%, VAN 5.0%).
DISCUSSION
Given the burden of CDI and its recurrence, clinical development of new CDI therapeutics remains an urgent unmet medical need. The data from this phase 3 trial, which was conducted to assess the safety and efficacy of SUR relative to VAN, demonstrated that SUR did not meet the primary efficacy endpoint (clinical cure at EOT) of noninferiority compared with VAN, nor did it meet the key secondary efficacy endpoints of clinical response over time and sustained clinical response superiority over VAN. Surotomycin demonstrated reduced CDI recurrence rates and improved clinical response for subjects with baseline BI/NAP1/027-positive samples compared with VAN, but these differences were not statistically significant.
Compared with the SUR phase 2 trial results [7], the CDI clinical cure rates in the current trial were lower across treatment groups (86.6% and 89.4% for SUR 250 mg and VAN, respectively, in the phase 2 trial; 79.0% and 83.6%, respectively, in the current trial). In addition, the CDI recurrence rates were lower and clinical failure rates were higher in the current trial for both treatments compared with the SUR phase 2 trial [7]. The observed differences in these efficacy parameters may be due to the different inclusion criteria of the trials (phase 2 trial: ≥4 UBMs were required for inclusion; current trial: ≥3 UBMs) as well as differences in the definition of cure (phase 2 trial: defined as <4 UBMs per 24-hour period for at least 2 consecutive days; current trial: ≤2 UBMs per 24-hour period for at least 2 consecutive days). Furthermore, in both the phase 2 and current trials, CDI diagnosis required only 1 toxin-positive result. More importantly, these diagnostic 1-step procedures reflected recommended methods at the time of protocol development. The most current CDI diagnosis recommendations include a 2-step procedure [11].
Overall, the cure rates at EOT, sustained clinical response, and CDI recurrence for VAN were similar to those of a previous phase 3 noninferiority trial for fidaxomicin [12]. In this current trial, the sustained clinical response for both VAN and SUR was ~61% by the end of the trial (Day 40–50); however, in the SUR phase 2 trial, sustained clinical response rates of 66.7% and 70.1% were reported for SUR 125 mg BID and 250 mg BID, respectively [7, 12]. The lower sustained clinical response rate in the current study may be due to the longer follow-up period used in this trial (30–40 days) compared with previous investigations (28 days) [7, 12].
The proportion of subjects from whom the epidemic BI/NAP1/027 strain was isolated (~25%) was slightly lower than in previous trials, which report values closer to one third [7, 12]. In the current trial, among BI/NAP1/027 strain-infected subjects, there was a higher clinical cure rate at EOT, sustained clinical response at the end of the trial, and lower recurrence rate in the SUR group than the VAN group. Vancomycin administration was associated with more favorable outcomes for these 3 efficacy parameters than SUR for non-BI/NAP1/027-infected subjects (Figure 4A and B). In contrast, in the phase 2 trial, a numerically higher clinical cure rate was seen in the VAN group than in the SUR 250 mg group for subjects infected with BI/NAP1/027 strain (91.3% vs 71.4%); however, the clinical cure rates were comparable for non-BI/NAP1/027 strain-infected subjects in both groups (SUR, 91.5%; VAN, 89.5% [7]). The differences in the BI/NAP1/027 strain-specific efficacy results may have contributed to the overall differences between the phase 2 and phase 3 SUR trial outcomes.
Throughout the current trial, SUR was generally well tolerated. The percentage of subjects with at least 1 AE and of drug-related AEs in this trial was similar between the 2 treatment groups. The number of deaths and discontinuations due to AEs was numerically higher in the SUR group than in the VAN group. None of the deaths were deemed treatment-related by the investigators.
Sparing of the Gram-negatives and bacteroidetes during CDI therapy is hypothesized to favor recovery of a healthy microbiota and to lower CDI recurrence [13]. However, in this trial, the in vitro SUR spectrum of activity and modest perturbation of gut microbiota in vivo [6] was not associated with decreased CDI recurrence rates. After the unfavorable phase 3 data, the SUR development program was discontinued.
CONCLUSIONS
Although SUR 250 mg BID was generally well tolerated, it did not meet the primary efficacy endpoint of noninferiority versus VAN for clinical cure at EOT or either of the key secondary endpoints, ie, superiority of SUR to VAN for clinical response over time and sustained clinical response at the end of the trial. Approximately 40% of subjects ultimately failed CDI therapy for both SUR and VAN at the end of the follow-up period.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
The trial and statistical analyses were performed by INC Research and Cubist Legacy before acquisition by Merck & Co., Inc.
Medical writing assistance was provided by Dr. Edward Rochford (Complete Medical Communications, Hackensack, NJ). This assistance was funded by Merck & Co., Inc., Kenilworth, NJ.
Financial support. This work was supported by Merck & Co., Inc. Kenilworth, NJ.
Potential conflicts of interest. V. B. has acted as a consultant for, and received payments for talks from, AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. K. M. M. has been a clinical trial investigator and participated in advisory boards for Merck & Co., Inc., for surotomycin and other products. U. S., M. J., A. A., L. C., D. G., K. B. L., and Y. M. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, and may own stock and/or stock options.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001–2010. Am J Infect Control 2014; 42:1028–32. [DOI] [PubMed] [Google Scholar]
- 2. Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis 2012; 55(Suppl 2):S65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med 2008; 359:1932–40. [DOI] [PubMed] [Google Scholar]
- 4. O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–24. [DOI] [PubMed] [Google Scholar]
- 5. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Citron DM, Tyrrell KL, Dale SE, et al. Impact of surotomycin on the gut microbiota of healthy volunteers in a phase 1 clinical trial. Antimicrob Agents Chemother 2016; 60:2069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee CH, Patino H, Stevens C, et al. Surotomycin versus vancomycin for Clostridium difficile infection: phase 2, randomized, controlled, double-blind, non-inferiority, multicentre trial. J Antimicrob Chemother 2016; 71:2964–71. [DOI] [PubMed] [Google Scholar]
- 8. Arora V, Kachroo S, Ghantoji SS, et al. High Horn’s index score predicts poor outcomes in patients with Clostridium difficile infection. J Hosp Infect 2011; 79:23–6. [DOI] [PubMed] [Google Scholar]
- 9. Snydman DR, Jacobus NV, McDermott LA. Activity of a novel cyclic lipopeptide, CB-183,315, against resistant Clostridium difficile and other Gram-positive aerobic and anaerobic intestinal pathogens. Antimicrob Agents Chemother 2012; 56:3448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Food and Drug Administration. Guidance for Industry: Drug-Induced Liver Injury: Premarketing Clinical Evaluation. Available at: http://www.fda.gov/downloads/Drugs/.../Guidances/UCM174090.pdf Accessed 25 August 2016
- 11. Crobach MJ, Planche T, Eckert C, et al. European Society of Clinical Microbiology and Infectious Diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 2016; 22(Suppl 4):S63–81. [DOI] [PubMed] [Google Scholar]
- 12. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 13. Seekatz AM, Young VB. Clostridium difficile and the microbiota. J Clin Invest 2014; 124:4182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.