Abstract
Mixed chimerism is a promising approach to inducing allograft and xenograft tolerance. Mixed allogeneic and xenogeneic chimerism in mouse models induced specific tolerance and global hyporesponsiveness, respectively, of host mouse NK cells. In this study, we investigated whether pig/human mixed chimerism could tolerize human NK cells in a humanized mouse model. Our results showed no impact of induced human NK cell reconstitution on porcine chimerism. NK cells from most pig/human mixed chimeric mice showed either specifically decreased cytotoxicity to pig cells or global hyporesponsiveness in an vitro cytotoxicity assay. Mixed xenogeneic chimerism did not hamper the maturation of human NK cells, but was associated with an alteration in NK cell subset distribution and IFN-γ production in the bone marrow. In summary, we demonstrate that mixed xenogeneic chimerism induces human NK cell hyporesponsiveness to pig cells. Our results support the use of this approach to inducing xenogeneic tolerance in the clinical setting. However, additional approaches are required to improve the efficacy of tolerance induction while assuring adequate NK cell functions.
Introduction
The use of xenogeneic organs could solve the severe shortage of organs for transplantation (1, 2). The pig is considered a promising candidate as a potential source animal (1, 2). Despite the progress in recent years (3–6), robust immunological rejection remains a major obstacle to xenotransplantation (7). An attractive approach to preventing xenograft rejection is tolerance induction, so that the human immune system is specifically unresponsive to the pig xenografts (1, 2, 8), avoiding the use of long-term immunosuppression while preserving the ability of the immune system to respond to pathogens.
Mixed chimerism is a state in which host and donor hematopoietic cells coexist (9). The achievement of sustained mixed xenogeneic chimerism by hematopoietic cell transplantation has been shown to prevent xenograft rejection in mouse models (10). Mixed xenogeneic chimerism in the rat→mouse and pig→mouse models leads to the tolerization of T cells and in rat→mouse chimeras, of B cells, which are the major cell types mediating xenograft rejection (11–15). Natural Killer (NK) cells have been implicated in xenograft rejection in rodents (16, 17) and primates (18, 19). We have previously shown in a mixed allogeneic chimerism model that specific tolerance of host NK cells could be induced (20). In a rat→mouse xenogeneic transplantation model we demonstrated that mixed xenogeneic chimerism induced host global unresponsiveness of NK cells, as they were unable to reject either donor rat or β2m (class I MHC)-deficient mouse bone marrow cells (21). Currently, it is unclear whether mixed chimerism can induce human NK cell tolerance to pig xenografts.
In this study we address this question using a humanized mouse model where pig and human mixed hematopoietic chimerism is induced (22). Our results show that induction of human NK cell development in pig/human mixed chimeras does not affect pig chimerism. Human NK cells from the majority of pig/human mixed chimeric mice show a trend of either specific loss of cytotoxicity to pig cells or global hyporesponsiveness. These data indicate that mixed xenogeneic hematopoietic chimerism can downregulate responses of human NK cells to pig cells.
Materials and Methods
Animals and tissues
NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl) and porcine cytokine-transgenic NSG mice (PCT-NSG, expressing pig IL-3, GM-CSF and stem cell factor) purchased from Jackson Laboratory (Bar Harbor, ME) were housed and bred in a specific pathogen-free environment. Human fetal liver tissues (gestational age, 17–23 weeks) were obtained from Advanced Bioscience Resource (Alameda, CA). Swine bone marrow cells were harvested from adult SLA-defined miniature swine (provided by Dr. David H. Sachs, Massachusetts General Hospital). Studies were approved by Institutional Review Boards and Animal Care and Use Committees at Columbia University.
Human fetal and porcine tissue transplantation
NSG or PCT-NSG mice (6–10 weeks old) were sublethally irradiated (1–1.2 Gy) by an X-Ray irradiator (RS-2000, Rad Source Technologies, Inc., Suwanee, GA). To generate pig/human mixed chimeric mice, fresh or cryopreserved pig bone marrow cells (1×108/mouse, SLA-DD) were injected to irradiated PCT-NSG mice followed by injection of fresh or cryopreserved fetal liver-derived CD34+ cells (1–2×105/mouse) through the tail vein 3 days later. To generate non-chimeric humanized mice, CD34+ human fetal liver cells (1–2×105) were injected alone.
Flow cytometry (FCM)
Levels of pig and human hematopoietic chimerism in transplanted mice were assessed by multicolor flow cytometry as described (23). Mice were tail bled at regular intervals after transplantation. Fluorochrome-labeled monoclonal antibodies were purchased from Biolegend (San Diego, CA), or BD Pharmingen (San Josa, CA) or obtained from Massachusetts General Hospital. FCM analysis was performed using a FACSCantoII or LSRII (BD), and data was analyzed by FlowJo software (TreeStar, Ashland, OR). Humanized mice were defined as chimeric when ≥0.003% pan pig-positive cells were found within peripheral blood white blood cells or white blood cells in organs (Supplemental Fig. 1). Human NK cells from humanized mice were defined as human CD45+CD19−CD14−CD3−CD56+ cells.
Induction of human NK cell reconstitution
Human NK cell reconstitution was induced 14 weeks following injection of fetal liver CD34+ cells, when porcine and human reconstitution were complete. Humanized mice first received hydrodynamic injection of plasmid encoding human Flt3L (50μg/mouse, Day 0) followed by injection of recombinant human IL-15 (rhIL-15) (Gemini Bio-Products, West Sacramento, CA)/recombinant human IL-15 receptor alpha Fc chimeric protein (rhIL-15Ralpha-Fc) (R&D Systems, Minneapolis, MN) complex 3, 7, 11, and 13 days later. To generate the complex, rhIL-15 and rhIL-15Ralpha-Fc were incubated at a ratio by weight of 1:2 in 100μL of PBS at 37°C for 30 minutes (24). Mice were injected intravenously with rhIL-15/rhIL-15Ralpha-Fc complex (0.5μg rhIL-15 and 1 μg rhIL-15Ralpha-Fc/mouse/injection). For simplicity, this complex is referred to as “IL-15” throughout this paper. Plasmid encoding human Flt3L was a gift from Dr. Jianzhu Chen (MIT, MA) and was prepared as described (25).
Human NK cell purification
Humanized mouse splenocytes were prepared and human NK cells were enriched using anti-human CD56 microbeads (Miltenyi Biotec). NK cells were enriched from human peripheral blood mononuclear cells (PBMCs) by depletion of human T cells using anti-human CD3-biotin antibody and anti-biotin microbeads followed by positive selection with anti-human CD56 microbeads. Purity of NK cells ranged 70–90%.
In vitro NK cytotoxicity assay
In initial experiments, cytotoxicity of NK cells was determined by the standard chromium (51Cr) release assay as described (21). Briefly, splenic NK cells isolated from chimeric and non-chimeric humanized mice in triplicate were serially diluted and co-incubated with various 51Cr-labeled target cells for 4 hours. Because flat or irregular titrations were often observed with chromium release assays of NK activity in our hands, we adapted a radioisotope-free method of measuring NK cell cytotoxicity that has previously been used T cell cytotoxicity assays (26). NK cell targets, including pig lymphoblasts, K562 cells and NOD mouse lymphoblasts, were stained with 5μM CSFE (see Supplemental Methods for details of generation of lymphoblasts). Human B cells, isolated from humanized mouse splenocytes or human peripheral blood, were stained with 0.5 μM CFSE and used as an internal control population. CFSE-labeled human B cells (CFSElow) were then mixed with NK cell targets (CFSEhigh) at a ratio of 1:1 and 1×104 of the mixed cells were incubated alone or with different numbers of human NK cells in duplicate in 200μL of medium for 4 hours. Cells were then harvested and analyzed by flow cytometry (see Supplemental Methods and Supplemental Fig. 2 for further details). Percentage of specific killing was calculated using the following formula as described (26):
Cytotoxicity was defined as measurable if the percentage of specific killing was >10%.
The ratio of the maximum killing of pig lymphoblasts to that of K562 cells by NK cells from the same mouse was used to represent the response status of the human NK cells. The ratios from non-chimeric and chimeric mice were compared. However, if the maximum killing of K562 cells by NK cells of the chimeric mouse was less than half that of the maximum killing of K562 cells by NK cells of the non-chimeric mice, NK cells were considered globally hyporesponsive and the mice with this characteristic were excluded from the comparison described above, as there were no instances of killing of pig lymphoblasts by NK cells that were hyporesponsive to K562 cells.
IFN-γ detection
Humanized mouse splenocytes or human PBMCs were stimulated with PMA (50ng/mL) and Ionomycin (500ng/mL) (Sigma) for 5 hours in the presence of Brefeldin A (Biolegend). Intracellular staining was then performed to detect human NK cell IFN-γ production. In some studies, human NK cells from human peripheral blood and humanized mouse spleen and bone marrow were co-cultured with pig lymphoblasts or K562 cells at a ratio of 1:1 for 4 hours and human NK cell IFN-γ production was determined using an IFN-γ capture assay kit (Miltenyi Biotec).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. Student’s t-test (two-tailed) or ANOVA was used for analyses. A p value of <0.05 was considered to be statistically significant. Data are presented as mean ± SEM (standard error of mean).
Results
Enhancing human NK cell reconstitution in humanized mice
Due to the absence of human IL-15 and the inability of human cells to respond to mouse IL-15 (27), reconstitution of human NK cells in humanized mice is very low (24, 27). We first characterized the human NK cell reconstitution induced by provision of human Flt3L and IL-15 in humanized mice. Humanized mice 14 weeks post-CD34 cell injection were given Flt3L and IL-15 (Methods and Materials). NK cells in various tissues were enumerated and their functions were analyzed (Fig. 1). Compared to control untreated or PBS-treated mice, mice receiving Flt3L and IL-15 showed a 2–6-fold increase in the percentages and absolute numbers of human NK cells (Fig. 2A). PMA/Ionomycin-induced production of IFN-γ by human NK cells from spleen of humanized mice was comparable to that produced by NK cells from human peripheral blood (Fig. 2B). Enriched human NK cells from the spleen of humanized mice were able to kill both K562 cells and pig lymphoblasts, while the killing of NOD lymphoblasts was very low (Fig. 2C). These data demonstrated that NK cells reconstituted in humanized mice were functionally intact and were able to kill xenogeneic pig cells, even though they had developed in the mouse xenogeneic environment. Furthermore, the human NK cells were unresponsive to the host, suggesting that they were either tolerant or unable to interact with mouse cells. The failure of normal human peripheral blood NK cells to kill NOD mouse lymphoblasts (Fig. 2C) is consistent with the latter possibility. Collectively, these data demonstrated that our humanized mouse model was suitable for investigation of the impact of mixed xenogeneic chimerism on the tolerance of human NK cells to pig cells.
Figure 1. Experimental design.
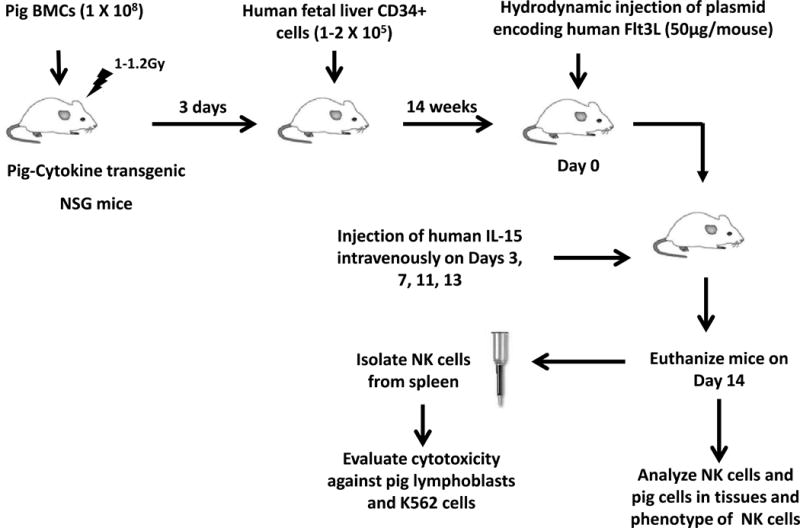
Pig cytokine-transgenic NSG (PCT-NSG) mice expressing pig IL3, GM-CSF and SCF were used. To generate pig/human mixed chimeric mice, pig bone marrow cells (1×108/mouse) were injected to irradiated PCT-NSG mice followed by intravenous injection of fresh or cryopreserved human fetal liver-derived CD34+ cells (1–2×105/mouse) through the tail vein. To generate non-chimeric humanized mice, PCT-NSG mice were injected with fresh or cryopreserved human fetal liver-derived CD34+ cells (1–2×105) alone. 14 weeks post-injection of CD34+ cells, hydrodynamic injection of plasmid encoding human Flt3L (50μg/mouse) was performed (Day 0) followed by intravenous injection of human IL-15 on Days 3, 7, 11 and 13. On Day 14, humanized mice were euthanized and NK cells were purified from the spleens using anti-human CD56 microbeads. Control NK cells were enriched from human PBMCs by depletion of human T cells using anti-human CD3-biotin antibody and anti-biotin microbeads followed by positive selection with anti-human CD56 microbeads. Purified human NK cells were then further used to evaluate cytotoxicity against pig lymphoblasts and K562 cells. Human NK cells and pig cells in various lymphoid tissues were analyzed by flow cytometry. For studies described in Figure 2, non-chimeric mice generated with injection of only human fetal liver-derived CD34+ cells were used.
Figure 2. Provision of exogenous human Flt3L and IL-15 enhances human NK cell reconstitution in humanized mice.
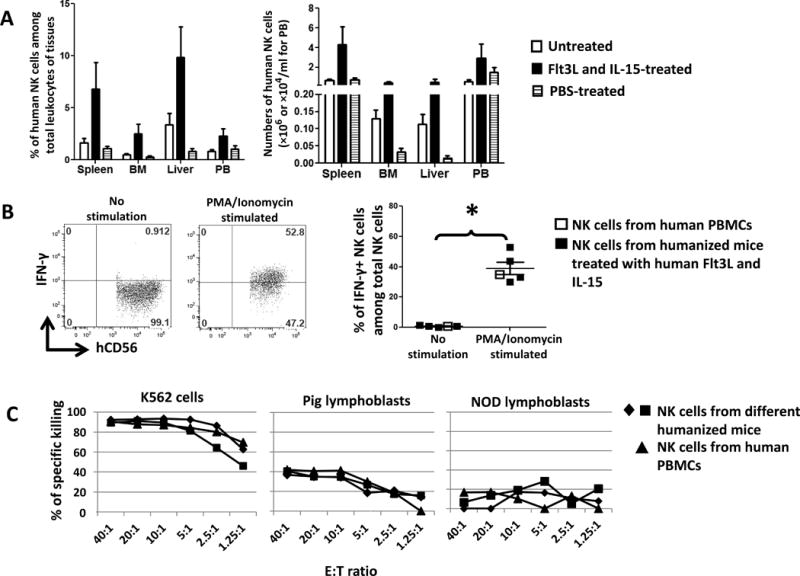
Humanized mice were generated by injection of human fetal liver-derived CD34+ cells to irradiated NSG mice. 14 weeks later, when mice were fully reconstituted by human cells, plasmid encoding human Flt3L was administered by hydrodynamic injection to these mice followed by injection of human IL-15. (A) Humanized mice without treatment (n=3) or treated with PBS (n=4) were used as controls for humanized mice treated with human Flt3L and IL-15 (n=4). Human NK cells in tissues were quantified. BM: bone marrow. PB: peripheral blood. Error bars represent SEM. *, p<0.05 with ANOVA, compared with PBS-treated group. (B) Splenocytes from humanized mice treated with human Flt3L and IL-15 were stimulated with PMA/Ionomycin for 5 hours in the presence of Brefeldin A followed by intracellular staining to determine the production of IFN-γ by human NK cells. Error bars represent SEM. *, p<0.05 with a Student’s t-test. NK cells from human PBMCs served as a control and were not included in the statistical analysis. (C) Human splenic NK cells from the spleen of humanized mice were isolated and their cytotoxic responses to pig lymphoblasts, K562 cells and NOD mice lymphoblasts were determined. Effector:target (E:T) ratio is shown on the x-axis. NK cells from human PBMCs served as a control. SP: spleen. BM: bone marrow. PB: peripheral blood.
Human NK cell reconstitution has no impact on pig/human mixed chimerism
We next investigated the impact of mixed xenogeneic hematopoietic chimerism on NK cell function and xenoresponsiveness. We reasoned that if human NK cells developing in the presence of pig/human mixed chimerism were tolerant to pig cells, inducing human NK cell reconstitution would not lead to a decrease in pig chimerism compared to that in pig/human mixed chimeric mice without induction of human NK cell reconstitution. To this end, we compared the pig chimerism in pig/human mixed chimeric humanized mice receiving and not receiving Flt3L and IL-15.
As shown in Fig. 3A, no significant differences in kinetics of pig peripheral blood chimerism were found between chimeric mice receiving Flt3L and IL-15 and control groups, although the numbers of pig cells in peripheral blood in all groups showed a gradual decrease over time. Similar numbers of pig cells were found in spleen, bone marrow and liver among chimeric mice receiving or not receiving Flt3L and IL-15 (Fig. 3B). Treatment with Flt3L and IL-15 had no direct effect on pig chimerism in mice that did not have a human immune system (Supplemental Fig. 3). While consistent with the possibility that human NK cells developing in the presence of mixed xenogeneic chimerism were hyporesponsive to pig cells, these data were inconclusive because of the loss of pig chimerism in control animals. Notably, mice receiving pig bone marrow cells alone had higher numbers of porcine cells in bone marrow and spleen than mixed chimeric mice (Fig. 3B), regardless of Flt3L and IL-15 treatment, indicating that human populations other than NK cells might destroy or out-compete pig hematopoietic cells in these tissues.
Figure 3. Human NK cell reconstitution has no apparent impact on pig/human mixed chimerism.
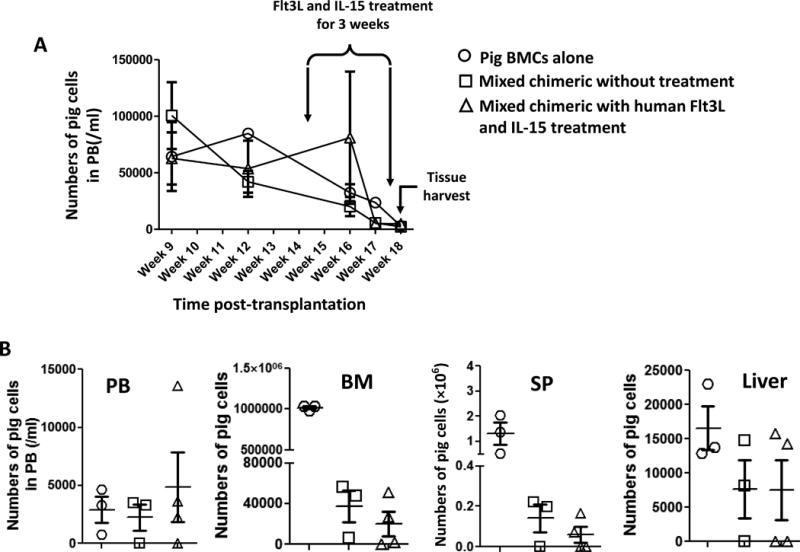
To determine the effects of induced human NK cell reconstitution on the persistence of pig chimerism, pig/human mixed chimeric humanized mice (n=4) were given plasmid encoding human Flt3L by hydrodynamic injection followed by injection of human IL-15 twice per week for 3 weeks. Pig/human mixed chimeric mice without Flt3L and IL-15 treatment (n=3) and mice transplanted with only pig bone marrow cells (n=3) were used as controls. (A) The pig chimerism in peripheral blood was followed and kinetics are shown. Timing of treatments to enhance human NK cell reconstitution and of tissue harvest for analysis are indicated. (B) After the mice were treated with human Flt3L and IL-15 for 3 weeks to induce human NK cell reconstitution, they were euthanized and pig cells in tissues were quantified. SP: spleen. BM: bone marrow. PB: peripheral blood. Each symbol represents an individual animal.
Mixed xenogeneic chimerism can lead to specific human NK cell unresponsiveness to pig cells or global hyporesponsiveness in vitro
We performed cytotoxicity assays to further determine the responses of human NK cells in pig/human mixed chimeric mice to pig cells (Fig. 1). We tested human NK cells from a total of 13 mixed chimeric mice in 4 separate experiments, including the experiment presented in Fig. 3. Treatment of chimeric and non-chimeric mice with human Flt3L and IL-15 led to increased percentages of human T cells and decreased percentages of human B cells in peripheral blood (Supplemental Fig. 4). NK cells from 3 of these mice showed markedly lower cytotoxicity to both pig cells and K562 cells than NK cells from non-chimeric mice. Since this pattern was not seen in any of 8 non-chimeric mice, these data suggest that the presence of porcine chimerism rendered human NK cells globally hyporesponsive (Fig. 4B and Supplemental Table 1). For the other 10 chimeric mice, cytotoxicity against K562 cells was ≥ 50% of that of non-chimeric controls and we compared the ratios of maximum killing of pig cells vs K562 cells to those of non-chimeric mice. Ratios for the chimeric mice were significantly lower than those for the non-chimeric humanized mice, demonstrating that mixed xenogeneic chimerism led to specific hyporesponsiveness of human NK cells to pig cells (Fig. 4A and B). However, some chimeric mice showed similar killing of pig lymphoblasts and K562 cells and some showed only reduced responsiveness to the pig (Fig. 4A), suggesting that incomplete tolerance was induced by porcine chimerism. We routinely determined the pig/human mixed chimerism in all tissues when human NK cells were isolated and tested for cytotoxicity (Supplemental Fig. 1). We did not detect any significant difference in cytotoxicity between mice with or without measurable porcine chimerism in the spleen (Supplemental Fig. 5). These data indicated that hyporesponsiveness of human splenic NK cells did not require the persistent presence of measurable pig chimerism in the spleen. No significant differences in NK and pig cell levels in tissues were found between the 3 chimeric mice showing global hyporesponsiveness and the 10 chimeric mice showing specific hyporesponsiveness. However, mice showing global hyporesponsiveness had altered NK cell phenotypes in their tissues (Supplemental Fig. 6).
Figure 4. Mixed xenogeneic chimerism induces hyporesponsiveness of human NK cells to pig cells.
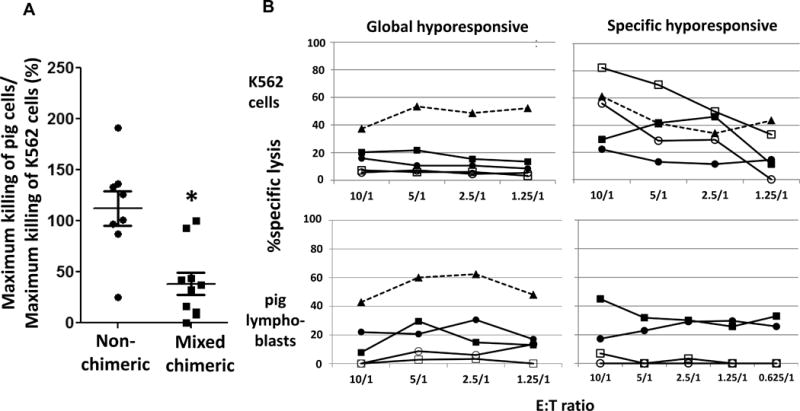
Pig/human mixed xenogeneic chimeric and control humanized mice were given hydrodynamic injection of plasmid encoding human Flt3L followed by injection of human IL-15 for 2 or 3 weeks. Human splenic NK cells were isolated and their cytotoxic responses in vitro to pig lymphoblasts and K562 cells were determined. NK cells from human PBMCs served as additional controls. (A) The ratio of (×100%) the maximum killing of pig lymphoblasts to that of K562 cells by NK cells from the same mouse is shown. Error bars represent SEM. Each symbol represents an individual animal. *, p<0.05, compared with non-chimeric mice with a Student’s t-test. (B) Examples of global hyporesponsiveness and specific hyporesponsiveness of splenic human NK cells to pig cells from two different experiments are shown. Cytotoxicity of NK cells to K562 cells and pig lymphoblasts are shown in the upper and lower panel, respectively. Filled triangle with dotted line = NK cells from human peripheral blood; filled square and circle = NK cells from two different non-chimeric mice; open square and circle = NK cells from two different chimeric mice.
Comparison of cytokine responses of human NK cells from chimeric and non-chimeric mice to pig lymphoblasts and K562 cells showed that a significant percentage of human peripheral blood NK cells expressed IFN-γ when stimulated with pig lymphoblasts or K562 cells (Fig. 5). However, the percentages of IFN-γ+ human NK cells from the spleen and bone marrow of both chimeric and non-chimeric mice in response to pig lymphoblast and K562 cell stimulation were much lower than those of the human peripheral blood NK cells, suggesting that the cytokine production function of human NK cells in humanized mice was suboptimal. No difference was detected between chimeric and non-chimeric mice in IFN-γ production by human splenic and bone marrow NK cells in response to pig lymphoblast stimulation. While no difference was detected between chimeric and non-chimeric mice in IFN-γ production by splenic NK cells stimulated with K562 cells, percentages of IFN-γ+ bone marrow NK cells in chimeric mice were significantly lower than those in non-chimeric mice (p<0.05, Fig. 5B), suggesting that global hyporesponsiveness was induced in bone marrow NK cells of mixed chimeras.
Figure 5. Cytokine responses of human NK cells from chimeric and non-chimeric mice to pig lymphoblasts and K562 cells in vitro.
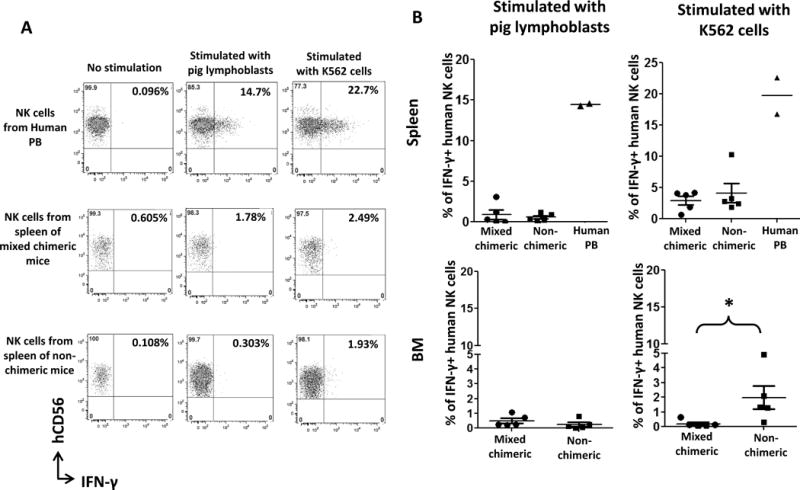
Human NK cells isolated from human peripheral blood or from spleen and bone marrow of chimeric and non-chimeric mice were co-cultured with pig cells or K562 cells at a ratio of 1:1 for 4 hours in vitro and production of IFN-γ was determined using an IFN-γ capture assay kit. (A) Representative flow cytometric data of IFN-γ production by NK cells from human peripheral blood and spleen of chimeric and non-chimeric mice in response to pig lymphoblast and K562 cell stimulation in vitro. (B) Group data of IFN-γ production by NK cells from human peripheral blood and spleen and bone marrow of chimeric and non-chimeric mice in response to pig lymphoblast and K562 cell stimulation in vitro. Data are combined from 2 experiments and there were 5 mice in each group. Values shown were adjusted by subtracting the percentages of IFN-γ+ NK cells without stimulation from those of NK cells stimulated with pig lymphoblasts or K562 cells. *, p<0.05, compared with non-chimeric mice with Mann Whitney test. PB: peripheral blood.
Phenotypic characterization of NK cells in mixed chimeras
We compared the immuno-phenotype of human NK cells from mixed chimeric and non-chimeric humanized mice from various tissues (Fig. 6). Human NK cells of the spleen and peripheral blood showed a more mature phenotype than those in bone marrow or liver, with greater KIR expression. In general, no differences were found in the expression of these markers in NK cells from the two groups of mice. Expression by human NK cells from chimeric mice of significant levels of CD94, NKG2D, NKp30, NKp46 and KIR, suggests that hyporesponsiveness to pig cells induced by mixed xenogeneic chimerism was not associated with an immature phenotype. Activation markers, such as CD25 and CD69 were not expressed, suggesting that human NK cells were not activated by pig cells in vivo in mixed chimeras. However, in bone marrow a significantly higher percentage of CD56brightCD16dim and lower percentage of CD56dimCD16bright human NK cell subsets was detected in chimeric compared to non-chimeric mice (Fig. 6). No significant difference in the CD56dimCD16dim NK subset was found between the chimeric and non-chimeric mice. However, the percentages of this population in bone marrow NK cells seemed to be higher than that recently reported for normal human bone marrow (28). Although there were no differences in any NK cell subsets in the peripheral blood of chimeric vs. non-chimeric mice, percentages of CD56dimCD16high and CD56dimCD16dim NK cells in human peripheral blood were significantly higher and lower, respectively, than those in the peripheral blood of both chimeric and non-chimeric mice.
Figure 6. Phenotypic characterization of NK cells in mixed chimeras.
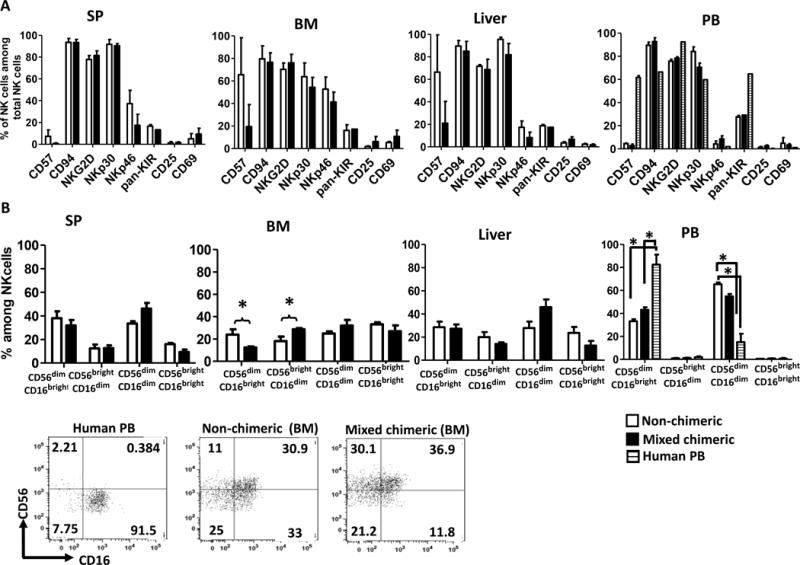
Pig/human mixed xenogeneic chimeras and non-chimeric mice were given hydrodynamic injection of plasmid encoding human Flt3L followed by injection of human IL-15 for 2 weeks. Mice were euthanized at the end of the 2-week Flt3L and IL-15 treatment and phenotype of human NK cells in various tissues was determined. (A) Phenotype of human NK cells in various tissues from pig/human mixed chimeric mice not showing global hyporesponsiveness (n=4) and non-chimeric mice (n=3). (B) Human NK cell subsets in various tissues identified by CD56 and CD16 expression. Representative flow cytometric dot plots from one experiment are shown to demonstrate the designation of NK cell subsets. Human peripheral blood NK cells (left) and bone marrow NK cells of non-chimeric and mixed chimeric mice are shown. Error bars represent SEM. *, p<0.05, Student’s t-test, comparison as specified in the figure. SP: spleen. BM: bone marrow. PB: peripheral blood.
Discussion
The relatively long-lasting pig/human mixed chimerism in our humanized mouse model permits analysis of the impact of this chimerism on human NK cell function. Global hyporesponsiveness of human NK cells in the presence of abundant murine recipient cells might have been predicted in humanized mice, based on our previous findings in the rat-to-mouse model, in which host mouse NK cells were rendered globally unresponsive by low levels of rat mixed chimerism (21). However, resting human peripheral blood NK cells show negligible cytotoxicity against NOD mouse lymphoblasts while demonstrating significant killing of pig lymphoblasts, suggesting a fundamental inability of human NK cells to interact with murine targets. Interactions between resting human NK cells and target cells leading to activation and killing involve multiple surface molecules such as LFA-1 and ICAM-1 interactions (29). Although LFA-1 on human NK cells is able to bind to mouse ICAM-1(30, 31), this has been shown to lead to cytolytic granule polarization but not degranulation (32). Incompatibility of receptors and ligands between human and mouse cells may therefore explain the low cytotoxicity of mouse lymphoblasts by human NK cells and cannot be construed as global hyporesponsiveness in humanized mice since intact cytotoxicity against pig and K562 cells was seen for these human NK cells. Since global unresponsiveness was a manifestation of NK cell tolerance in rat-mouse chimeras, failure of human-mouse interactions may explain the failure to induce global hyporesponsiveness of human NK cells in the murine environment. Our results indicate that human NK cells developing in the presence of mixed xenogeneic chimerism may become hyporesponsive to pig cells, as demonstrated by the decreased cytotoxicity to pig cells in vitro of NK cells developing in mixed chimeras compared to those developing in control humanized mice. Thus, induction of mixed xenogeneic chimerism may be an approach to inducing human NK cell tolerance in addition to T and B cell tolerance to xenografts in humans, although additional approaches may be needed to improve its reliability.
In a rat-to-mouse BMT model, recipient mouse NK cells became tolerant to rat cells in association with global hyporesponsiveness (21). In contrast, recipient NK cells were specifically tolerant to donor cells in mixed allogeneic chimeras (20). We hypothesized that NK cells that receive unopposed activating signals from a xenogeneic cell population become anergic. Inhibitory receptors on murine NK cells are quite broad in their class I allorecognition (33), and fully allogeneic class I molecules reduce NK-mediated marrow destruction compared to that of class I MHC-deficient cells (34, 35). Xenogeneic MHC molecules tend not to interact with inhibitory NK-cell receptors (36, 37), whereas activating ligands often activate xenogeneic receptors (18, 38–40). Thus, in rat-mouse mixed xenogeneic chimeras, most murine NK cells may undergo unopposed activation by xenogeneic rat hematopoietic cells, resulting in a state of ‘anergy’ in all NK cells and global hyporesponsiveness (21). This hypothesis is in keeping with the ‘disarming’ model of NK-cell tolerance (41) and with dominant tolerance induced class I-deficient cells in mixed chimeras (42, 43).
Many previously published studies demonstrated that inhibitory receptors on mature human NK cells were not able to recognize pig MHC molecules, while human NK activating receptors can recognize their ligands expressed on pig cells (18, 19, 44–46). If our observations in the rat-to-mouse model apply to human NK cells, reduced human NK cell responses to pig cells would be expected to be associated with global hyporesponsiveness in pig/human mixed chimeric mice. However, our data showing partial or complete hyporesponsiveness of human NK cells to pig cells with normal reactivity to K562 cells in the majority of pig/human mixed chimeric mice suggest that some human NK cells receive inhibitory signals from the pig. Indeed, a limited number of studies suggested that certain unidentified ligands for human inhibitory killer cell immunoglobulin-like receptors (KIRs) could be expressed by pig aortic endothelial cells, protecting them from human NK cell killing (47, 48).
Human KIR polymorphism may explain the different impact of pig/human mixed chimerism on human NK cells observed in different experiments. The human KIR family contains 14 highly polymorphic genes which encode both inhibitory and activating receptors. Some genes have more than 50 alleles (49). Each inhibitory KIR recognizes distinct subsets of HLA class I molecules and some ligands are still unidentified (49). Swine leukocyte antigens (SLA) and HLA share considerable sequence homology (50, 51). If there are indeed some pig SLA-human KIR interactions, outcomes would be expected to vary with the genotype of the particular pig-human pair being studied. Further studies are warranted to address this hypothesis.
We observed a lower percentage of CD56dimCD16bright and a higher percentage of CD56brightCD16dim human NK cell subsets in the bone marrow of mixed chimeric compared to non-chimeric mice. Human peripheral blood CD56dimCD16bright NK cells are characterized by high cytotoxicity and low cytokine production while CD56brightCD16dim NK cells, in contrast, are characterized by low cytotoxicity and high cytokine production (52, 53). Similar characteristics were recently reported for these NK cell subsets in human bone marrow (28). Thus our observation that NK cells in bone marrow of chimeric mice had decreased ability to produce IFN-γ despite containing a higher percentage of CD56brightCD16dim NK cells compared to non-chimeric mice seems inconsistent with the published results. The discrepancy may reflect the type of stimulation used for cytokine measurement, as cytokine stimulation was used in the published reports (52, 53), whereas we used cellular stimulation. It is also possible that the higher percentage of CD56brightCD16dim human NK cells in bone marrow of chimeric mice reflects shedding of CD16 from NK cells upon encounter with pig cells, as reported for human NK cells encountering target cells (54). While metalloproteinase-17 (ADAM17)-mediated shedding of CD16 has been shown in human NK cells in responses to cytokine and tumor stimulation in vitro (55), its role in tolerance induction of human NK cells is unknown. However, it is tempting to speculate that loss of the CD16 activation molecule could contribute to NK cell tolerance/loss of function in mixed chimeras.
Our data, to our knowledge, are the first to demonstrate that mixed xenogeneic hematopoietic chimerism leads to either specific hyporesponsiveness to pig cells or global hyporesponsiveness of human NK cells. Although these data support the use of this approach to xenograft tolerance, improvements are required to assure tolerance of human NK cells with normal function. Whether induction of mixed xenogeneic chimerism could tolerize human NK cells in a pre-established immune system following conditioning or how pre-existing NK cells impact pig bone marrow cell engraftment are remaining issues to address. Given the variable hyporesponsiveness of human NK cells to pig cells resulting from mixed xenogeneic chimerism in our study, more reliable solutions, such as the use of transgenic donor pigs expressing HLA class I molecules with broad NK inhibitory activities, such as HLA-E/human β2 microglobulin/leader peptide trimers (56), should be explored.
Supplementary Material
Acknowledgments
We thank Dr. Jianzhu Chen for providing the plasmid encoding recombinant human Flt3L, Drs. David H. Sachs and Scott Arn for providing pig tissues and reagents, and Drs. Emmanuel Zorn and Tomer Granot for critical review of the manuscript. We thank Ms. Deborah Peniche for expert assistance with the manuscript.
This work was supported by National Institutes of Health Grant P01AI045897-14 and R01 AI084903 (to M.S.). P.V and G.C were supported by the Dutch Cancer Society (KWF; project UVA 2010-4648). M.H was supported by Austrian Science Fund (FWF) J3416-B19. S.C was supported by the NIH/NHLBI Ruth L. Kirschstein National Research Service Award (T35).
Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050 and S10OD020056. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BMT
bone marrow transplantation
- BM
bone marrow
- BMCs
bone marrow cells
- NK
cells natural killer cells
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
- Supplemental methods, figure legends and table legend
Reference list
- 1.Yang Y-G, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;07(07):519–31. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 2.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014;258(1):241–58. doi: 10.1111/imr.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwase H, Kobayashi T. Current status of pig kidney xenotransplantation. International Journal of Surgery. 2015;23(Part B):229–33. doi: 10.1016/j.ijsu.2015.07.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier RPH, Navarro-Alvarez N, Morel P, Schuurman H-J, Strom S, Bühler LH. Current status of hepatocyte xenotransplantation. International Journal of Surgery. 2015;23(Part B):273–9. doi: 10.1016/j.ijsu.2015.08.077. [DOI] [PubMed] [Google Scholar]
- 5.Murthy R, Bajona P, Bhama JK, Cooper DKC. Heart Xenotransplantation: Historical Background, Experimental Progress, and Clinical Prospects. The Annals of Thoracic Surgery. 2016;101(4):1605–13. doi: 10.1016/j.athoracsur.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, Wang W, Yu L, Wang B. Pig islets xenotransplantation: recent progress and current perspectives. Frontiers in Surgery. 2014;1(7) doi: 10.3389/fsurg.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper DKC, Ekser B, Tector AJ. Immunobiological barriers to xenotransplantation. International Journal of Surgery. 2015;23(Part B):211–6. doi: 10.1016/j.ijsu.2015.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachs DH, Sykes M, Yamada K. Achieving tolerance in pig-to-primate xenotransplantation: Reality or fantasy. Transpl Immunol. 2009;21(2):101–5. doi: 10.1016/j.trim.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14(4):417–24. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 10.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–70. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 11.Abe M, Qi J, Sykes M, Yang Y-G. Mixed chimerism induces donor-specific T-cell tolerance across a highly disparate xenogeneic barrier. Blood. 2002;99(10):3823–9. doi: 10.1182/blood.v99.10.3823. [DOI] [PubMed] [Google Scholar]
- 12.Aksentijevich II, Sachs DH, Sykes M. Humoral tolerance in xenogeneic BMT recipients conditioned by a nonmyeloablative regimen. Transplantation. 1992;53(5):1108–14. doi: 10.1097/00007890-199205000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Ohdan H, Yang Y-G, Shimizu A, Swenson KG, Sykes M. Mixed chimerism induced without lethal conditioning prevents T cell– and anti-Galα1,3Gal–mediated graft rejection. J Clin Invest. 1999;104(3):281–90. doi: 10.1172/JCI6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharabi Y, Aksentijevich I, Sundt TM, Sachs DH, Sykes M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med. 1990;172(1):195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y-G, deGoma E, Ohdan H, Bracy JL, Xu Y, Iacomini J, et al. Tolerization of anti-Galalpha1–3Gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med. 1998;187(8):1335–42. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin D, Zeng H, Ma L, Shen J, Xu H, Byrne GW, et al. Cutting Edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. J Immunol. 2004;172(12):7235–8. doi: 10.4049/jimmunol.172.12.7235. [DOI] [PubMed] [Google Scholar]
- 17.Nikolic B, Cooke DT, Zhao G, Sykes M. Both γδ T cells and NK cells inhibit the engraftment of xenogeneic rat bone marrow cells and the induction of xenograft tolerance in mice. J Immunol. 2001;166(2):1398–404. doi: 10.4049/jimmunol.166.2.1398. [DOI] [PubMed] [Google Scholar]
- 18.Forte P, Lilienfeld BG, Baumann BC, Seebach JD. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J Immunol. 2005;175(8):5463–70. doi: 10.4049/jimmunol.175.8.5463. [DOI] [PubMed] [Google Scholar]
- 19.Lilienfeld BG, Garcia-Borges C, Crew MD, Seebach JD. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J Immunol. 2006;177(4):2146–52. doi: 10.4049/jimmunol.177.4.2146. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Ohdan H, Manilay JO, Sykes M. NK cell tolerance in mixed allogeneic chimeras. J Immunol. 2003;170(11):5398–405. doi: 10.4049/jimmunol.170.11.5398. [DOI] [PubMed] [Google Scholar]
- 21.Kawahara T, Rodriguez-Barbosa JI, Zhao Y, Zhao G, Sykes M. Global unresponsiveness as a mechanism of natural killer cell tolerance in mixed xenogeneic chimeras. Am J Transplant. 2007;7(9):2090–7. doi: 10.1111/j.1600-6143.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 22.Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, et al. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103(10):3964–9. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 23.Onoe T, Kalscheuer H, Danzl N, Chittenden M, Zhao G, Yang Y-G, et al. Human natural regulatory T cell development, suppressive function, and postthymic maturation in a humanized mouse model. J Immunol. 2011;187(7):3895–903. doi: 10.4049/jimmunol.1100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pek EA, Chan T, Reid S, Ashkar AA. Characterization and IL-15 dependence of NK cells in humanized mice. Immunobiology. 2011;216(1–2):218–24. doi: 10.1016/j.imbio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A. 2009;106(51):21783–8. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–50. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stabile H, Nisti P, Morrone S, Pagliara D, Bertaina A, Locatelli F, et al. Multifunctional human CD56low CD16low natural killer cells are the prominent subset in bone marrow of both healthy pediatric donors and leukemic patients. Haematologica. 2015;100(4):489–98. doi: 10.3324/haematol.2014.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryceson YT, March ME, Ljunggren H-G, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214(1):73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston SC, Dustin ML, Hibbs ML, Springer TA. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990;145(4):1181–7. [PubMed] [Google Scholar]
- 31.March ME, Long EO. β2 integrin induces TCRζ-Syk-phospholipase C-γ phosphorylation and paxillin-dependent granule polarization in human NK cells. J Immunol. 2011;186(5):2998–3005. doi: 10.4049/jimmunol.1002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryceson YT, March ME, Barber DF, Ljunggren H-G, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202(7):1001–12. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renard V, Cambiaggi A, Vély F, Bléry M, Olcese L, Olivero S, et al. Transduction of cytotoxic signals in natural killer cells: a general model of fine tuning between activatory and inhibitory pathways in lymphocytes. Immunol Rev. 1997;155(1):205–21. doi: 10.1111/j.1600-065x.1997.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 34.Bix M, Liao N-S, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349(6307):329–31. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 35.Höglund P, Sundbäck J, Olsson-Alheim MY, Johansson M, Salcedo M, Öhién C, et al. Host MHC class I gene control of NK-cell specificity in the mouse. Immunol Rev. 1997;155(1):11–28. doi: 10.1111/j.1600-065x.1997.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 36.Forte P, Baumann BC, Weiss EH, Seebach JD. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am J Transplant. 2005;5(9):2085–93. doi: 10.1111/j.1600-6143.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 37.Forte P, Pazmany L, Matter-Reissmann UB, Stussi G, Schneider MKJ, Seebach JD. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167(10):6002–8. doi: 10.4049/jimmunol.167.10.6002. [DOI] [PubMed] [Google Scholar]
- 38.Christiansen D, Mouhtouris E, Milland J, Zingoni A, Santoni A, Sandrin MS. Recognition of a carbohydrate xenoepitope by human NKRP1A (CD161) Xenotransplantation. 2006;13(5):440–6. doi: 10.1111/j.1399-3089.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 39.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21(5):228–34. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura MC, Naper C, Niemi EC, Spusta SC, Rolstad B, Butcher GW, et al. Natural killing of xenogeneic cells mediated by the mouse Ly-49D receptor. J Immunol. 1999;163(9):4694–700. [PubMed] [Google Scholar]
- 41.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6(7):520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 42.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207(10):2065–72. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu MF, Raulet DH. Class I-deficient hemopoietic cells and nonhemopoietic cells dominantly induce unresponsiveness of natural killer cells to class I-deficient bone marrow cell grafts. J Immunol. 1997;158(4):1628–33. [PubMed] [Google Scholar]
- 44.Tran PD, Christiansen D, Winterhalter A, Brooks A, Gorrell M, Lilienfeld BG, et al. Porcine cells express more than one functional ligand for the human lymphocyte activating receptor NKG2D. Xenotransplantation. 2008;15(5):321–32. doi: 10.1111/j.1399-3089.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 45.Lilienfeld BG, Schildknecht A, Imbach LL, Mueller NJ, Schneider MKJ, Seebach JD. Characterization of porcine UL16-binding protein 1 endothelial cell surface expression. Xenotransplantation. 2008;15(2):136–44. doi: 10.1111/j.1399-3089.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- 46.Sommaggio R, Cohnen A, Watzl C, Costa C. Multiple receptors trigger human NK cell-mediated cytotoxicity against porcine chondrocytes. J Immunol. 2012;188(5):2075–83. doi: 10.4049/jimmunol.1100433. [DOI] [PubMed] [Google Scholar]
- 47.Kwiatkowski P, Artrip JH, John R, Edwards NM, Wang S-F, Michler RE, et al. Induction of swine major histocompatibility complex class I molecules on porcine endothelium by tumor necrosis factor-alpha reduces lysis by human natural killer cells. Transplantation. 1999;67(2):211–8. doi: 10.1097/00007890-199901270-00005. [DOI] [PubMed] [Google Scholar]
- 48.Itescu S, Artrip JH, Kwiatkowski P, Wang S-F, Minanov O, Morgenthau A, et al. Lysis of pig endothelium by IL-2 activated human natural killer cells is inhibited by swine and human major histocompatibility complex (MHC) class I gene products. Ann Transplant. 1997;2(1):14–20. [PubMed] [Google Scholar]
- 49.Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology. 2011;132(3):315–25. doi: 10.1111/j.1365-2567.2010.03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chardon P, Rogel-Gaillard C, Cattolico L, Duprat S, Vaiman M, Renard C. Sequence of the swine major histocompatibility complex region containing all non-classical class I genes. Tissue Antigens. 2001;57(1):55–65. doi: 10.1034/j.1399-0039.2001.057001055.x. [DOI] [PubMed] [Google Scholar]
- 51.Renard C, Vaiman M, Chiannilkulchai N, Cattolico L, Robert C, Chardon P. Sequence of the pig major histocompatibility region containing the classical class I genes. Immunogenetics. 2001;53(6):490–500. doi: 10.1007/s002510100348. [DOI] [PubMed] [Google Scholar]
- 52.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 53.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56bright subset. Blood. 2001;97(10):3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 54.Grzywacz B, Kataria N, Verneris MR. CD56dimCD16+ NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia. 2007;21(2):356–9. doi: 10.1038/sj.leu.2404499. [DOI] [PubMed] [Google Scholar]
- 55.Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17) Blood. 2013;121(18):3599–608. doi: 10.1182/blood-2012-04-425397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss EH, Lilienfeld BG, Müller S, Müller E, Herbach N, Keler B, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87(1):35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


