Abstract
A bidirectional association between type 2 diabetes (T2DM) and depression has been consistently reported. Depression is associated with worse biomedical outcomes and increased mortality. The mechanisms underlying the association of T2DM with depression remain unclear. One possible question we can address is the extent to which the co-occurrence of diabetes and depression is due to correlated genetic and/or environmental risk factors. In this study, we performed structural equation model fitting to population-level data from the Swedish (n=68 606) and Danish (n=95 403) twin registries. The primary outcomes were clinical diagnosis of T2DM and depression using national hospital discharge registries. The phenotypic correlation between T2DM and depression is modest in both samples. In the Swedish sample, unique environmental effects explain a greater proportion of the covariance in males, whereas the association is primarily attributed to genetic effects in females. In the Danish sample, genetic effects account for the majority of the covariance in both males and females. Qualitative genetic sex differences are observed in both samples. We believe this is the first study to demonstrate significant genetic overlap between T2DM and depression.
Introduction
Cross-sectional and longitudinal studies have consistently reported an association between type 2 diabetes (T2DM) and depression,1, 2 with up to 60% increased risk for developing T2DM in individuals with depression and 15% increased risk for incident depression in those with T2DM.3, 4 Depression has been associated with adverse effects on diabetes outcomes including suboptimal glycaemic control, complications and higher rates of mortality. Tentative evidence suggests a common biological pathway between T2DM and depression, as people with depression have increased levels of inflammation, hyperactivity in the hypothalamic–pituitary adrenal axis and sympathetic nervous system.5 Those biological processes also underlie T2DM.6
A significant genetic correlation between T2DM and depression would provide evidence in support of a common genetic pathway to both disorders. Although it is known that genetic factors are aetiologically important in both T2DM and depression, it is unclear to what extent the T2DM-depression association correlated genetic or environmental factors. Twin studies provide a valuable approach to investigate genetic influences on complex traits or disorders,7 as well as the genetic architecture of comorbidity. They use the similarity within and between diseases across monozygotic (MZ) and dizygotic (DZ) twins to estimate the relative importance of the effects of genetic, shared and individual-specific environmental factors on the variance and covariance of the disorders.
Two twin studies have addressed the genetic comorbidity of T2DM and depression. The Vietnam Era Twin Study of Aging reported a significant odd ratio between T2DM and depression (1.7 (95% confidence interval: 1.1–2.7)) in a sample restricted to males aged 50–59. They concluded that there was no significant genetic correlation (0.19 (0–0.46)) or unique environmental correlation (0.09 (0–0.45)) between T2DM and depression.8 A study based on the Screening Across the Lifespan Twin (SALT) study reported a significant phenotypic correlation (0.20 (0.09–0.33)) in combined analyses of male and female twins aged 40 years and older. They concluded that there was significant environmental correlation (0.54 (0.02–0.88)) between T2DM and depression.9
Although current evidence does not support a common genetic pathway hypothesis, a genetic model that incorporates possible quantitative and qualitative sex differences in genetic and environmental effects is called for, given the substantially higher prevalence rate of comorbid T2DM and depression in females compared with males.10, 11, 12 We therefore aimed to examine the genetic aetiology of the T2DM-depression association in two large population samples of twins using sex-limitation genetic modelling. In this study we combined the data from the Swedish and Danish twin registries, as they have a similar ethnic population structure.
Materials and methods
Sample
The Swedish Twin Registry (http://ki.se/twinreg) covers over 99% of all twins born in Sweden between 1886 and 2000 (~160 000 individuals).13 This study will include data from the following two cohorts: (i) SALT (n=44 113) and (ii) Swedish Twin Studies of Adults: Genes and Environment (STAGE; n=24 493). The SALT cohort consists of twins born between 1886 and 1958,13, 14 whereas the STAGE cohort consists of twins born between 1959 and 1985.15 The project was approved by the Regional Research Ethics Board at Karolinska Institute and the Steering Board of the Swedish Twin Registry.
The Danish Twins Registry covers twin births in Denmark since 1870, with 170 000 individuals registered as of January 2011.16 This study will include all birth cohorts (1870–1930, 1931–1952, 1952–1982 and 1983–2000), excluding participants aged <18 at time of data retrieval. The project was approved by the Danish Data Protection Agency.
Primary variables from hospital registry records
The Swedish National Patient Registry has coverage of inpatient care for medical and psychiatric disorders since 1963 on a regional basis, extended to complete coverage in 1987 and further expanded to include outpatient care in 2001. Disorders were coded according to the International Classification of Disease (ICD) 8th, 9th or 10th Editions. The Swedish Twin Registry is linked to the National Patient Registry using a unique personal registration number assigned to all residents of Sweden. Data were extracted from 1969 to 31 December 2010.
The Danish National Patient Register initially had coverage of inpatient care for somatic disorders in 1977, and extended to complete coverage of all inpatient and outpatient care for medical and psychiatric disorders since 1995.17 Disorders were coded according to the ICD 8th or 10th Editions. The Danish Twin Registry is linked to the National Patient Registry using a unique personal identification number recorded in the Danish Civil Registration System. Data were extracted from 1977 to 1 April 2011.
The primary outcomes are ICD diagnoses of T2DM and depression from the respective National Hospital Discharge Registry, defined a prior to maximise statistical power. T2DM is defined by the following codes: (i) ICD-8: 250; (ii) ICD-9: 250.00, 250.02, 250.10, 250.12, 250.20, 250.22, 250.30, 250.32, 250.40, 250.42, 250.50, 250.52, 250.60, 250.62, 250.70, 250.72, 250.80, 250.82, 250.90, 250.92 and (iii) ICD-10: E11. Depression is defined by the following codes: (i) ICD-8: 296.0, 296.2, 296.9, 298.0, 300.4; (ii) ICD-9: 296.2, 296.3, 300.4, 301.10, 301.12, 301.13, 309.10, 311 and (iii) ICD-10: F32, F33, F34.1 and F38.1 (Supplementary Table 1).
Secondary variables
Zygosity of same-sex twin pairs was based on standard self-report items and, when validated with biological makers, has an accuracy of 95–99% in the Swedish Twin Registry18 and 96% in the Danish Twin Registry.19 Demographic details included age and sex. The demographic data were retrieved on 31 December 2010 for the Swedish twin sample and 11 October 2014 for the Danish twin sample.
Statistical analysis
Genetic model fitting
The classical twin method is based on comparison of MZ twins who share 100%, whereas DZ twins share on average 50% of segregating genes. In the univariate ACE model, individual differences in a trait are assumed to arise from: (i) additive genetic influences (A), (ii) shared environmental influences (C) and (iii) unique environmental influences (E). In bivariate twin analysis, in addition to the variance components of the individual traits, the main goal is to decompose the phenotypic correlation between them into parts due to correlating addictive genetic (rA), shared environmental (rC) and unique environmental (rE) effects predisposing to both traits.7 Quantitative sex differences are modelled by specifying sex-specific ACE paths for T2DM and depression as well as rA, rC and rE.20 The power is derived from different MZ-DZ correlation ratios across sex. Qualitative sex differences are incorporated by specifying free correlational paths between (i) all male and female genetic factors or (ii) all male and female common environmental factors in DZ opposite-sex twin pairs. The power is derived from differential within-trait and cross-trait correlations in opposite-sex compared with same-sex DZ pairs.20
Genetic model fitting analysis was performed in the programme OpenMx.21 A liability threshold model was used, assuming that the risk to T2DM and depression is each normally distributed in the general population with the disorder manifesting when a certain threshold of risk is exceeded.22 Their joint distribution is assumed to have a bivariate normal distribution, with the relative proportions of concordant (both twins above or below the thresholds) and discordant pairs informing on the correlation between the liabilities. Given the range of birth year from 1896 to 1985 in the Swedish Twin Registry and from 1870 to 2000 in the Danish Twin Registry, age at the time when the demographic data were retrieved was modelled as a covariate on the thresholds.
Two criteria were used to choose the best-fitting, parsimonious model: (i) differences in minus twice the log-likelihood (−2LL) yielding a statistic equivalent to a χ2-test, with degrees of freedom equal to the difference in the numbers of parameters and (ii) Akaike's Information Criterion (AIC),23 with lower values indicating a better balance between explanatory power and parsimony. A difference in AIC at least 10 indicates substantial support in favour of the more parsimonious model.
Secondary analysis
The genetic model fitting analysis was repeated and restricted to individuals born after 1950 in both the Swedish and Danish samples.
Results
Descriptive finding
The Swedish Twin Registry from the SALT and STAGE cohorts consists of 68 606 twin individuals with known age, sex, zygosity, T2DM and depression. This results in 50 082 twin pairs and 18 524 single twins (Table 1). The mean age was 58.5 (17.6) as of 31 December 2010 when the demographic data were retrieved.
Table 1. Descriptive summary of MZ and DZ twins in Swedish and Danish twin samples, stratified by sex.
| Sample |
Swedish |
Danish |
|||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| Number of paired twins Number of single twins | MZ | 6524 2335 | 9270 2131 | 11 006 59 | 11 110 59 |
| DZ | 7440 3757 | 9770 3421 | 19 538 130 | 17 640 113 | |
| Opposite-sex | 17 078 6880 | 34 900 848 | |||
| Total | 50 082 18 524 | 94 194 1209 | |||
| Age, mean (s.d.) | MZ | 54.9 (17.9) | 55.0 (18.7) | 58.3 (16.8) | 59.5 (18.3) |
| DZ | 60.3 (16.8) | 61.3 (18.0) | 60.5 (16.1) | 63.0 (17.7) | |
| Opposite-sex | 59.2 (16.5) | 58.6 (15.7) | |||
| Total | 58.5 (17.6) | 60.0 (16.8) | |||
| Prevalence of T2DM (%)a | MZ | 5.5 | 4.2 | 4.1 | 3.3 |
| DZ | 6.9 | 5.2 | 4.7 | 4.2 | |
| Opposite-sex | 5.8 | 3.1 | |||
| Prevalence of depression (%)a | MZ | 3.7 | 5.8 | 2.8 | 4.7 |
| DZ | 3.7 | 5.7 | 3.2 | 5.7 | |
| Opposite-sex | 4.9 | 3.8 | |||
| Proband wise concordance rate for T2DM (Number of concordant pair, discordant pair) | MZ | 0.42 (69, 190) | 0.43 (75, 195) | 0.37 (83, 287) | 0.33 (60, 247) |
| DZ | 0.20 (49, 398) | 0.23 (53, 350) | 0.14 (64, 764) | 0.18 (66, 605) | |
| Opposite-sex | 0.18 (91, 813) | 0.13 (66, 886) | |||
| Proband wise concordance rate for depression (Number of concordant pair, discordant pair) | MZ | 0.21 (22,174) | 0.22 (57, 399) | 0.19 (29, 243) | 0.23 (59, 400) |
| DZ | 0.06 (8, 249) | 0.05 (13, 489) | 0.05 (16, 591) | 0.16 (77, 837) | |
| Opposite-sex | 0.09 (37, 706) | 0.09 (61, 1170) | |||
Abbreviations: DZ, dizygotic twins; MZ, monozygotic twins; T2DM, type 2 diabetes.
In the genetic modelling, the thresholds and other estimates apply to the sample at mean age. For example, with the effect of age being estimated at −0.03 for males in the Swedish Twin Registry, T2DM prevalence rates will be 1.3% at age 40 and 9.0% at age 70.
The Danish Twin Registry consists of 115 886 twin individuals, of whom 95 403 individuals have known age, sex, zygosity, T2DM and depression status and are aged over 18. This results in 94 194 twin pairs and 1209 single twins (Table 1). The mean age was 60.0 (16.8) as of 11 October 2014 when the demographic data were retrieved.
In both the Swedish and Danish samples, the prevalence rates for T2DM and depression were similar for males and females (Table 1). Mean-centred age was incorporated as a covariate, and thus, the thresholds and other estimates apply to a sample at mean age. To illustrate, the prevalence rate for T2DM in males for the Swedish sample is 4.7% (z-value=1.67) at age 59. With the effect of age being estimated at –0.03, T2DM prevalence rates will be 1.3% at age 40 (z-value=2.24) and 9.0% at age 70 (z-value=1.34).
Logistic regression analysis revealed a positive relationship between T2DM and depression in males and females in the Swedish (Rph: males 0.13 (0.08–0.14); females 0.16 (0.12–0.17) and Danish (Rph: males 0.16 (0.12–0.20); females 0.15 (0.12–0.20)) samples. Tetrachoric correlations stratified by zygosity and sex are summarised in Supplementary Table 2.
Genetic model fitting in the Swedish twin sample
The starting point was an ACE model that included both quantitative and qualitative sex differences in genetic factors (model IS: −2LL=52 061.97; df=137 186; AIC=−222 310). The effect of common environment was negligible, shown by a non-significant decline in fit of the AE model (model 2S: −2LL=52 063.00; df=137 192; AIC=−222 321; χ2=1.04; P=0.98). To test the significance of qualitative sex differences, all genetic factors were constrained to correlate at 0.50 across males and females in opposite-sex pairs model, resulting in a significantly poorer fit of the AE model (model 3S: −2LL=52 116.61; df=137 196; AIC=−222 275; χ2= 53.61; P<0.05). All A and E paths are therefore estimated freely for males and females with no further constraints, as it was not possible to equate them without a significant worsening of fit.
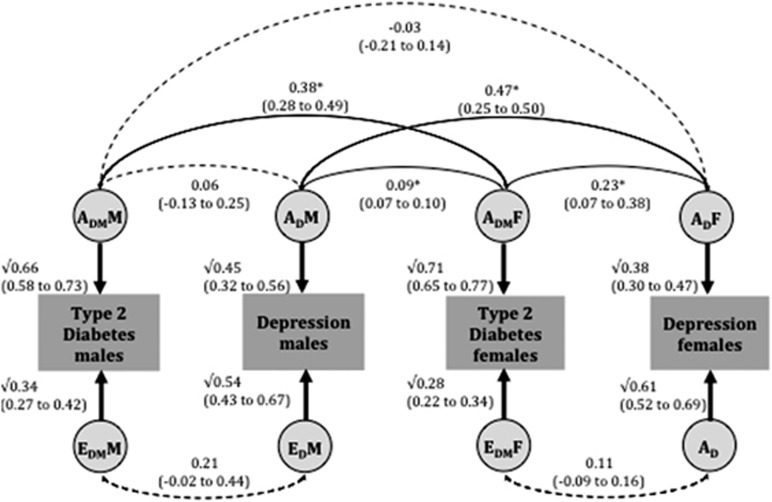
The best-fitting model is therefore the full sex-limitation bivariate AE model (Figure 1), with both quantitative and qualitative sex differences. The heritability estimates for clinical diagnoses of T2DM and depression were 66% (58–73%) and 45% (32–56%) in males and 71% (65–77%) and 38% (30–47%) in females, respectively. The genetic correlation between T2DM and depression was non-significant in males (0.06 (−0.13–0.25)) but significant in females (0.23 (0.07–0.38)). In males, the phenotypic correlation between T2DM and depression was 31% due to genetic factors and 69% due to individual-specific environmental factors, whereas in females, these values were 75% and 25%, respectively.
Figure 1.
Parameters estimates from bivariate AE twin models for type 2 diabetes and depression, using the Swedish twin sample, with age as a covariate. Best-fit full sex-limitation bivariate AE model fit to Swedish data in opposite-sex dizygotic twins. Asterisk indicates a significant pathway. A indicates addictive genetic effects; E for unique environmental effects; subscript DM for type 2 diabetes; subscript D for depression DM; M for males and F for females. Additive genetic and unique environment contributions to type 2 diabetes and depression are indicated by ADMM, ADM, EDMM and EDM in males, and by ADMF, ADF, EDMF and EDF in females, respectively.
Under the assumption of random mating, all correlations between genetic factors are expected to be 0.50 in same-sex DZ sibling pairs.24 The estimated correlation between male and female genetic factors for T2DM (ADMM and ADMF) was 0.38 (0.28–0.49) and for depression (ADM and ADF) is 0.47 (0.25–0.50; Figure 1). They are close to the expected value of 0.50, suggesting little or no qualitative sex difference within diabetes and depression, respectively. The estimated correlations between the genetic factors for (i) male T2DM and female depression (ADMM and ADF) and (ii) male depression and female T2DM (ADM and ADMF) were −0.03 (−0.21–0.14) and 0.09 (0.07–0.10), respectively. They are substantially different from 0.50, indicating that different genetic factors are at play in males and females when it comes to explaining the T2DM-depression association (qualitative sex difference).
Genetic model fitting in the Danish twin sample
The same approach was adopted in the Danish sample. The starting model was the ACE model with both quantitative and qualitative sex differences in genetic factors (model ID: −2LL=58 776.30; df=176 878; AIC=−294 979.7). The effect of common environment was again negligible, as shown by a non-significantly decline in fit (model 2D: −2LL=58 778.83; df=176 884; AIC=−294 989.2; χ2=2.52; P=0.87). The effect of qualitative sex difference was important, as when all genetic factors were constrained to correlate at 0.50 across males and females in the opposite-sex pair model, a significantly poorer fit of the AE model resulted (model 3D: −2LL=58 838.40; df=176 888; AIC=−294 937.6; χ2=62.10; P<0.05). All A and E paths are therefore estimated freely for males and females with no further constraints.
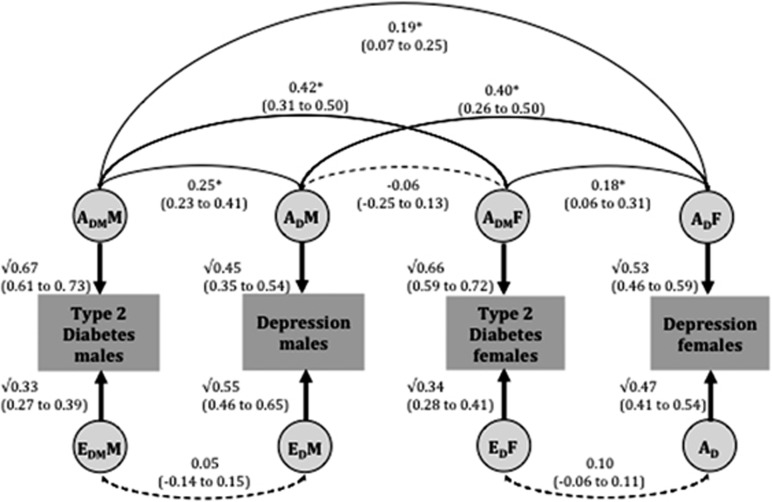
The best-fitting model is also the full sex-limitation bivariate AE model (Figure 2), with both quantitative and qualitative sex differences. The heritability estimates for clinical diagnoses of T2DM and depression were 67% (61–73%) and 45% (35–54%) in males and 66% (59–72%) and 53% (46–59%) in females, respectively. In both males and females, the genetic correlation between T2DM and depression was moderate and significant (males: 0.25 (0.23–0.41); females: 0.18 (0.06–0.31)). The phenotypic correlation was primarily attributed to shared genetic effects in both sexes (males: 87% females: 74%).
Figure 2.
Parameters estimates from bivariate AE twin models for type 2 diabetes and depression, using the Danish twin sample, with age as a covariate. Best-fit full sex-limitation bivariate AE model fit to Danish data in opposite-sex dizygotic twins. Asterisk indicates a significant pathway. A indicates addictive genetic effects; E for unique environmental effects; subscript DM for type 2 diabetes; subscript D for depression DM; M for males and F for females. Additive genetic and unique environment contributions to type 2 diabetes and depression are indicated by ADMM, ADM, EDMM and EDM in males, and by ADMF, ADF, EDMF and EDF in females, respectively.
The estimated correlation between male and female genetic factors for T2DM (ADMM and ADMF) was 0.42 (0.31–0.50) and for depression (ADM and ADF) was 0.40 (0.26–0.50; Figure 1). They are close to the expected value of 0.50, suggesting little or no qualitative sex difference within diabetes and depression, respectively. The estimated correlations between the genetic factors for (i) male T2DM and female depression (ADMM and ADF) and (ii) male depression and female T2DM (ADM and ADMF) were 0.19 (0.07–0.25) and −0.06 (−0.25–0.13), respectively. These are substantially different from 0.50, indicating that different genetic factors are involved in explaining the T2DM-depression association across sex (qualitative sex difference).
Secondary analysis
In samples restricted to individuals born after 1950, the best-fitting model is also the full sex-limitation bivariate AE model in both the Swedish and Danish samples, with both quantitative and qualitative sex differences (data not shown). In the Swedish sample, the phenotypic correlation between T2DM and depression was significant in both males (0.21 (0.13–0.30)) and females (0.25 (0.17–0.33)). The heritability estimates for T2DM and depression were 73% (58–81%) and 52% (50–67%) in males and 72% (58–84%) and 40% (30–49%) in females, respectively. The genetic correlation between T2DM and depression was significant for females (males: 0.00 (−0.24–0.32); females: 0.49 (0.26–0.73)). There is no major difference between the findings from the primary and secondary analysis.
In the Danish sample, the phenotypic correlation between T2DM and depression was significant in both males (0.11 (0.02–0.19)) and females (0.13 (0.04–0.22)). The heritability estimates for T2DM and depression were 78% (67–85%) and 45% (31–58%) in males and 78% (62–89%) and 47% (36–57%) in females, respectively. The genetic correlation between T2DM and depression was no longer significant (males: 0.07 (−0.18–0.32); females: 0.21 (−0.06–0.47)), in comparison with the primary analysis, as the 95% confidence intervals cross zero.
Discussion
To the best of our knowledge, this is the first study to examine the genetic aetiology of the T2DM-depression comorbidity using hospital registry data from two large population samples of male and female twins covering the entire adult age range. The most notable feature of our finding is the similarity between the results from the Swedish and Danish samples. Both samples support the notion that qualitative genetic sex differences are at play in the genetic overlap between T2DM and depression. The main difference is the proportion of the T2DM-depression association due to shared genetic factors between Swedish and Danish samples. In the Swedish sample, the phenotypic relationship is mainly due to unique environment factors in males and shared genetic factors in females. In the Danish sample, it is due to shared genetic effects in both sexes. The observations were attenuated in the analyses restricted to individuals born after 1950. Although this could be a genuine difference, the 95% confidence intervals around the genetic correlations are overlapping between the primary and secondary analyses. In addition, the secondary analysis is half of the initial sample size for the Swedish sample (primary analysis: n=68 606; secondary analysis: n=35 912) and two fifths for the Danish sample (primary analysis: n=95 403; secondary analysis: n=40 984), leading to reduced power. The secondary analysis further strengthens the validity of our primary findings, as any observed cohort effect may potentially be secondary to multiple residual confounders. The findings, however, did not differ significantly between the entire sample and those born after 1950, especially in the Swedish sample.
Previous literature
Our results differ from previous findings. The Vietnam Era Twins Study of Aging8 suggests that there is no evidence of common genetic factors in contributing to the T2DM-depression association. The finding is difficult to interpret as it was restricted to males aged 50–59, relied on a much smaller sample and used self-reported data to construct the diagnosis of T2DM and depression.
The SALT study (using a cohort subsample included in our Swedish sample) previously reported a significant contribution of unique environmental factors in the T2DM-depression association, but insignificant contribution of genetic factors.9 Discrepancies in results could be due to reduced power, narrower age range and the use of computerised interview-based Composite International Diagnostic Inventory-Short Form instead of hospital registry data.
Implication
This is the first study examining genetic overlap in the T2DM-depression comorbidity in two large, population-based twin registries. Our finding of qualitative genetic sex differences implies that although the comorbidity is mainly due to correlated genetic risk factors, these factors are not necessarily the same in males and females. Possible biological support for this observed latent effect is given by findings regarding the activation of the hypothalamic–pituitary adrenal axis, which has been postulated to have a major role in the T2DM and depression comorbidity.25 A preliminary study reported sex difference between hypothalamic–pituitary adrenal axis activity and adrenergic polymorphisms in 189 patients with depression.26 Increased hypothalamic–pituitary adrenal axis activity was associated with the ADRA2A genotype in males and ADRB2 genotype in females.
Other support for the observed qualitative sex differences in comorbidity is given by findings regarding visceral obesity, which has been suggested as a biological explanatory link between T2DM and depression comorbidity.25, 27 Anthropometric traits such as waist-to-hip ratio are well-established proxy measures of abdominal fat distribution.28, 29 Sex-specific dimorphic genetic effects have been reported for waist-to-hip ratios.30 Of particular interest, a female-only association was reported for the peroxisome proliferator-activated receptors region.31 Proliferator-activated receptors-γ has a well-established role in T2DM treatment and has been implicated in its pathology.32 It has also been postulated as a potential target for the treatment of depression, as it is closely related to parainflammation and endoplasmic reticulum stress, processes that can potentially interfere with normal stress response in a depressive illness.33, 34 Interestingly, the glucocorticoid receptor gene have been linked to both T2DM35 and depression,36 and its polymorphism has been associated with β-cell function in females, but not in males.37
The findings from this study have potential clinical relevance. Our study demonstrates that the T2DM-depression comorbidity may be genetic in origin, with different genetic factors being at play in males and females. Future research would involve developing sex-specific biopsychosocial explanatory models and conducting sex-specific analysis in molecular genetic studies. Understanding the underlying mechanism of the T2DM and depression comorbidity will provide useful insight into identifying potential biological mechanisms that may be accessible to interventions.
Limitations
Our findings should be interpreted in the context of potentially significant limitations. No discrimination of types of diabetes was made in ICD-8 (1965) and the loss of participants at the time of retrieval of hospital registry data (2010 for the Swedish Twin Registry and 2011 for the Danish Twin Registry) may lead to misclassification of diabetes type. This is probably modest, given that T2DM comprises 90% of adults with diabetes.38 Estimations obtained from a single database should, however, be interpreted with caution.39 In addition, the mean age of onset for T2DM has been reported to be 46-year old40 and we have included all individuals aged over 18 in both samples. It is therefore possible that individuals without a diagnosis of T2DM still have the propensity to develop the illness.
Another important limitation of our study is the use of national health registries. The Swedish and Danish National Hospital Discharge Registries do not include visits to general practitioners and have been shown to capture more severe cases.41 Our constructs of T2DM and depression might therefore capture a more severe spectrum of the disorders that warrant outpatient care and/or hospital admission. In addition, there is a small possibility of misclassifications of cases as controls, such as when a participant had a past history of depression before the inception of the registry and did not subsequently have further episodes of depression requiring outpatient or inpatient care.
Studies have also shown that males were less likely to seek help.42 The prevalence rates for diabetes and depression were similar across the sexes (Table 1). Anthropometric traits such as body mass index have not been adjusted for in this study and we need to note the limitation that the observed genetic overlap can in part be due to the respective correlation of these traits with T2DM43 and depression.44 This and previous studies on T2DM and depression have focused on European-descent samples and replication in Asian and African descent populations are needed.
Conclusion
This study uses two large, independent population-based twin samples to explore the genetic overlap between T2DM and depression. Our results suggest a genetic aetiology to the T2DM-depression comorbidity in males and females. It further suggests that different genetic risk factors may be involved in this comorbidity in males and females. T2DM and depression are chronic conditions with major public health impact. Understanding the pathophysiology of the T2DM and depression comorbidity, the basis for possible including sex differences, is critical for developing appropriate and effective interventions for this complex patient group.
Acknowledgments
The project is based on data from the Swedish Twin Registry and Danish Twin Registry and we would like to thank all participants. Open access for this article was funded by King's College London. CK, NP, KC, KI and FR designed the protocol. CK wrote the manuscript and performed the statistical analysis. FR supervised the statistical analysis. All authors reviewed/edited the manuscript. Dr Carol Kan currently receives salary support from Novo Nordisk UK Research Foundation and was funded by National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust in the past. Professor Khalida Ismail and Dr James MacCabe are part funded by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008; 299: 2751–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PC, Chan YT, Chen HF, Ko MC, Li CY. Population-based cohort analyses of the bidirectional relationship between type 2 diabetes and depression. Diabetes Care 2013; 36: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotella F, Mannucci E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res Clin Pract 2013; 99: 98–104. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008; 31: 2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 2012; 379: 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laake JP, Stahl D, Amiel SA, Petrak F, Sherwood RA, Pickup JC et al. The association between depressive symptoms and systemic inflammation in people with type 2 diabetes: findings from the South London Diabetes Study. Diabetes Care 2014; 37: 2186–2192. [DOI] [PubMed] [Google Scholar]
- NMC LR. Methodology for Genetic Studies of Twins and Families in Neurogenetics. Kluwer Academic Publishers: The Netherlands, 1992. [Google Scholar]
- Scherrer JF, Xian H, Lustman PJ, Franz CE, McCaffery J, Lyons MJ et al. A test for common genetic and environmental vulnerability to depression and diabetes. Twin Res Hum Genet 2011; 14: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Heh V, Prom-Wormley E, Kendler KS, Pedersen NL. Association between major depression and type 2 diabetes in midlife: findings from the screening across the lifespan twin study. Psychosom Med 2015; 77: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Moran E, Satylganova A, Orueta J, Nuno-Solinis R. Prevalence of depression in adults with type 2 diabetes in the Basque Country: relationship with glycaemic control and health care costs. BMC Public Health 2014; 14: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24: 1069–1078. [DOI] [PubMed] [Google Scholar]
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006; 23: 1165–1173. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Lichtenstein P, Svedberg P. The Swedish Twin Registry in the third millennium. Twin Res 2002; 5: 427–432. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet 2006; 9: 875–882. [DOI] [PubMed] [Google Scholar]
- Furberg H, Lichtenstein P, Pedersen N, Thornton L, Bulik C, Lerman C et al. The STAGE cohort: a prospective study of tobacco use among Swedish twins. Nicotine Tob Res 2008; 10: 1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skytthe A, Kyvik K, Bathum L, Holm N, Vaupel JW, Christensen K. The Danish Twin Registry in the new millennium. Twin Res Hum Genet 2006; 9: 763–771. [DOI] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011; 39: 30–33. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Int Med 2002; 252: 184–205. [DOI] [PubMed] [Google Scholar]
- Christiansen L, Frederiksen H, Schousboe K, Skytthe A, von Wurmb-Schwark N, Christensen K et al. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res 2003; 6: 275–278. [DOI] [PubMed] [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G x E interaction. Twin Res Hum Genet 2006; 9: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika 2011; 76: 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS. Inheritance of liability to certain diseases estimated from incidence among relatives. Ann Hum Genet 1965; 29: 51–76. [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika 1987; 52: 317–332. [Google Scholar]
- Jacquard A. The Genetic Structure of Populations. Springer-Verlag: Berlin, Germany, 1974. [Google Scholar]
- Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological basis of depression in adults with diabetes. Curr Diab Rep 2010; 10: 396–405. [DOI] [PubMed] [Google Scholar]
- Haefner S, Baghai TC, Schule C, Eser D, Spraul M, Zill P et al. Impact of gene-gender effects of adrenergic polymorphisms on hypothalamic-pituitary-adrenal axis activity in depressed patients. Neuropsychobiology 2008; 58: 154–162. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torréns JI, Kravitz HM et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care 2004; 27: 2856–2862. [DOI] [PubMed] [Google Scholar]
- Ludescher B, Machann J, Eschweiler GW, Vanhofen S, Maenz C, Thamer C et al. Correlation of fat distribution in whole body MRI with generally used anthropometric data. Invest Radiol 2009; 44: 712–719. [DOI] [PubMed] [Google Scholar]
- Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjöström L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg. Sweden1984 1984; 289: 1257–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015; 518: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall JC, Winkler TW, Kutalik Z, Berndt SI, Jackson AU, Monda KL et al. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet 2013; 9: e1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010; 42: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani MS, Javadi-Paydar M, Gharedaghi MH, Fard YY, Dehpour AR. Antidepressant-like effect of pioglitazone in the forced swimming test in mice: the role of PPAR-gamma receptor and nitric oxide pathway. Behav Brain Res 2011; 224: 336–343. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Kenna HA, Williams KE, Powers B, Wroolie T, Schatzberg AF. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. ScientificWorldJournal 2010; 10: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel R, Reis AF, Dubois-Laforgue D, Bellanne-Chantelot C, Timsit J, Velho G. The N363S polymorphism in the glucocorticoid receptor gene is associated with overweight in subjects with type 2 diabetes mellitus. Clin Endocrinol 2003; 59: 237–241. [DOI] [PubMed] [Google Scholar]
- van West D, Van Den Eede F, Del-Favero J, Souery D, Norrback KF, Van Duijn C et al. Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology 2006; 31: 620–627. [DOI] [PubMed] [Google Scholar]
- van Raalte DH, van Leeuwen N, Simonis-Bik AM, Nijpels G, van Haeften TW, Schafer SA et al. Glucocorticoid receptor gene polymorphisms are associated with reduced first-phase glucose-stimulated insulin secretion and disposition index in women, but not in men. Diabet Med 2012; 29: e211–e216. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- Orueta JF, Nuno-Solinis R, Mateos M, Vergara I, Grandes G, Esnaola S. Monitoring the prevalence of chronic conditions: which data should we use? BMC Health Serv Res 2012; 12: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman RJ, Mainous AG 3rd, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005; 3: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver MI, Pearson N, Coe N, Gunnell D. Help-seeking behaviour in men and women with common mental health problems: cross-sectional study. Br J Psychiatry 2005; 186: 297–301. [DOI] [PubMed] [Google Scholar]
- Drong AW, Lindgren CM, McCarthy MI. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther 2012; 92: 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Kubzansky LD, Koenen KC, Liang L, Tchetgen Tchetgen EJ, Cornelis MC et al. Revisiting mendelian randomization studies of the effect of body mass index on depression. Am J Med Genet Neuropsychiatr Genet 2015; 168: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.