Abstract
Background
Outpatient therapies for urinary tract infections (UTIs) are becoming limited due to antimicrobial resistance. The purpose of this paper is to report how the incidence of hospitalizations for UTIs have varied over time in both men and women and across age groups. We also explore how the severity for UTI hospitalizations has changed and describe the seasonality of UTI hospitalizations.
Methods
Using the Nationwide Inpatient Sample, we compute a time-series of UTI incidence and subdivide the series by age and sex. We fit a collection of time-series models to explore how the trend and seasonal intensity varies by age and sex. We modeled changes in severity using regression with available confounders.
Results
In 2011, there were approximately 400000 hospitalizations for UTIs with an estimated cost of $2.8 billion. Incidence increased by 52% between 1998 and 2011. The rate of increase was larger among both women and older patients. We found that the seasonal intensity (summer peaks and winter troughs) increased over time among women while decreasing among men. For both men and women, seasonality decreased with advancing age. Relative to controls and adjusted for demographics, we found that costs among UTI patients grew more slowly, patients left the hospital earlier, and patients had lower odds of death.
Conclusions
Incidence of UTI hospitalization is increasing and is seasonal, peaking in the summer. However, the severity of UTI admissions seems to be decreasing, indicating that patients previously treated as outpatients may now be admitted to the hospital due to increasing antimicrobial resistance.
Keywords: healthcare costs, seasonality, time series, trends, urinary tract infections
Urinary tract infections (UTIs) are among the most common of all bacterial infections [1]. Half of all women experience at least 1 UTI by the age of 35 [2], and approximately 20% of women between the ages of 18 and 24 have a UTI annually [3, 4]. Urinary tract infections are a common reason for healthcare visits. In the United States, UTIs result in an estimated 7 million office visits, 1 million emergency department visits, and over 100000 hospitalizations with an associated annual cost of $1.6 billion [2, 5, 6].
The majority of UTIs are treated on an outpatient basis [7]. However, resistance to first-line oral antimicrobials that are used to treat UTIs is increasing [4, 8–14], and this resistance complicates outpatient treatment approaches: indeed, increasing antimicrobial resistance may have reduced the efficacy of traditional outpatient treatments [7, 14, 15]. As the number of antimicrobials resistant to outpatient therapies has risen, the number of hospitalizations for UTIs has also grown. Between 2000 and 2009, hospitalizations for UTIs increased dramatically [16]. However, it is not known how such an increase in hospitalizations has affected the estimates of healthcare costs attributable to UTIs. Furthermore, it is not clear what subpopulations of patients are driving this growth in incidence: the epidemiology of UTIs differs between men and women and younger and older patients. In addition, although the incidence of UTIs appears to be seasonal [17–21], it is not clear how seasonality affects hospitalizations for UTIs, especially with respect to different populations of patients.
The purpose of this study is to describe the trends and seasonal patterns in the incidence of UTI hospitalizations by age group and sex. In addition, we describe trends in length of stay, inpatient mortality, and healthcare costs for hospitalizations associated with UTIs.
METHODS
Data Source and Case Definition
Hospitalization data were obtained through the Agency for Healthcare Research and Quality Nationwide Inpatient Sample (NIS) for the years 1998 to 2011. The NIS contains a 20% stratified sample of all hospitals in the United States. Each record represents a single hospitalization that includes diagnoses, procedures, demographic, and other information about the patient. Cases were defined as any inpatient stay with a primary diagnosis International Classification of Diseases, Ninth Revision (ICD-9) code of 599.0 (“Urinary tract infection, site not specified”). We excluded records for patients under 18 years of age or that did not include values for month, year, age, and patient sex. Case counts were normalized to incidence rates with the midyear population estimates from the Census Bureau by each specific combination of sex and age category. Age categories were defined as 18–29, 30–39, 40–49, 50–59, 60–69, 70–79, and over 90 years old. To facilitate comparisons among the groups, the incidence in some analyses was converted to age-sex-specific Z-scores (Z = (observation – mean)/standard deviation).
Statistical Analysis
Trend Estimation
An autoregressive moving average (ARMA) model with a seasonal autoregressive component of order 1 was used to characterize the data. A linear trend was incorporated as an exogenous regressor in the ARIMA model to account for the increase in UTI cases. Based on the ARMA framework, we can estimate the trend effect while simultaneously controlling for the temporal correlation inherent in time-series data. A separate model was fit for each combination of sex and age (eg, males 18–29, males 30–39…), where the standardized incidence was regressed on a year index (year + (month – 1)/12) and the month of the patient’s discharge. The year variable accounts for long-term changes over time, and the month variable captures seasonality—annual periodicities as reflected in changes by month.
Seasonal Estimation
To further examine UTI seasonality, we detrended the series: a linear trend was fit to each of the subseries described above, and the residuals were obtained. The resulting residuals are a series that has a constant mean (no trend) but retains the seasonal fluctuations. For each series, we computed the yearly maximum, minimum, and range observed in these residuals. We also computed the mean patient age within each of the age groups used to create the series. We stratified the resulting data by sex. We estimated the effects of increasing age and increasing year by regressing the observed maximum, minimum, and range on the mean age and the year with separate models for men and women.
Severity Estimation
Next, we consider length of stay, inpatient mortality, and costs for UTI and non-UTI admissions. We used these measures of severity as opposed to an index such as the Charlson or Elixhauser comorbidity coding systems due to concerns about the lack of time invariance of the comorbidity systems. Specifically, changes over time would have affected cases and controls differently. In contrast, length of stay, mortality, and costs (after adjustment for inflation) exist on a standard scale that does not vary over time, and any changes would affect cases and controls in the same way. We used data from 100% of UTI cases, but we only retained data on a randomly selected 10% subsample of controls to reduce the computational demands of model fitting. Because the 10% subsample is approximately 7 times larger than the number of UTI cases, there is little effect of this sampling on our estimation. We excluded any of the cases or controls that had a labor and delivery diagnosis code on their record (ICD 9 codes: 650, 651, 652, 669.5, 669.6, or 669.7), to reduce confounding by these stays in young women. We only considered length of stay in cases in which the patient was discharged alive to avoid truncation due to death. Total charges were converted to total cost using the Healthcare Cost and Utilization Project (HCUP)-provided cost-to-charge ratios. When possible, we used the all-payer hospital-specific ratio. If the hospital-specific ratio was not reported, we used the group-average all-payer ratio. These ratios are only available for years 2001–2011. After conversion to total costs, we applied the Consumer Price Index for Medical Care [22] to convert all dollar amounts to constant December 2011 dollars.
We estimate changes in length of stay, mortality, and costs using traditional linear regression or logistic regression models, as appropriate. We regressed each outcome on the patient age (an indicator for each decade), sex, number of procedures performed during the stay, month of year, an indicator for UTI as primary diagnosis, the year, and the interaction between year and the UTI indicator. The primary coefficient of interest is on this interaction term: it reflects the slope difference between the linear trend of non-UTI and UTI patients. For the regression parameter estimates, we used heteroskedastic-consistent standard errors.
Sensitivity Analysis
Because it is possible that some of the change in incidence of UTIs could have been associated with changes in coding or diagnostic approaches, as a sensitivity analysis, we considered trends in incidence for pyelonephritis (ICD-9-CM codes of 590.xx) relative to trends for UTIs. Pyelonephritis is a more severe diagnosis, and regardless of antimicrobial resistance patters of the causative agent, it is more likely to lead to hospitalization than the diagnosis of an UTI. Thus, we computed monthly incidence series for pyelonephritis and UTIs and estimated the trend using the ARMA framework explained above.
RESULTS
For the years 1998 to 2011, there were 108672713 hospital admissions in the NIS. Of these, 960516 were for UTIs in adults. The data required to construct the time series (age, sex, admission year, and month) were present on 860870 of these records. Somewhat smaller samples were used for the severity models due to the additional variables required (eg, length of stay, mortality) (sample sizes are reported with the regressions).
Summary Statistics
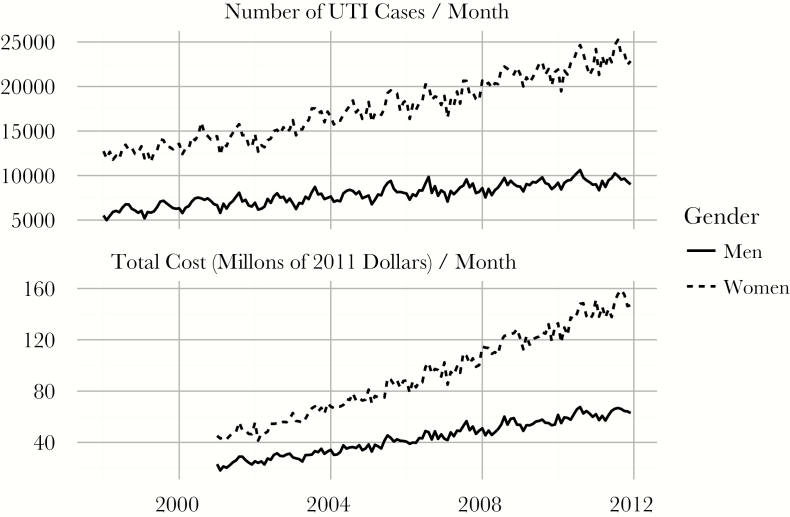
Weighted summary statistics of the sample are included in Table 1. Between 1998 and 2011, the number of UTI admissions increased from 264404 (12.9 per 10000 people) to 436635 (18.4 per 10000 people). The majority of the increase in admissions occurred among women (67.7% in 1998, 71.4% in 2011). The mean age of UTI patients with a primary diagnosis of a UTI increased from 73.2 to 74.7 years. Mean length of stay decreased from 5.29 to 4.24 days. For UTI hospitalizations, average real total costs increased from $3368 to $6425 between 2001 and 2011. The 20th and 80th percentiles of costs in 2011 were $3113 and $8409, respectively. The median real total costs increased from $2365 to $5019. A plot of total charges over time is shown in Figure 1.
Table 1.
Weighted Baseline Characteristics of UTI Admissions in NIS, 1998–2011
| Variable Name | Count/Mean | Percent/SD |
|---|---|---|
| Age | 74.03 | 16.62 |
| Female | 2935344 | 69.05% |
| Race | ||
| White | 2454310 | 74.90% |
| Black | 424712 | 12.96% |
| Hispanic | 245948 | 7.51% |
| Asian | 62150 | 1.90% |
| Native American | 16535 | 0.50% |
| Other | 73252 | 2.24% |
| Length-of-Stay | 4.77 | 5.11 |
| Died | 74480 | 1.75% |
| Total Charges | ||
| Mean | 13671 | 18940 |
| Median | 8682 | |
| Number of Procedures | 0.50 | 1.09 |
Abbreviations: NIS, nationwide inpatient sample; SD, standard deviation; UTI, urinary tract infection.
Figure 1.
Urinary tract infection (UTI) incidence and total cost of hospitalizations by sex, 1998–2011. Incidence is the number of cases per 10000 people in the community by sex, and real total costs are converted to costs using the Healthcare Cost and Utilization Project cost-to-charge ratio and are normalized to constant December 2011 dollars. Solid lines denote the male series, whereas dotted lines represent the female series.
Trend Estimation
Incidence of hospital admissions for UTIs increased in both men and women of all ages (Table 2). The number of cases increased by 76% over the study period, and incidence (population adjusted) increased by 52%. Incidence rates accelerated with advancing age: the growth rate for 18- to 29-year-old women was 7.9% of a standard deviation per year, whereas the growth rate for 80- to 89-year-old women was 23.1%. Although UTI incidence was rising for all sex and age groups, the average rate of increase for women was approximately twice the rate of increase in men. For example, the rate of increase for 50- to 59-year-old men was 9.8% of a standard deviation per year and for 50- to 59-year-old women, 19.2%.
Table 2.
Yearly Trend Estimates From ARMA Models Expressed as the Percentage of a Standard Deviation Increase per Year in UTI Hospitalization Incidence
| Age Group | Trend in Men | Trend in Men, Standard Error | Trend in Women | Trend in Women, Standard Error |
|---|---|---|---|---|
| 18–29 | 6.8% | 2.1% | 7.9% | 3.0% |
| 30–39 | 2.2% | 2.5% | 14.6% | 2.4% |
| 40–49 | 6.9% | 2.4% | 17.1% | 2.2% |
| 50–59 | 9.8% | 2.9% | 19.2% | 1.9% |
| 60–69 | 6.8% | 3.4% | 19.7% | 2.0% |
| 70–79 | 12.3% | 3.2% | 22.4% | 1.4% |
| 80–89 | 14.4% | 2.6% | 23.1% | 1.6% |
| 90+ | 12.3% | 2.4% | 21.4% | 1.7% |
Abbreviations: ARMA, autoregressive moving average; UTI, urinary tract infection.
Seasonal Estimation
The incidence of UTI hospitalizations is highly seasonal and our seasonality findings are reported in Table 3. Urinary tract infections peak in the summer months and the nadir occurs during the winter. The incidence of admissions for UTIs exhibits a stronger seasonal effect for women than for men. Seasonality is most pronounced among younger patients, and it diminishes with advancing age. Among women, for each year of age there was a decrease of 2.6% of a standard deviation in the range of the seasonal intensity. Among men, for each year of age there was a decrease of 1.0% of a standard deviation. During our study period, the seasonal intensity changed. Among women, the seasonality increased: the incidence of UTIs for women at the beginning of our sample was less seasonal than at the end. In contrast, among men, the seasonality diminished. Specifically, with each passing year between 1998 and 2011, the average seasonal intensity increased by 3.0% of a standard deviation in women and decreased by 4.5% of a standard deviation in men.
Table 3.
Effects of Age and Admission Year on Seasonal Intensity of UTI Hospitalization Incidence in Men and Women
| Variable Name | Range | Minimum | Maximum | |||
|---|---|---|---|---|---|---|
| Effect in Men (SD) | Effect in Women (SD) | Effect in Men (SD) |
Effect in Women (SD) | Effect in Men (SD) | Effect in Women (SD) | |
| Patient age | −0.010 (0.003)* | −0.026 (0.002)* | 0.004 (0.002)† | 0.012 (0.002)* | −0.007 (0.003)‡ | −0.014 (0.001)* |
| Admission year | −0.045 (0.015)‡ | 0.030 (0.010)‡ | 0.024 (0.011)§ |
−0.015 (0.009)† | −0.021 (0.014) | 0.015 (0.008)† |
| R2 | 0.177 | 0.689 | 0.072 | 0.388 | 0.080 | 0.471 |
Abbreviations: SD, standard deviation; UTI, urinary tract infection.
*P < .001.
† P < .1.
‡ P < .01.
§ P < .05.
Severity Estimation
Length of stay decreased in both UTI and non-UTI hospitalizations. However, in a model adjusting for age (grouped by decade), sex, year, month of year, number of procedures, and a primary diagnosis of an UTI, we found that the length of stay decreased faster for patients with a primary diagnosis of an UTI (Table 4). Specifically, a non-UTI patient stayed an average of 13.1 (P < .0001) fewer hours in 2011 compared with 1998; however, UTI patients stayed an average of 27.7 (P < .0001) fewer hours—a difference of 14.6 hours (P < .0001).
Table 4.
Adjusted Changes in Severity Measures for Control and UTI Patients per Year Between 1998 and 2011a
| Variable Name | Change in Controls | Change in UTI Patients | Difference (SD) | Difference as Percentage |
|---|---|---|---|---|
| Cost (dollars) (n = 6374024) |
576.71 | 317.24 | −259.47 (2.64)* | −45% |
| Length-of-stay (hours) (n = 8517901) |
−0.936 | −1.968 | −1.032 (0.024)* | +10% |
| Inpatient death (n = 8734469) |
0.97 | 0.92 | 0.95 (95% CI, .94–.95)* | −5% |
Abbreviations: CI, confidence interval; SD, standard deviation; UTI, urinary tract infections.
aNote that the “change in UTI patients” column is simply the combination of the “change in controls” and “difference” columns (addition for linear models, multiplication for the odds ratios).
*P < .0001.
Inpatient mortality decreased substantially between 1998 and 2011 (Table 4). In general, the odds of inpatient death for non-UTI patients decreased by 3% per year. Urinary tract infection patients, on the other hand, observed an extra 5% (odds ratio, 0.95; 95% condidence interval [CI], .94–.95) reduction in the odds of death per year for a total of 8% decrease in the odds of death per year for UTI patients. Thus, for non-UTI patients, their odds of death were 35% lower in 2011 than in 1998. In contrast, for UTI patients, their odds of death were 68% lower in 2011 than in 1998.
Costs increased for all patients between 1998 and 2011 (Table 4). Among non-UTI patients, there was an average yearly increase of $577 (P < .0001). In contrast, UTI patients had yearly increases of $317, a difference of $259 (P < .0001) per year.
In 2011, more than 436437 patients were admitted with a primary diagnosis of UTI. These hospitalizations resulted in charges of $9.7 billion and a real total cost of $2.8 billion for these UTI admissions. The mean real cost per case has increased by 90.8% and non-UTI mean real cost has increased by more than 123.0% between 2001 and 2011.
Sensitivity Analysis
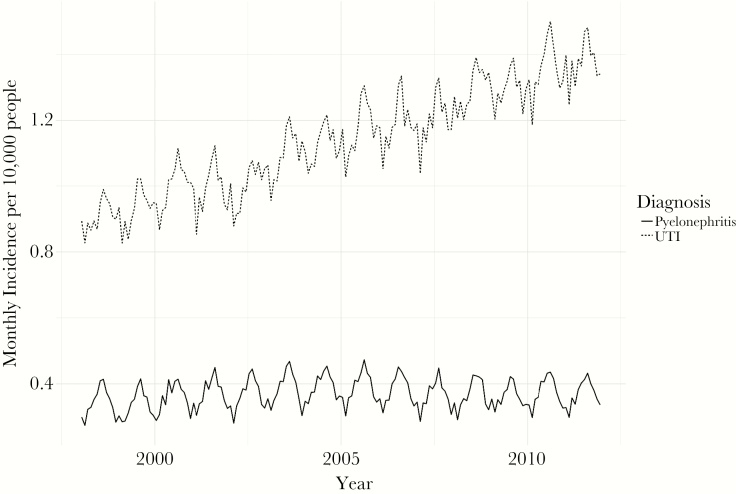
The incidence rate for pyelonephritis was 4.7 cases per 10000 people in 1998, and it increased by 2.7% to 4.8 cases per 10000 people in 2011. Monthly incidence is shown in Figure 2. Our regression analysis showed the monthly incidence increased by 0.002 more cases per 10000 people every year (95% CI, −.001 to .005). This compares to the 42.6% increase in incidence for UTIs between 1998 and 2011, and our regression analysis showed an increase in the monthly incidence of UTIs of 0.04 more cases per 10000 people every year (95% CI, .028–.044).
Figure 2.
Urinary tract infection (UTI) and pyelonephritis incidence, 1998–2011. Incidence is the number of cases per 10000 people by month.
DISCUSSION
We found a dramatic increase in the incidence of hospitalizations attributable to UTIs from 1998 to 2011: cases increased by 76% and incidence increased by 52%. The greatest increase in the number of hospitalizations for UTIs occurred among women, which is not surprising. Although UTIs are most common in younger women, our results demonstrated that most of the increase in UTI hospitalizations occurred among older women (eg, patients older than 70). Urinary tract infection hospitalizations also increased for men, especially among older men. These dramatic changes in incidence of UTI hospitalizations that we report highlight the need to re-estimate costs attributable to UTIs. An average hospitalization with a primary diagnosis of UTI cost $3368 in 2001 and $6424 in 2011 (constant December 2011 dollars). The 436437 cases hospitalized in 2011 resulted in a total cost of $2.8 billion in healthcare cost.
These increases in incidence were only observed among UTIs. The incidence of pyelonephritis remained relatively flat between 1998 and 2011. In addition, the rate of growth over time in pyelonephritis incidence was not statistically significantly different from zero (0.002 [95% CI, −.001 to .005] higher monthly incidence per 10000 people), whereas UTI incidence was increasing (0.04 [95% CI, .028–.044] higher monthly incidence per 10000 people every year). Assuming that patients with pyelonephritis are more likely to be hospitalized than patients with UTIs because of their more severe symptoms, regardless of the resistance of the causative pathogen, we believe that these findings are consistent with our theory that antimicrobial resistance was driving some of the increase in incidence in hospitalizations for patients with a primary diagnosis of an UTI between 1998 and 2011. The relatively faster growth in incidence for UTIs compared with pyelonephritis is also suggestive of antimicrobial resistance as a driver of the dramatic increase in the incidence of hospitalizations for UTIs rather than changes in coding or changes in diagnostic practices.
Our study period coincided with reports of increases in antimicrobial resistance for agents commonly used to treat UTIs [8, 12–14, 16]. Thus, the increase in incidence of admissions we report is most likely associated with the inability to treat UTIs in outpatient settings due to increased resistance. If some of the increase in incidence in admissions was driven by patients who would have otherwise been treated as an outpatient, we would expect to see a trend toward the admission of less-severe patients, ie, patients who were only admitted for treatment with intravenous (IV) antimicrobials. Indeed, in terms of severity, we found that the patients admitted at the beginning of our study period differed from those at the end. Specifically, compared with controls, costs grew at a 45% slower annual rate for UTI patients, length of stay fell 110% faster for UTI patients, and the odds of death for UTI patients fell at a 105% faster rate. We posit that these less severe patients represent patients who would have previously been treated as outpatients with oral antimicrobial agents.
The dramatic increase in hospitalizations for UTIs suggests the need for oral antibiotics or IV treatments convenient to administer in outpatient settings that are effective against uropathogens resistant to first-line antimicrobial treatments. Converting only a small fraction of patients from inpatient to outpatient treatment would lead to a large number of patients being able to avoid a hospitalization, given that the median UTI hospitalization costs $4500, whereas the cost for outpatient treatment is significantly less expensive. Shifting patients from inpatient admissions to outpatient treatment could lead to considerable cost savings. In lieu of new oral antibiotics, innovative approaches to provide IV antibiotic therapy at home or in outpatient settings provides alternative cost-saving approaches; however, payer barriers remain an important limitation [23].
For both men and women, the annual rate of growth in incidence of admissions for UTIs increased with age. For the oldest men in our study population, the UTI rate increased at twice the rate of the youngest men. For the oldest women, the UTI rate increased at almost 3 times the rate of the youngest women. Thus, older patients seem to be disproportionally contributing to the increase in hospitalizations. If the increase in incidence of admissions for UTI is being driven at least in part by antimicrobial resistance, it follows that older patients are more likely to be affected, given that older patients are more likely to have contact with the healthcare system and are more likely to be exposed to multidrug-resistant organisms. However, given that we did observe an increase in admissions among women of all ages, as multidrug resistance spreads, we may see a further increase in hospitalizations among younger women.
We observed a strong pattern of seasonality for UTI hospitalizations among both men and women of all ages: UTI incidence increases in the summer and decreases in the winter. Reports of seasonality have mostly been restricted to single centers [17, 18]. However, Internet search terms for UTI are seasonal in countries around the world [24]. Also of note, we found that the changes in the degree of seasonality vary by sex. During our study period, the seasonality of hospitalizations for UTIs decreased with age for men and women: younger men and women experience more seasonality than older men and women. However, over time, the seasonal range increased for women and decreased for men: specifically, for each year of our study period, UTIs became more seasonal for women and less seasonal for men. Although the differences in risk factors for and epidemiology of UTIs between men and women are well known [7, 25, 26], our findings suggest additional differences, highlighting the need to think about different preventive approaches for men and women. Prevention approaches also may need to differ by age group.
Although it is unclear why the incidence for UTIs is seasonal, weather has been associated with the seasonality of several diseases [20, 27–30]. It is noteworthy to mention that blood stream infections with Gram-negative organisms are seasonal and are associated in some cases with higher temperatures [19, 21, 31, 32], and most of these blood stream infections could have originated as UTIs. Further exploration of the seasonality of UTIs by, for example, adding weather data, may lead to better understanding of the cause of this seasonality of UTIs.
Our study has limitations. First, we exclusively used administrative data and did not have medication or microbiology data, and thus we could not directly incorporate information regarding therapies or antimicrobial drug resistance. In addition, we were unable to review charts to validate the assignment of diagnostic codes. Second, in our database, we only observed events from inpatient admissions and did not observe outpatient UTI incidence, and the majority of patients were treated on an outpatient basis; thus, our study was focused only on more severe or difficult-to-treat cases. Third, our data source did not include a unique identifier to link visits over time. Thus, we could not identify patients first treated as outpatients and later admitted to the hospital for UTIs, nor could we investigate the incidence among patients with a history of recurrent UTI admissions. Fourth, the decreases in length of stay that we observed may have beeen due to improvements in treatment that occurred during the study period.
Despite our limitations, we show that the incidence of admissions for UTIs have increased dramatically, especially among older patients and women. We also show that patients admitted for UTIs, on average, appear to be relatively less severe than in prior years. Our results are consistent with what we would expect to find from increasing antimicrobial resistance, ie, increasing rates of hospitalization, increasing total cost, and decreasing average severity among patients admitted with a primary diagnosis of an UTI. Although, our results provide evidence that antimicrobial resistance may be driving healthcare costs, future studies with antimicrobial data at the patient level are needed to confirm our findings.
CONCLUSIONS
Finally, our results demonstrate the need for (1) new oral or single-dose antimicrobial agents that can administered in outpatient settings, (2) focused stewardship efforts on patients with UTIs, and (3) innovative approaches to treat UTI patients with resistant pathogens on an outpatient basis. Reducing hospitalizations due to resistant organisms may represent “low-hanging fruit” in an effort to control hospitalization costs and prevent hospitalizations.
Acknowledgments
Financial support. This work was funded by the National Heart, Lung and Blood Institute at the National Institutes of Health (Grant number K25HL 122305; to L. A. P.), the University of Iowa Health Ventures’ Signal Center (to P. M. P.), and a dissertation fellowship from the University of Iowa College of Pharmacy (to J. E. S.).
Potential conflicts of interest. All authors: No reported conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nicolle LE. Epidemiology of urinary tract infections. Clin Microbiol Newsletter 2002; 24:135–40. [Google Scholar]
- 2. Foxman B, Barlow R, D’Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 2000; 10:509–15. [DOI] [PubMed] [Google Scholar]
- 3. Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med 1996; 335:468–74. [DOI] [PubMed] [Google Scholar]
- 4. Brown PD, Freeman A, Foxman B. Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin Infect Dis 2002; 34:1061–6. [DOI] [PubMed] [Google Scholar]
- 5. Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med 1993; 329:1328–34. [DOI] [PubMed] [Google Scholar]
- 6. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002; 113:5S–13S. [DOI] [PubMed] [Google Scholar]
- 7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 8. Karlowsky JA, Jones ME, Thornsberry C, et al. Prevalence of antimicrobial resistance among urinary tract pathogens isolated from female outpatients across the US in 1999. Int J Antimicrob Agents 2001; 18:121–7. [DOI] [PubMed] [Google Scholar]
- 9. Kahlmeter G. Prevalence and antimicrobial susceptibility of pathogens in uncomplicated cystitis in Europe. The ECO.SENS study. Int J Antimicrob Agents 2003; 22:49–52. [DOI] [PubMed] [Google Scholar]
- 10. Kahlmeter G; ECO.SENS An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother 2003; 51:69–76. [DOI] [PubMed] [Google Scholar]
- 11. Metlay JP, Strom BL, Asch DA. Prior antimicrobial drug exposure: a risk factor for trimethoprim-sulfamethoxazole-resistant urinary tract infections. J Antimicrob Chemother 2003; 51:963–70. [DOI] [PubMed] [Google Scholar]
- 12. Zhanel GG, Hisanaga TL, Laing NM, et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents 2006; 27:468–75. [DOI] [PubMed] [Google Scholar]
- 13. Olson RP, Harrell LJ, Kaye KS. Antibiotic resistance in urinary isolates of Escherichia coli from college women with urinary tract infections. Antimicrob Agents Chemother 2009; 53:1285–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012; 56:2181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raz R, Chazan B, Kennes Y, et al. Empiric use of trimethoprim-sulfamethoxazole (TMP-SMX) in the treatment of women with uncomplicated urinary tract infections, in a geographical area with a high prevalence of TMP-SMX-resistant uropathogens. Clin Infect Dis 2002; 34:1165–9. [DOI] [PubMed] [Google Scholar]
- 16. Zilberberg MD, Shorr AF. Secular trends in gram-negative resistance among urinary tract infection hospitalizations in the United States, 2000–2009. Infect Control Hosp Epidemiol 2013; 34: 940–6. [DOI] [PubMed] [Google Scholar]
- 17. Anderson JE. Seasonality of symptomatic bacterial urinary infections in women. J Epidemiol Community Health 1983; 37:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stamm WE, McKevitt M, Roberts PL, White NJ. Natural history of recurrent urinary tract infections in women. Rev Infect Dis 1991; 13:77–84. [DOI] [PubMed] [Google Scholar]
- 19. Perencevich EN, McGregor JC, Shardell M, et al. Summer Peaks in the incidences of Gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:1124–31. [DOI] [PubMed] [Google Scholar]
- 20. Falagas ME, Peppas G, Matthaiou DK, et al. Effect of meteorological variables on the incidence of lower urinary tract infections. Eur J Clin Microbiol Infect Dis 2009; 28:709–12. [DOI] [PubMed] [Google Scholar]
- 21. Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Seasonal variation in Escherichia coli bloodstream infection: a population-based study. Clin Microbiol Infect 2009; 15:947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers: Medical Care (CPIMEDSL) Available at: https://fred.stlouisfed.org/series/CPIMEDSL Accessed 18 July 2016.
- 23. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med 2015; 12:e1001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rossignol L, Pelat C, Lambert B, et al. A method to assess seasonality of urinary tract infections based on medication sales and google trends. PLoS One 2013; 8:e76020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hooton TM. Uncomplicated urinary tract infection. N Engl J Med 2012; 366:1028–37. [DOI] [PubMed] [Google Scholar]
- 26. Scholes D, Hooton TM, Roberts PL, et al. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 2005; 142:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falagas ME, Theocharis G, Spanos A, et al. Effect of meteorological variables on the incidence of respiratory tract infections. Respir Med 2008; 102:733–7. [DOI] [PubMed] [Google Scholar]
- 28. Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect 2012; 18:946–54. [DOI] [PubMed] [Google Scholar]
- 29. Fisman DN. Seasonality of infectious diseases. Annu Rev Public Health 2007; 28:127–43. [DOI] [PubMed] [Google Scholar]
- 30. Fisman DN, Lim S, Wellenius GA, et al. It’s not the heat, it’s the humidity: wet weather increases legionellosis risk in the greater Philadelphia metropolitan area. J Infect Dis 2005; 192:2066–73. [DOI] [PubMed] [Google Scholar]
- 31. Freeman JT, Anderson DJ, Sexton DJ. Seasonal peaks in Escherichia coli infections: possible explanations and implications. Clin Microbiol Infect 2009; 15:951–3. [DOI] [PubMed] [Google Scholar]
- 32. Schwab F, Gastmeier P, Meyer E. The warmer the weather, the more Gram-negative bacteria—impact of temperature on clinical isolates in intensive care units. PLoS One 2014; 9:e9115. [DOI] [PMC free article] [PubMed] [Google Scholar]