Abstract
Advancing paternal and maternal age have both been associated with risk for autism spectrum disorders (ASD). However, the shape of the association remains unclear, and results on the joint associations is lacking. This study tests if advancing paternal and maternal ages are independently associated with ASD risk and estimates the functional form of the associations. In a population-based cohort study from five countries (Denmark, Israel, Norway, Sweden and Western Australia) comprising 5 766 794 children born 1985–2004 and followed up to the end of 2004–2009, the relative risk (RR) of ASD was estimated by using logistic regression and splines. Our analyses included 30 902 cases of ASD. Advancing paternal and maternal age were each associated with increased RR of ASD after adjusting for confounding and the other parent's age (mothers 40–49 years vs 20–29 years, RR=1.15 (95% confidence interval (CI): 1.06–1.24), P-value<0.001; fathers⩾50 years vs 20–29 years, RR=1.66 (95% CI: 1.49–1.85), P-value<0.001). Younger maternal age was also associated with increased risk for ASD (mothers <20 years vs 20–29 years, RR=1.18 (95% CI: 1.08–1.29), P-value<0.001). There was a joint effect of maternal and paternal age with increasing risk of ASD for couples with increasing differences in parental ages. We did not find any support for a modifying effect by the sex of the offspring. In conclusion, as shown in multiple geographic regions, increases in ASD was not only limited to advancing paternal or maternal age alone but also to differences parental age including younger or older similarly aged parents as well as disparately aged parents.
Introduction
The associations between older paternal age and autism spectrum disorders (ASD) is now well established,1, 2, 3, 4, 5 including two recent population-based studies.4, 5 A risk increase with advancing maternal age has also been shown.6 There are, however, important issues to resolve. Meta-analyses have shown considerable study heterogeneity2, 6 and it is still not clear whether paternal and maternal ages represent independent risk factors.7 Most importantly, there is limited information about the combined effect of paternal and maternal age as most studies have considered these as independent. There have been attempts to address this question4, 8 but with no clear conclusion (Supplementary Appendix A). For schizophrenia, parental age risk has been proposed through different biological mechanisms4, 9, 10 and an association with parental age differences has also been suggested.11 A large US study reported increased risk for adverse perinatal outcomes for mothers older than their partner.12 Even the largest population-based studies have been limited by size and thereby unable to reliably examine the risk from parental ages across the entire age distribution, or to properly separate the independent and combined effects of paternal and maternal ages.
Using a large population-based cohort across several geographic regions, the aim of this study was to test if advancing paternal and maternal age are independently associated with risk for ASD and to estimate the functional form of the association between parental age and ASD.
Materials and methods
The study builds on the 'International Collaboration for Autism Registry Epidemiology (ICARE)', combining population-based cohorts across several geographic regions and health systems with the purpose of studying risk factors for ASD.13 Ethics committee approval, with waiver for informed consent, was obtained by each site. The ASD prevalence varies across sites13 but time trends and rates are consistent with contemporary reports from other populations.14 For details see Schendel et al.13
Study population
The study population comprised all live-born singletons in Denmark, Norway and Sweden between 1985 and 2004. Data from Western Australia (WA) included all 1985–1999 singleton live-born births among the non-aboriginal population. Data from Israel comprised all 1993–2004 singleton live-born Jewish births with a subsequent diagnosis of ASD and a random sample of controls born in the same period. We excluded twins because they are highly correlated for ASD risk,15 thereby violating the assumption of independent observations, and they are also associated with maternal age and birth complications16 which could introduce bias.
ASD outcome, parental age and covariate information
Children were followed from birth to reported diagnosis of ASD through 2004 in WA; 2006 in Norway and 2009 in Denmark, Israel and Sweden. Sweden and Denmark provided diagnoses from medical registers whereas in Israel, Norway and WA, it was derived from government-maintained service/benefits registers. Case identification and registry reporting procedures have been described previously13 and Supplementary Appendix B provides details on the reliability and validity of reported diagnoses.
Parental ages, sex and birth year were obtained from birth or civil registers.
Statistical methods
Logistic regression was used to calculate odds-ratios as estimates of relative risk (RR). To achieve a qualitative (descriptive) interpretation of the functional form of ASD risk across parental ages we fitted splines.17, 18 To quantify the association, the RR of ASD was presented by categories of parental age. All statistical tests were performed by using the two-sided 5% level of significance and associated two-sided 95% Wald-type confidence intervals (CIs) were calculated. We did not adjust for multiplicity of statistical tests. Besides the exclusion of twins, it was not possible to identify family members in the data and correct for possible correlations between family members in the analyses.
Because data from Israel were obtained by using a case–control design with known sampling probabilities for cases and controls, rather than a birth cohort design, we included the sampling weights in the analyses.19 To adjust for the variation introduced by the sampling weights, robust standard errors were used.20
Paternal and maternal age independently
To visualize the shape and functional form of the RRs, we fitted splines to continuous paternal and maternal ages separately, adjusting for site, sex and potential confounding factors: the categorical age of the other parent (<20, 20–24, 25–29, 30–34, 35–39, ⩾40 for mothers and <20, 20–24, 25–29, 30–34, 35–39, 40–44 and ⩾45 for fathers), and child's birth year (4-year intervals). To quantify the risk, RRs were calculated by fitting logistic regression considering parental age categorically (mothers: <20, 20–29, 30–39, ⩾40; fathers: <20, 20–29, 30–39, 40–49 and ⩾50). All models had separate intercept parameters for each site, birth year (4-year intervals) and for male and female offspring.
Subgroup analyses
We further adjusted for the effect of the partner's age by analyzing parental age in sub-groups of the other parent's age. To avoid bias from uneven distribution of ASD cases in the different sub-groups we created sub-groups of maternal age with approximately equal numbers of ASD cases resulting in maternal age cut-offs of <27, 27–31 and >31 years. For each sub-group, we fitted the logistic spline models, both crude and adjusted. We used the same approach to define sub-groups of paternal age (<29, 29–34 and >34) while analyzing the maternal age.
Paternal and maternal age jointly
In a new approach enabled by the large sample size we analyzed paternal and maternal ages jointly on a bivariate continuous scale using thin-plate splines21 adjusting for sex and birth year categorically as above. The thin-plate spline is a two-dimensional smoother wherein the curvature is estimated in local regions moving over the bivariate (two-dimensional) surface of parental age. This is a descriptive statistical approach displaying the functional form of the relationship between bivariate paternal–maternal age and ASD with a qualitative interpretation rather than focusing on numerical estimates.
Finally, to determine the importance of independent and joint parental age effects on ASD risk, we fitted logistic regression models including separate covariates for the mean of the parent's ages (capturing the direct effect of ageing) and the difference in age between the parents (capturing effects not necessarily associated with ageing). We compared the goodness-of-fit of the models when including the two components together and each one separately by calculating the Akaike information criterion.
Supplementary analyses
We estimated the RR for male and female offspring separately. We repeated all analyses for autistic disorder (AD) only (excluding Israel where this information was not available).
The logistic regression model, assumes that the time at risk is the same for all subjects or that the length of follow-up does not affect the risk. If this assumption is violated, bias may be introduced. For this purpose, birth year was included in all models. We also performed a sensitivity analysis including Denmark and Sweden only, as both of these countries had information about date of diagnosis. Here we used Cox regression with age as the underlying time-scale and compared results with the logistic regression results. This approach adjusts for calendar effects as well.22
Site heterogeneity was addressed by visual inspection of the graphs of the spline predictions and by verifying pooled results through the use of models that excluded any potentially heterogeneous sites.
Data management
Statistical analyses were performed using SAS 9.3. Thin-plate splines were fitted using the gamm4, v0.1-6 package23 running R software 2.15.2.24
Results
The cohort comprised of 5 766 794 births (Denmark:1 270 229; Israel: 1 015 130; Norway: 1 121 392; Sweden: 2 023 017; and WA: 337 026). There were 30 902 (0.54%) children with ASD, and 10 128 (0.18%) with AD. Distributions of births, ASD cases, birth year and parental age are presented in Table 1 (site-specific data: Supplementary Table 1).
Table 1. Study population characteristics: covariate distributions by parental age.
| Variable | Interval (years)a | Births (N) | Male (%) | ASD N (%) | AD N (%) | Maternal age (year)b | Paternal age (year)b | Birth yearb |
|---|---|---|---|---|---|---|---|---|
| All Births | 5 766 794 | 51.2 | 30 902 (0.54) | 10 128 (0.18) | 29 (21–38) | 31 (23–42) | 1995 (1986–2004) | |
| Maternal age | <20 | 128 021 | 49.6 | 684 (0.53) | 207 (0.16) | 19 (17–19) | 23 (18–31) | 1994 (1985–2003) |
| 20–29 | 3 125 863 | 51.1 | 16 275 (0.52) | 5093 (0.16) | 26 (21–29) | 29 (23–37) | 1994 (1986–2003) | |
| 30–39 | 2 374 625 | 51.4 | 13 007 (0.55) | 4485 (0.19) | 33 (30–38) | 35 (28–44) | 1996 (1986–2004) | |
| ⩾40 | 138 284 | 49.6 | 936 (0.68) | 343 (0.25) | 41 (40–44) | 42 (32–52) | 1998 (1986–2004) | |
| Paternal age | <20 | 27 303 | 52.7 | 174 (0.64) | 54 (0.20) | 19 (16–24) | 19 (17–19) | 1993 (1985–2003) |
| 20–29 | 2 156 618 | 51 | 10 963 (0.51) | 3298 (0.15) | 25 (20–31) | 27 (22–29) | 1994 (1986–2003) | |
| 30–39 | 2 993 109 | 51.2 | 15 842 (0.53) | 5316 (0.18) | 30 (24–37) | 33 (30–39) | 1996 (1986–2004) | |
| 40–49 | 540 627 | 51.6 | 3497 (0.65) | 1288 ( 0.24) | 36 (26–42) | 42 (40–48) | 1996 (1986–2004) | |
| ⩾50 | 49 137 | 50.7 | 426 (0.87) | 172 (0.35) | 36 (26–44) | 52 (50–61) | 1997 (1986–2004) |
Abbreviations: ASD: autism spectrum disorder; AD: autistic disorder; N: number of individuals.
Maternal age <20 years and⩾40 years include mothers aged 15–19 years and 40–55 years, respectively; paternal age <20 years and⩾50 years include fathers aged 14–19 years and 50–71 years, respectively.
Mean (10th percentile, 90th percentile).
Independent effects of paternal and maternal age
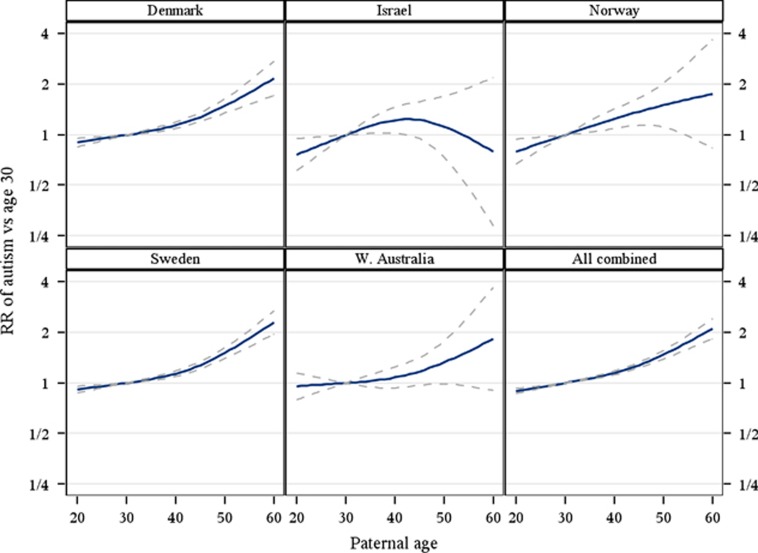
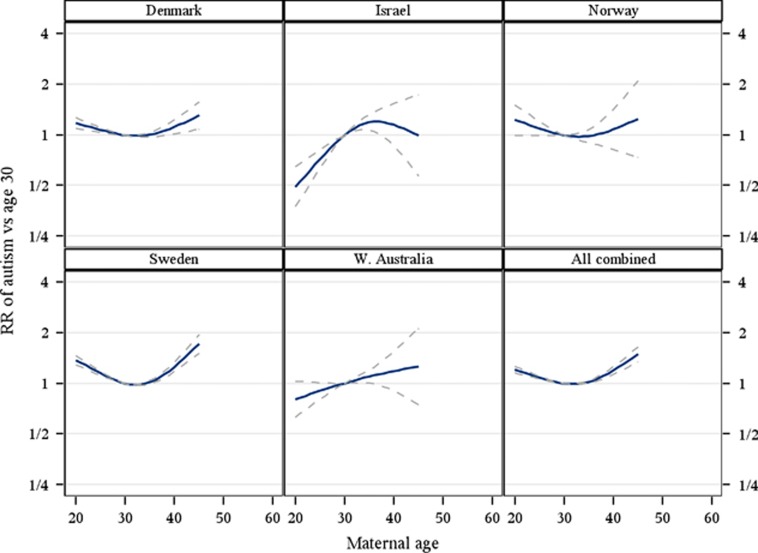
Qualitatively, there was a monotonic increase in ASD risk with increasing paternal age (Figure 1). In contrast, except for Israel and WA, there was a U-shaped association with maternal age with the lowest risk observed shortly after the age of 30. Relative to the age of 30 there was a statistically significantly increased risk of ASD associated with advancing, as well as younger maternal age (Figure 2, Table 2).
Figure 1.
Relative risk for autism spectrum disorder with increasing paternal age, for each site separately and combined. Point estimates and pointwise two-sided 95% confidence intervals. Relative risk calculated using the parental age of 30 years as reference. Relative risk estimated by ordinary logistic regression adjusting for age of the mother (<20, 20–24, 25–29, 30–34, 35–39 and ⩾40 years), sex (male/female), birth year (4-year intervals) and, for all sites combined, site (Denmark, Israel, Norway, Sweden and Western Australia).
Figure 2.
Relative risk for autism spectrum disorder with increasing maternal age, for each site separately and combined. Point estimates and pointwise two-sided 95% confidence intervals. Relative risk calculated using the parental age 30 as reference. Relative risk estimated by ordinary logistic regression adjusting for age of the father (<20, 20–24, 25–29, 30–34, 35–39, 40–44 and ⩾45 years), sex (male/female), birth year (4-year intervals) and, for all sites combined, site (Denmark, Israel, Norway, Sweden and Western Australia).
Table 2. Relative risk for ASD and AD, with associated two-sided 95% confidence intervals by maternal and parental age.
| Outcome | Age intervala | Maternal age crude | Maternal age adjustedb | Paternal age crude | Paternal age adjustedb |
|---|---|---|---|---|---|
| ASD | <20/20–29 | 1.16 (1.07–1.26) | 1.18 (1.08–1.29) | 1.23 (1.05–1.43) | 1.08 (0.92–1.27) |
| 30–39/20–29 | 1.02 (0.99–1.04) | 0.98 (0.95–1.01) | 1.01 (0.99–1.04) | 1.05 (1.02–1.08) | |
| 40–49/20–29c | 1.34 (1.24–1.44) | 1.15 (1.06–1.24) | 1.27 (1.22–1.32) | 1.28 (1.22–1.34) | |
| ⩾ 50/20–29 | 1.64 (1.47–1.82) | 1.66 (1.49–1.85) | |||
| AD | <20/20–29 | 1.11 (0.96–1.27) | 1.17 (1.01–1.36) | 1.15 (0.88–1.51) | 1.05 (0.78–1.40) |
| 30–39/20–29 | 1.11 (1.07–1.16) | 0.96 (0.91–1.01) | 1.13 (1.08–1.18) | 1.11 (1.06–1.17) | |
| 40–49/20–29c | 1.60 (1.43–1.78) | 1.15 (1.01–1.29) | 1.57 (1.47–1.67) | 1.52 (1.40–1.64) | |
| ⩾ 50/20–29 | 2.18 (1.87–2.54) | 2.05 (1.75–2.41) |
Abbreviations: AD, autistic disorder; ASD, autism spectrum disorder.
Relative risks compared with 20–29 year
Adjusted for site, sex, birth year (4-year-intervals), age of the other parent (mother: <20, 20–29, 30–39 and ⩾40 years; father: <20, 20–29, 30–39, 40–49 and ⩾50 years), sex (male/female), birth year (4-year intervals) and site (Denmark, Israel, Norway, Sweden, Western Australia).
For maternal age this is 40–49 is⩾40 year.
RRs in 10-year age categories are presented in Table 2. For example, relative to fathers aged 20–29 years, fathers 50 years or older had a statistically significantly increased risk for offspring with ASD (RR=1.66 95% CI:1.49–1.85). Relative to mothers aged 20–29 years, mothers younger than 20 years had a statistically significantly increased risk for offspring with ASD (RR=1.18 95% CI:1.08–1.29) (Table 2).
Similar patterns of association, but with slightly higher RRs for the highest parental ages, were evident for AD (Supplementary Figures 1 and 2, Table 2).
Sub-group analyses
An increase in ASD risk with advancing paternal age was evident in all three sub-groups of maternal age (<27, 27–31 and >31 years) (Supplementary Figure 3, right panel). For the oldest mothers (>31 years) the effect of advancing paternal age was delayed compared with mothers younger than 32 years, with no increase in risk before the father turned 40. For the two youngest sub-groups (mothers 31 years or younger), the increases RRs by advancing paternal age were similar (overlapping RRs), including a statistically significant decrease in RR when fathers were younger than 30 years.
The ASD risk by maternal age association was similarly U-shaped in each sub-group of paternal age (<29, 29–34 and >34 years) (Supplementary Figure 3, left panel), with increasing RR for both younger and older mothers, and, in each sub-group, the lowest RR for parents of similar age.
Joint effects of paternal and maternal ages
Couples of mothers 20–39 years of age and fathers 20–49 years of age generated 94.6% of all births. Among all children, 2.2% had a mother younger than 20 years and 2.4% had a mother 40 years or older; 0.9% had a father older than 50 years (Supplementary Table 2). The bivariate distribution of number of births by parental age across all sites is presented in Supplementary Figure 4.
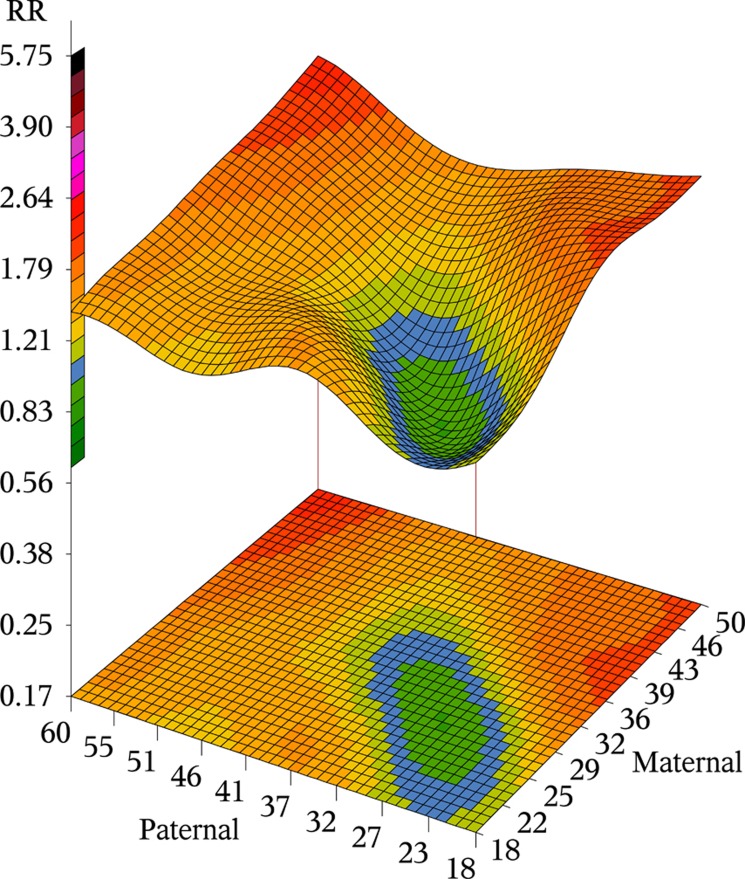
Using couples wherein the parents were both aged 25 years as the reference, the RR of ASD as a function of the joint influence of paternal and maternal age is displayed in Figure 3. Colors ranging from light green toward the yellow and red indicate increasing RR, while dark green indicates decreasing RR compared with the 25-year-old parents. The corresponding pointwise upper and lower 95% CIs are presented in Supplementary Figure 5.
Figure 3.
Relative risk (RR) for autism spectrum disorder by paternal and maternal age jointly. RR calculated by using the maternal and paternal ages of 25 years as reference. RR estimated by ordinary logistic regression adjusting for sex (male/female), birth year (4-year intervals), and, site (Denmark, Israel, Norway, Sweden and Western Australia). Blue color indicate RR≈1, light green, to yellow and red indicate increasing RR>1.
The joint effect of paternal and maternal ages on the RR of ASD (Figure 3) shows an inverse-shaped form compared with the age of parenting distribution (Supplementary Figure 4). That is, lowest risk corresponded to couples that generated the majority of births, specifically, 29–39-year-old fathers and 25–35-year-old mothers. RR increased in all directions from this region as the parental age difference increased. Furthermore, the RR pattern revealed that for mothers older than 40 years the RR increased in a U-shaped pattern with younger and older partner. For fathers 45 years or older the RR increased monotonically with increasing maternal age.
Illustrated in Figure 3, the highest ASD risk was evident among couples characterized by fathers older than ~45 years, independent of maternal age; fathers 35–44 years with mothers at least 10 years younger; mothers 30–39-years old with fathers at least 10 years younger. These couples corresponded to 7% of all births. In addition, among couples with fathers younger than 45 years and mothers younger than 40 years, the RR increased with increasing difference in age between the parents.
Examining whether mean parental age or parental age-difference explained most of the variation in ASD risk, there was strong support that they both were important, jointly (Supplementary Table 3).
Supplementary analyses
The RRs for ASD were similar for male and female offspring (Supplementary Figure 6). Cox regression using data from Denmark and Sweden produced RRs very similar to the RRs from the logistic regression models (Supplementary Table 4).
By visual inspection, the functional form of the RR for paternal age was similar across sites. For maternal age, results in Israel appeared to diverge from the other sites. This did not, however, bias the pooled results (Supplementary Figure 7). After excluding Israel, (a) the functional form of the RR for maternal age was similar (Supplementary Figure 7), (b) the RR associated with advancing maternal age in 10-year age categories showed similar results (data not shown) and (c) the three-dimensional figure for the bivariate effect of paternal and maternal ages was very similar (Supplementary Figure 8).
For all sites combined, the joint effect of paternal and maternal age on AD risk was qualitatively similar to that of ASD (Supplementary Figure 9).
Discussion
This study provides the strongest evidence to date supporting the hypothesis that advanced parental ages at the time of birth are independently associated with risk for ASD in the offspring; with no support for any modification by the sex of the child. There was also evidence for a combined parental age effect. The risk was highest when both parents were older, but the risk was also increased among disparately aged parents. Furthermore for all sites combined, and in each of the Scandinavian populations, there was an increased ASD risk for mothers younger than ~30 years; this was not observed in the Israeli population, which warrants further investigation.
The observed associations between parental age and ASD risk were similar in magnitude to those reported in earlier meta-analyses,2, 6 and with RRs higher for AD compared with ASD. The slight differences in RR between ASD and AD may indicate an effect of co-occurring intellectual disability (ID), more frequently reported in AD.25, 26 An earlier study showed higher RR for maternal and paternal age in children with ID.27 While we did not have data on ID in our database; data from a previously published study from Sweden28 estimated the proportion of children with ID among AD cases to 43% and only 15% among children with ASD, excluding AD.
Comparing fathers and mothers over the same age range, the RRs with advancing age were of similar magnitude. This suggests that advancing paternal age may contribute more to the risk than advancing maternal age overall, due to the longer male child-bearing potential. Human29, 30 and animal31 studies provide support for the hypothesis that de novo mutations contribute to the association between paternal age and ASD. In contrast, mechanisms mediating the effect of advancing maternal age on ASD risk have not been frequently investigated. Advancing maternal age has been associated with chromosomal changes32, 33 and genomic modifications.34
Our study, however, may provide insights into other potential etiological mechanisms underlying paternal and maternal age-associated factors in ASD risk. Our analyses showed that there was an increase in risk also when the difference between the parental ages was moderate-to-large (10 years or more) (Supplementary Figure 4). This suggests that the increase in risk is not attributable to advancing parental age per se, and that the risk increase cannot be explained solely by an accumulation of point mutations or other genomic alterations in the parents. Our data currently do not allow for further exploration of this phenomenon, but the mechanism underlying such reproductive strategies should be considered further. It is possible that the partners in these disparately aged couples represent other socio-economic (SES), genetic, and/or psychological characteristics that increase their risk of having children with ASD. Genetic shared influences (pleiotrophy) between ASD and uncommon reproductive ages may harbour potential biological mechanisms.35
The role of low SES in the association between parental age and ASD has not yet been resolved, and can potentially effect the associations in different ways. Firstly, SES can be a source for ascertainment bias (for example, in settings where access to health care is differential with respect to SES, as in the USA). In this situation, adjusting for maternal SES is warranted to control for confounding between maternal age and ASD. A study from Denmark36 examining multiple indices of SES and risk for ASD concluded that SES have little or no role in the risk of ASD, at least where access to the health-care system is equally available for all and is free of charge.
Secondly, younger parental age can have consequences effecting education, employment and other markers of SES. Therefore, while a low SES may be a risk factor for ASD, it may lie on the same causal path as younger parental age and act as a factor modifying the risk of ASD. Such a complex interplay between SES and maternal age can be accompanied by other risks for poor outcome, including maternal infection and poorer health monitoring. In this situation, adjusting for SES, as a mediator between maternal age and ASD, would be erroneous and may even introduce bias in the maternal age-ASD risk estimate.
Alternatively, low SES may be a proxy for other shared factors that may contribute to ASD risk, for example, genetic confounding. In this situation, adjustment for SES may be warranted as well. Therefore, the decision to adjust, or not, for SES deserves careful consideration in future studies of ASD. A recent publication has discussed this issue, also in the context of parental age, did not adjust for SES and reported a U-shaped relationship.4 Other studies that adjusted for SES mostly report a monotone association with maternal age.2 A Swedish study showed a U-shaped association with maternal age for ASD with co-morbid ID but not for ASD without.27
Social phobia and traits such as shyness and aloofness that may limit cross-sex interactions have been described for parents of children with ASD.37, 38 These factors could influence the age when a person establishes a relationship and has children, confounding the association between parental age and risk of ASD. A US study with nine million pregnancies showed altered risks of fetal death, preterm birth and small size for gestational age with increasing parental age differences.12 More generally, large trait differences between partners have been shown to be associated with adverse pregnancy outcomes. For example, in a large study from the UK, an increasing father–mother height difference was associated with abnormal pregnancy outcomes.39 Also, higher fertility has been seen in men with partners about 6 years younger and in women with partners ~4 years older, indicating fitness-benefits with certain combinations of parental age.40 Environmental and perinatal factors may also contribute cumulatively or at specific ages; pre- and perinatal complications are associated with ASD risk41 and a U-shaped risk profile has been reported for preterm birth and birth weight in relation to parental age.42
Study strengths include the large sample size, with prospective follow-up, in national cohorts, and along with rigorous data harmonization and quality control processes.13 The associations between parental age and ASD were similar regardless of whether clinical or service/benefits registers were used for case ascertainment. The sites' publicly financed health systems minimizes any potential bias due to differential access to health care.
The ASD prevalence observed was highest in Denmark and Sweden, somewhat lower in WA, and lowest in Israel and Norway. The range of prevalence rates in the contributing sites (12–84 per 10 000) is consistent with rates reported in epidemiological studies of ASD during the same decades.43 Rates in Denmark and Sweden are similar to those reported in newer epidemiological surveys.43 Prevalence's and time trends across sites are discussed in detail in an earlier methods paper.13
Study limitations include sparse data for extreme parental ages, despite the large sample size. Also we lack information about potentially confounding variables such as SES and parental psychiatric history.35, 44 We cannot rule out the possibility that other factors associated with parental age (for example, length of marriage or partnership, obstetric complications, gestational age and birth weight) have an important role in explaining our results. Time-trends are also possible sources of bias. Differences in year of birth and length of follow-up may explain part of the site-to-site variations. Using date of diagnosis would have allowed for a more detailed statistical adjustment for length of follow-up, but the assumptions about the data distribution used here follow the main approach used by most earlier studies.1, 2, 30, 45 Sweden and Denmark provided diagnostic information from medical registers whereas for Israel, Norway and WA, diagnostic information was derived from service/benefits registers. Service/benefit registers could potentially be biased towards cases associated with co-morbid ID. We did not have individual level information on co-morbid ID in ASD cases. Nevertheless, validation studies, from Sweden46 and Denmark47 reported rates of ID in ASD cases that were comparable to, or even somewhat lower than, reports in recent epidemiological studies identifying ASD through screening and diagnostic evaluations.48, 49 Analyses restricted to Sweden and Denmark produced RRs very similar to the RRs observed for all sites (Supplementary Table 4). Thus, while we could not completely rule out bias due to service registers potentially including a higher proportion of ASD cases with co-morbid ID, we found no evidence that this could substantially effect the overall results of the present study.
Conclusion
Shown in multiple geographic regions, increases in ASD risk was not only limited to advancing paternal or maternal age alone but also to differences in parental age including younger or older similarly aged parents as well as disparately aged parents.
These results suggest that multiple mechanisms are contributing to the association between parental age and ASD risk.
Acknowledgments
We gratefully acknowledge Sara Pettersson, Physiotherapist, Unit for children and adults with autism, Uppsala health and habilitation center, for excellence in providing data extraction from medical journals and supporting the validation of the Swedish ASD diagnoses (Supplementary Appendix B). Sara Pettersson did not receive any personal compensation for her work associated with this manuscript ICARE was funded by Autism Speaks grant nos 6230, 6246, 6247, 6248, 6249, 6251 and 6295. MR, Autism Speaks Portfolio Manager for ICARE, participated in the interpretation of the data; preparation, review and approval of the manuscript; and the decision to submit the manuscript for publication. Autism Speaks did not contribute to the design and conduct of the study; or collection, management and analysis of the data. SS and AR are supported by the Beatrice and Samuel A Seaver Foundation.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Grether JK, Anderson MC, Croen LA, Smith D, Windham GC. Risk of autism and increasing maternal and paternal age in a large north American population. Am J Epidemiol 2009; 170: 1118–1126. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol Psychiatry 2011; 16: 1203–1212. [DOI] [PubMed] [Google Scholar]
- Parner ET, Baron-Cohen S, Lauritsen MB, Jørgensen M, Schieve LA, Yeargin-Allsopp M et al. Parental age and autism spectrum disorders. Ann Epidemiol 2012; 22: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry 2014; 71: 301–309. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, Sjölander A et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry 2014; 71: 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Hultman CM, Kolevzon A, Gross R, Maccabe JH, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2012; 51: 477–486.e1. [DOI] [PubMed] [Google Scholar]
- Durkin MS, Maenner MJ, Newschaffer CJ, Lee L-C, Cunniff CM, Daniels JL et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol 2008; 168: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res 2010; 3: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry 2001; 58: 361–367. [DOI] [PubMed] [Google Scholar]
- Ek M, Wicks S, Svensson AC, Idring S, Dalman C. Advancing paternal age and schizophrenia: the impact of delayed fatherhood. Schizophr Bull 2014; 41: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. Parental age difference and schizophrenia. Br J Psychiatry 2004; 184: 540–541. [DOI] [PubMed] [Google Scholar]
- Kinzler WL, Ananth CV, Smulian JC, Vintzileos AM. Parental age difference and adverse perinatal outcomes in the United States. Paediatr Perinat Epidemiol 2002; 16: 320–327. [DOI] [PubMed] [Google Scholar]
- Schendel DE, Bresnahan M, Carter KW, Francis RW, Gissler M, Grønborg TK et al. The International Collaboration for Autism Registry Epidemiology (iCARE): multinational registry-based investigations of autism risk factors and trends. J Autism Dev Disord 2013; 43: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh Y-J, Kim YS, Kauchali S, Marcín C et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res 2012; 5: 160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet Part B Neuropsychiatr Genet 2011; 156B: 255–274. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Chauhan SP. Epidemiology of twinning in developed countries. Semin Perinatol 2012; 36: 156–161. [DOI] [PubMed] [Google Scholar]
- Smith PL. Splines as a useful and convenient statistical tool. Am Stat 1979; 33: 57–62. [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd Springer, 2009. Corr. 3rd printing, 5th Printing. [Google Scholar]
- Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime Data Anal 2000; 6: 39–58. [DOI] [PubMed] [Google Scholar]
- Barlow WE. Robust variance estimation for the case-cohort design. Biometrics 1994; 50: 1064–1072. [PubMed] [Google Scholar]
- Wood SN. Thin plate regression splines. J R Stat Soc Ser B Stat Methodol 2003; 65: 95–114. [Google Scholar]
- Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 1997; 145: 72–80. [DOI] [PubMed] [Google Scholar]
- Wood S, Scheipl F gamm4: Generalized additive mixed models using mgcv and lme4. 2013. Available at http://cran.r-project.org/web/packages/gamm4/index.html (accessed 13 September 2013).
- Anon. Development Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2005. Available at http://www.R-project.org (accessed 10 November 2011).
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry 2005; 162: 1133–1141. [DOI] [PubMed] [Google Scholar]
- Carlsson LH, Norrelgen F, Kjellmer L, Westerlund J, Gillberg C, Fernell E. Coexisting disorders and problems in preschool children with autism spectrum disorders. Sci World J 2013; 2013: 213979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idring S, Magnusson C, Lundberg M, Ek M, Rai D, Svensson AC et al. Parental age and the risk of autism spectrum disorders: findings from a Swedish population-based cohort. Int J Epidemiol 2014; 43: 107–115. [DOI] [PubMed] [Google Scholar]
- Sandin S, Nygren K-G, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA 2013; 310: 75–84. [DOI] [PubMed] [Google Scholar]
- Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature 2012; 488: 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S et al. Advancing paternal age and autism. Arch Gen Psychiatry 2006; 63: 1026–1032. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Foldi CJ, Chong S, Whitelaw E, Moser RJ, Burne THJ et al. Increased de novo copy number variants in the offspring of older males. Transl Psychiatry 2011; 1: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RH. Meiotic errors in human oogenesis and spermatogenesis. Reprod Biomed Online 2008; 16: 523–531. [DOI] [PubMed] [Google Scholar]
- Ginsburg C, Fokstuen S, Schinzel A. The contribution of uniparental disomy to congenital development defects in children born to mothers at advanced childbearing age. Am J Med Genet 2000; 95: 454–460. [PubMed] [Google Scholar]
- Kaytor MD, Burright EN, Duvick LA, Zoghbi HY, Orr HT. Increased trinucleotide repeat instability with advanced maternal age. Hum Mol Genet 1997; 6: 2135–2139. [DOI] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev 2009; 19: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol 2005; 161: 916–925, discussion 926–928. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry 1997; 154: 185–190. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: evidence from a family study of multiple-incidence autism families. Am J Psychiatry 1999; 156: 557–563. [DOI] [PubMed] [Google Scholar]
- Mascie-Taylor CG, Boldsen JL. Assortative mating, differential fertility and abnormal pregnancy outcome. Ann Hum Biol 1988; 15: 223–228. [DOI] [PubMed] [Google Scholar]
- Fieder M, Huber S. Parental age difference and offspring count in humans. Biol Lett 2007; 3: 689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med 2007; 161: 326–333. [DOI] [PubMed] [Google Scholar]
- Abel EL, Kruger M, Burd L. Effects of maternal and paternal age on Caucasian and native American preterm births and birth weights. Am J Perinatol 2002; 19: 49–54. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 2003; 33: 365–382. [DOI] [PubMed] [Google Scholar]
- Frans EM, Sandin S, Reichenberg A, Långström N, Lichtenstein P, McGrath JJ et al. Autism risk across generations: a population-based study of advancing grandpaternal and paternal age. JAMA Psychiatry Chic Ill 2013; 70: 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasanfar R, Haddad SA, Tolouei A, Ghadami M, Yu D, Santangelo SL. Paternal age increases the risk for autism in an Iranian population sample. Mol Autism 2010; 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idring S, Rai D, Dal H, Dalman C, Sturm H, Zander E et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PloS One 2012; 7: e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen MB, Jørgensen M, Madsen KM, Lemcke S, Toft S, Grove J et al. Validity of childhood autism in the Danish Psychiatric Central Register: findings from a cohort sample born 1990-1999. J Autism Dev Disord 2010; 40: 139–148. [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. Morb Mortal Wkly Rep Surveill Summ 2012; 61: 1–19. [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh Y-J, Fombonne E, Laska E, Lim E-C et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry 2011; 168: 904–912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.