Abstract
Depression is a common debilitating human disease whose etiology has defied decades of research. A critical bottleneck is the difficulty in modeling depressive episodes in animals. Here, we show that a transgenic mouse with chronic forebrain expression of a dominant negative mutant of Polg1, a mitochondrial DNA (mtDNA) polymerase, exhibits lethargic behavioral changes, which are associated with emotional, vegetative and psychomotor disturbances, and response to antidepression drug treatment. The results suggested a symptomatic similarity between the lethargic behavioral change that was recurrently and spontaneously experienced by the mutant mice and major depressive episode as defined by DSM-5. A comprehensive screen of mutant brain revealed a hotspot for mtDNA deletions and mitochondrial dysfunction in the paraventricular thalamic nucleus (PVT) with similar defects observed in postmortem brains of patients with mitochondrial disease with mood symptoms. Remarkably, the genetic inhibition of PVT synaptic output by Cre-loxP-dependent expression of tetanus toxin triggered de novo depression-like episodes. These findings identify a novel preclinical mouse model and brain area for major depressive episodes with mitochondrial dysfunction as its cellular mechanism.
Introduction
Despite decades of research, the clinical time course of depression, most notably its spontaneous episodic recurrent nature has been difficult to model in animals. Current animal models of depression primarily use acute stress-induced behavioral changes.1 However, roughly half of depression patients experience relapses2 that are not formally preceded by overt stressful events,3 and genetic factors have a role in some aspects of the depression time course.4 An additional limiting factor hampering the establishment of more accurate depression models in animals is the lack of valid verification tests. Forced swimming and tail suspension tests, which are widely used as behavioral tests for depression, are assays for the pharmacological effects of antidepressants, not for depression itself.1 Likewise, the sucrose preference test, used to measure anhedonia in disease models, can be confounded by appetite.5 More recent depression mouse models involving chronic mild stress or social defeat stress are improvements but remain variations of the acute stress tests, and furthermore do not recapitulate the episodic time course.
Recently, the roles of mutations of Mendelian disease loci in complex disease risk have drawn attention.6, 7, 8 Such mutations may have pleiotropic effects and confer risk for multiple diseases including neuropsychiatric disorders. One example is chronic progressive external ophthalmoplegia (CPEO),9 a Mendelian-inheritance mitochondrial disease. Mutations of several genes including POLG1 encoding the catalytic subunit of mitochondrial DNA (mtDNA) polymerase are causative for CPEO.10 In addition to muscular symptoms, these mutations cause a wide range of neuropsychiatric symptoms including epilepsy, sensory ataxia, neuropathy and Parkinsonism. A retrospective study reported that depression is the most frequent neuropsychiatric syndrome of mitochondrial disease seen in more than half of patients with mitochondrial diseases.9, 10, 11, 12 Deleted mtDNAs (Δ-mtDNAs) were observed in the postmortem brains of patients with depressive episodes.13, 14 Thus, we examined the role of CPEO mutations in brain circuits as a candidate entry point to modeling depression phenotypes in mice.
Though knock-in mice of Polg1 were reported,15, 16 expression of mutant Polg1 in non-neuronal tissues hampers behavioral analyses.17 We thus generated a mutant mouse line expressing proofreading-deficient Polg1 in forebrain neurons by the CaMKIIα promoter18 to avoid complications in analysis. In our initial study, we analyzed trait-dependent phenotypes and found unusual activity patterns in daytime and estrus cycle-associated behavioral activity changes.18 During the course of that study, however, we noticed that female mutant mice showed a remarkable propensity for spontaneous recurrent episodes of lethargy, which we operationally defined as hypoactivity. Here, we expanded the time course of the behavioral analyses to more than 6 months and characterized the hypoactivity episodes of the mice showing they were both spontaneous and recurrent. Further analysis showed reasonable concordance with clinical depression symptoms in reference to the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, 5th edition),5 which is commonly used in neuroscience, genetics and clinical psychiatry. We then mapped the brain regions accumulating Δ-mtDNAs to the paraventricular thalamic nucleus (PVT), a brain area not previously known to be associated with depression, and provided causal evidence for the PVT in initiation of the episodes.
Materials and methods
The Wako Animal Experiment Committee, RIKEN, approved all animal experiment protocols. The study using human samples was approved by the Wako first ethics committee of RIKEN. Materials and methods are described in detail in Supplementary Methods.
Animals
All mutant Polg1 transgenic mice (Polg1 mutant mice) used were heterozygotes. Polg1 mutant mice chronically express a proofreading-deficient Polg1 transgene in forebrain neurons by the CaMKIIα promoter at expression levels comparable to endogenous Polg1, and Δ-mtDNAs were accumulated in the brain of mutant mice.18 For AAV (adeno-associated virus)-mediated, region-specific inhibition of neural transmission, heterozygous CaMKIIα promoter–loxP–STOP–loxP–tTA (Tg2) and TetO–TeTX (Tg3) transgenic mice19 were crossed to obtain the double transgenic mice (Tg2/+Tg3/+), which expressed TeTX (tetanus toxin) depending on Cre recombinase.
Long-term recording of wheel-running activity
Recoding and analyses of wheel-running activity were performed as described previously.18
Behavioral test battery during episodic hypoactivity
We recorded the wheel-running activity for 30 weeks and conducted a behavioral test battery when an animal exhibited episodic hypoactivity. On the first day of the test battery, open-field, Y-maze, and forced swimming tests were performed. Tail suspension and treadmill tests were carried out on the second day.
Electroencephalogram/electromyogram recording and sleep analysis
Electroencephalogram and electromyogram signals were recorded using telemetry transmitters. The polysomnographic data in 10-s epochs were classified as either wake, non-rapid eye movement or rapid eye movement sleep with the aid of a sleep scoring software.
Escitalopram and lithium treatment
Escitalopram oxalate was given in drinking water containing 150 μg ml−1 escitalopram base.
Lithium chloride was given in the diet (1.7 g kg−1), and a salt solution (450 mm NaCl) as well as water were provided according to Engel et al.20 We administrated these agents in a two-period cross-over design with a baseline measurement.
Mapping of Δ-mtDNA accumulation
Coronal sections (40 μm thick) of fresh-frozen brains were microdissected into small pieces (300 × 300 μm2). The pieces were treated with Proteinase K, and amount of Δ-mtDNAs in each piece was determined by qPCR as described previously.18
Cytochrome c oxidase/succinate dehydrogenase immunohistochemistry
Paraformaldehyde-fixed, OCT compound-embedded mouse brains and formalin-fixed, paraffin-embedded human postmortem brain tissues of patients with mitochondrial diseases and controls were sectioned and treated for antigen retrieval. Primary antibodies used were monoclonal anti-MTCO1 and anti-SDHA (anti-SDH subunit A).
Virus injection into mouse PVT
Recombinant AAV2 expressing Cre recombinase and EGFP were injected into the PVT of Tg2/+Tg3/+ mice (at AP −1.7 mm, ML 0.0 mm and DV 3.2 mm from the bregma).
Results
Depressive episode-like behavioral change in Polg1 mutant mice
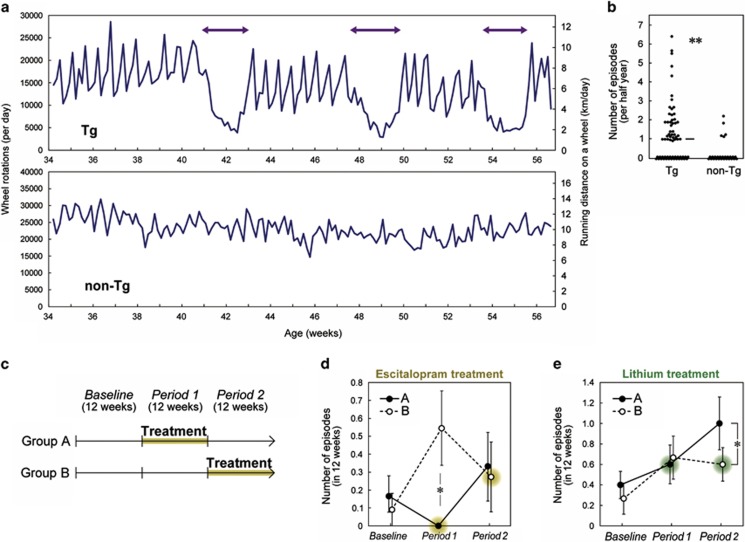
We expanded the time of behavioral analyses of previously constructed Polg1 mutant mice and observed that 60% of female mice showed noticeable, episodic behavioral change in wheel running around once every 6 months. A single episode lasted ~2–3 weeks with a spontaneous start and end (Figure 1a and Supplementary Figure 1a). On the basis of a Relative Strength Index analysis (Supplementary Figure 1a), female Polg1 mutant mice had lethargic episodes more often than control mice (Figure 1b) and most episodes occurred in mice older than 30 weeks (Supplementary Figure 1b). Several individuals experienced two or more (that is, recurrent) episodes of hypoactivity during the 6-month observation (Figure 1a and Supplementary Figure 1a). No episodes defined by Relative Strength Index were observed in male Polg1 mutant mice (n=33) or ovariectomized female mutant mice (n=14; P<0.01 compared with sham-operated mice; Supplementary Table 1).
Figure 1.
Depression-like hypoactivity episodes in Polg1 mutant mice. (a) Representative recordings of wheel-running activity of a female Polg1 mutant mouse (Tg) and a non-Tg mouse. Left–right arrows depict the duration of hypoactivity episode. Nominal running distance is given on the right. (b) Frequency of the hypoactivity episodes. Each circle indicates one mouse measured for more than 4 months. The horizontal line indicates median. Female Polg1 mutant mice showed hypoactive episodes more than non-Tg control mice. **P<0.0001. The details of statistical tests are provided in Supplementary Table 1. (c) Experimental design of escitalopram and lithium trials. n=11 or 12 per group for the escitalopram trial, and n=11 per group for the lithium trial. (d) Effect of escitalopram treatment on episode frequency. There was a tendency for a group × period interaction (P=0.052). The episode was significantly less frequent during treatment (Group A in Period 1) than the other group (Group B in Period 1; *P<0.05). Data are expressed as mean±s.e.m. (e) Effect of lithium treatment on episode frequency. There was a significant group × period interaction (P<0.05). The episode tended to be more frequent after the termination of the treatment (Group A in Period 2) than the other group during treatment (Group B in Period 2; *P<0.05).
We videotaped the wheel-running behavior of female Polg1 mutant mice (n=6) for 24 h per day over 2 months and observed three hypoactivity episodes. No neurological behaviors suggestive of epilepsy, ataxia, hypotonia or Parkinsonism symptoms were observed during the hypoactivity episodes as well as before and after their occurrence (Supplementary Movies 1–3).
To examine whether the hypoactivity episode was likely to be a depressive episode-like behavioral change and whether the episodes responded to antidepression drug treatment, we tested the effects of escitalopram, a selective serotonin reuptake inhibitor, and lithium, a mood stabilizer that mitigates depressive episodes in bipolar disorder. We administered escitalopram to mice (Supplementary Figures 2a–d) in a two-period cross-over design, with each period lasting for 12 weeks (Figure 1c). After a 12-week baseline measurement, mice were randomly divided into two groups (A and B). Episodes were less frequent during treatment compared with the control group (Figure 1d). Lithium was also tested in a two-period cross-over design. We recorded a more frequent occurrence of episodes after termination of lithium treatment, suggesting that drug withdrawal could have triggered new episodes (Figure 1e and Supplementary Figure 2e), a phenomenon that has been observed in depressive patients.21
Physiological characterization of depressive episode-like behavioral change
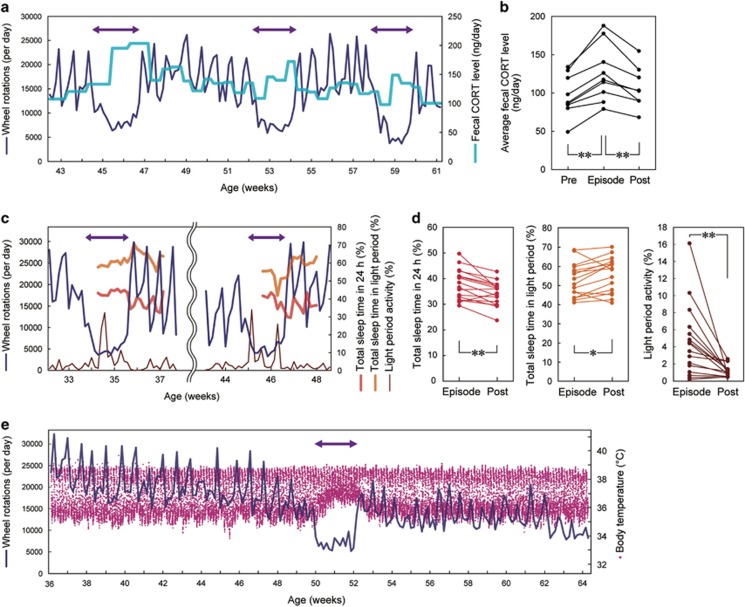
There are no established biomarkers for depression at present, but measurable alterations in cortisol levels, sleep–wake patterns and body temperature (Tb) in depressive patients have been consistently documented.22, 23, 24 To measure corticosterone, the major glucocorticoid in rodents, over several months, we sampled the feces of Polg1 mutant mice 1–2 times a week. We detected higher corticosterone levels during the episodes that are consistent with clinical observations (Figures 2a and b). We also analyzed sleep–wake patterns during and after single episodes using telemetry probes to record electroencephalogram and electromyogram activity. During the episodes, we observed an increase in total sleep time, but sleep time specifically in the light phase when mice are normally resting was decreased (Figures 2c and d). This sleep pattern disturbance during the episodes was also seen in the wheel-running activity data (Figure 2d). We also measured the core Tb of mutant mice using a telemetry probe placed in the abdominal cavity. Abnormal Tb dynamics were observed during episodes, including a damped diurnal rhythm of Tb due to a higher minimum Tb (Figure 2e and Supplementary Figure 3a) or a sharp drop of Tb in the early morning (Supplementary Figure 3b). Conversely, hyperthermia always accompanied episodes of hypoactive wheel running.
Figure 2.
Physiological analyses of hypoactivity episodes. (a and b) Increased fecal corticosterone level during the episodes. (a) A representative recording. Feces were sampled once or twice a week. (b) Cumulative data for all the episodes analyzed. The average values for 7 days before (‘Pre'), during (‘Episode') and following (‘Post') the hypoactivity episodes are shown, respectively. **P<0.01. (c and d) Sleep disturbance during the episodes; hypersomnia in 24 h, but insomnia in the light period. (c) A representative recording of total sleep time in 24 h and in 12-h light period, as well as wheel-running activity in 12-h light period. (d) Cumulative data for all the episodes analyzed. *P<0.05, **P<0.01. (e) Altered body temperature during the episodes: higher minimal temperature or damped circadian amplitude of body temperature. Core body temperature was measured every 10 min. An image magnified along the temporal axis is shown in Supplementary Figure 3a.
Comprehensive behavioral analysis of depression-like episodes
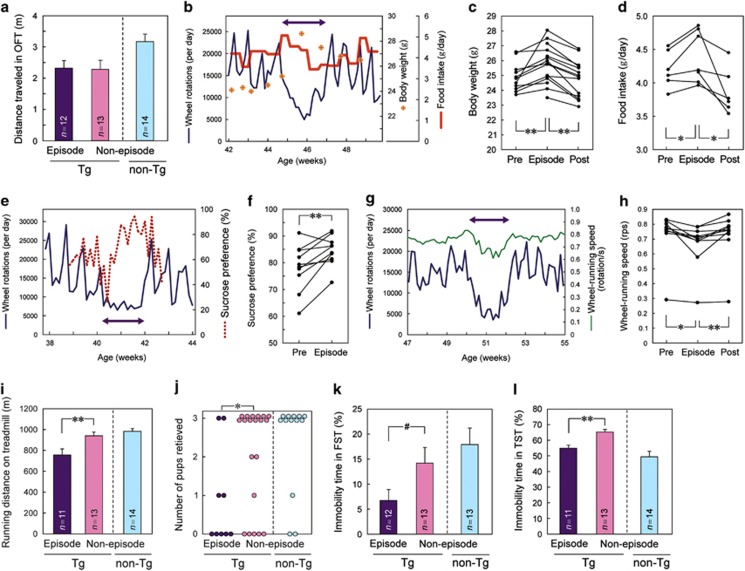
Using the radio telemetry system, we recorded the ambulatory activity of Polg1 mutant mice housed in cages without a running wheel and observed that hyperthermia bouts did not accompany hypoactive ambulation (Supplementary Figure 3c). The open-field test did not show alterations in distance traveled during the episodes of hypoactive wheel running (Figure 3a). These results indicated that the episodic reduction in wheel-running activity was not owing to a general loss of physical activity. Unlike other forms of locomotor activity, wheel running is well established to be a voluntary, motivated behavior for rodents.25, 26 Thus, the hypoactivity in wheel running observed in Polg1 mutant mice might reflect a loss of hedonic interest, one of the core clinical symptoms of depression.5
Figure 3.
Behavioral analyses of hypoactivity episodes. (a) No significant change in total distance traveled in an open-field test during the episodes as compared with Tg mice in non-episode state. Data for non-Tg mice were shown just as a reference. (b–d) Increased body weight and food intake during the episodes. (b) A representative recording. (c and d) Cumulative data for all episodes tested. The average values for 7 days before (‘Pre'), during (‘Episode') and following (‘Post') the hypoactivity episodes are shown, respectively. *P<0.05, **P<0.01. (e and f) Increased sucrose preference during the episodes. (e) A representative recording. A left–right arrow depicts the duration of a hypoactivity episode. (f) Cumulative data for all episodes tested. **P<0.01. (g and h) Slower wheel-running speed during the episodes. (g) A representative recording. (h) Cumulative data for all episodes tested. Data for non-Tg mice were shown as a reference. *P<0.05, **P<0.01. (i) Reduced endurance on a treadmill during the episodes. Values are means+s.e.m. (n=11–14). Data for non-Tg mice were shown as a reference. **P<0.01. (j) Impaired maternal behavior during the episodes. The numbers of newborn pups retrieved within 20 min were plotted. *P<0.05. (k and l) Reduced immobility time during the episodes in forced swimming (k) and tail suspension tests (l). Values are means+s.e.m. (n=11–14). Data for non-Tg mice were shown as a reference. #P<0.1, **P<0.01. FST, forced swimming test; OFT, open-field test; TST, tail suspension test.
We next investigated whether episodes were associated with vegetative, psychomotor or emotional signs of depression, which were described as characteristic symptoms in the DSM-5 diagnosis of depression.5 Polg1 mutant mice gained significant weight during the episodes (Figures 3b and c) and food intake as well as sucrose consumption increased (Figures 3b and d–f). During the episodes, the mutant mice had slower running speeds compared with non-episode periods (Figures 3g and h), indicating psychomotor retardation. In a treadmill endurance test, the mutant mice showed marked fatigue and reached exhaustion earlier during the episodes (Figure 3i). Attention or concentration was evaluated by five-choice serial reaction time task and Y-maze test, which also measured impulsivity and working memory, respectively. Significant changes during the episode were not detected in either test (Supplementary Figures 4a–c). To assess the intrinsic motivation and social function of the female mutant mice, we performed a pup-retrieving assay27 during the episode. Polg1 mutant mice in the episodes retrieved significantly fewer pups than mutant mice in the non-episode state (Figure 3j). The latency to sniff pup(s) for the first time was unchanged during the episode (Supplementary Table 1). The result of the assay indicates that during the episode even fundamental social parental functions are impaired.
We also examined the forced swimming and tail suspension tests that are currently in use as traditional measures of depression. In these tests, the mutant mice showed shorter immobility time during episodes compared with the non-episode state (Figures 3k and l) in contrast to longer immobility time observed in other models.
Identification of the PVT as a hotspot for mtDNA deletions
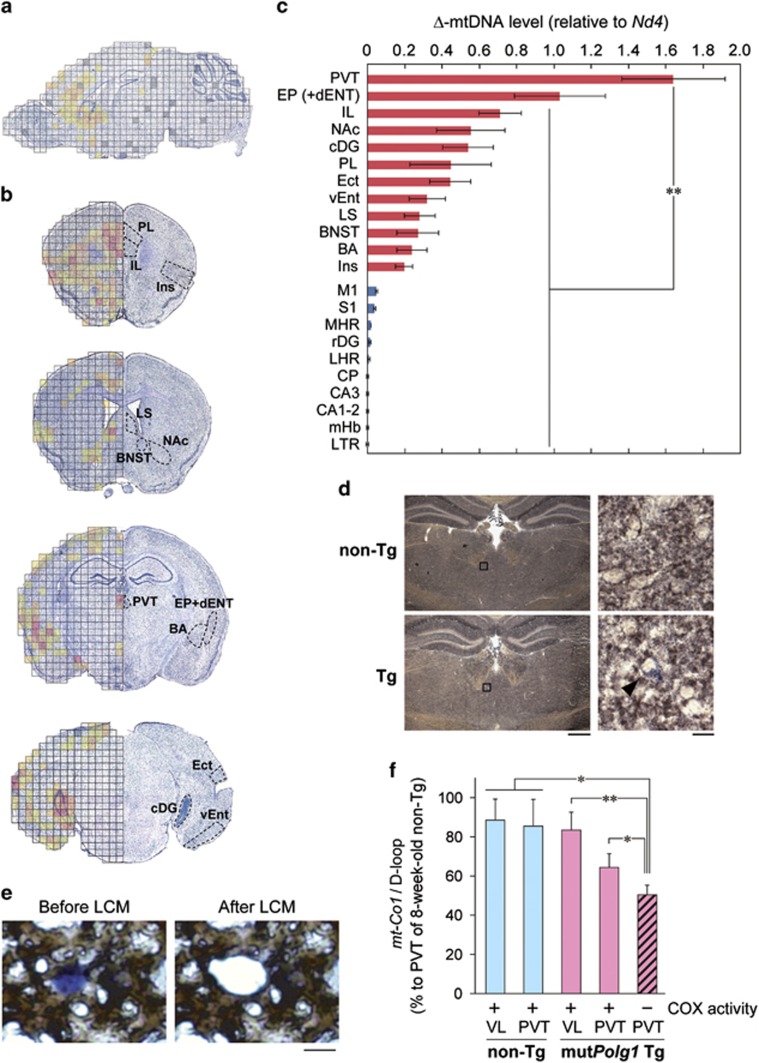
In CPEO, detection of Δ-mtDNAs in skeletal muscle is diagnostic. We hypothesized that in Polg1 mutant mice, the selective accumulation of Δ-mtDNA in particular brain areas could impair specific mood-related neural circuits resulting in depressive episodes. To test this hypothesis, we conducted a comprehensive screen of Δ-mtDNA levels over the entire brain of Polg1 mutant mice. Brain sections were cut into tiny pieces using a laser microdissection system, and the Δ-mtDNA level for each piece was assayed using quantitative PCR (Figure 4a and Supplementary Figure 5). We analyzed four coronal sections and found a high amount of Δ-mtDNAs concentrated in several forebrain regions (Figure 4b). We chose 12 regions with the highest levels of Δ-mtDNAs (Figure 4b) and 10 control regions (Supplementary Figure 6), and quantified Δ-mtDNA levels in the regions of interest. The highest levels of Δ-mtDNA accumulation by far were observed in the PVT, a part of the epithalamus (Figure 4c). There was no significant correlation between Δ-mtDNA level and expression level of the mutant Polg1 transgene (Supplementary Figure 5d). Together, the screen revealed that across all brain areas examined, the PVT appears to be the most susceptible locus to accumulate Δ-mtDNAs in the mutant mice.
Figure 4.
Accumulation of mtDNA deletions in the PVT of Polg1 mutant mice. (a) Comprehensive mapping of Δ-mtDNA of a Polg1 mutant mouse. Δ-mtDNA levels are shown in the following colors: red (high), orange, light orange, gold, yellow (low) and no color (unquantifiable). Gray, not determined. (b) Mapping of Δ-mtDNA levels on four coronal sections of a Polg1 mutant mouse. Regions with higher levels of Δ-mtDNA are marked. (c) Quantitative Δ-mtDNA levels in regions of interest, where higher (red) and lower (blue) levels of Δ-mtDNA are suggested. n=5 (individual mice) per region. **P<0.01. (d) Activity staining of SDH (blue) and COX (brown) of coronal sections at the level of PVT. The right images are magnified views of the boxed regions (left). A cell without COX activity (COX-negative cell) in the PVT is indicated by an arrowhead. Scale bars, 0.5 mm (left) and 10 μm (right). (e) Laser capture microdissection (LCM) of COX-negative cells after COX activity staining. Scale bar, 10 μm. (f) Quantification of Δ-mtDNA levels in LCMed COX-negative and -positive cells. The copy number ratio of mt-Co1/D-loop in LCMed cells inversely reflects the Δ-mtDNA level, and the ratio for the PVT of an 8-week-old wild-type mouse, assumed to have no deletion, is set to 100%. For example, the mt-Co1/D-loop ratio of 60% means that 40% of mtDNA molecules are Δ-mtDNAs. Data are expressed as means±s.e.m. of three mice. *P<0.05, **P<0.01. BA, basal amygdala; BNST, bed nucleus of the stria terminalis; CA, corpus ammonium; cDG, dentate gyrus, caudal part; CP, caudate putamen; dENT, entorhinal area, deep layer; Ect, ectorhinal area; EP, endopiriform nucleus; IL, infralimbic cortex; Ins, insular cortex; LHR, lateral hypothalamic region; LS, lateral septum; LTR, lateral thalamic region; M1, primary motor cortex; mHb, medial habenula; MHR, midline hypothalamic region; Δ-mtDNA, deleted mtDNA; NAc, nucleus accumbens; PL, prelimbic cortex; PVT, paraventricular thalamic nucleus; rDG, dentate gyrus, rostral part; S1, primary somatosensory cortex; vENT, entorhinal area, ventral part; VL, ventral lateral nucleus of the thalamus (See Supplementary Table 3).
PVT neurons have localized mitochondrial dysfunction
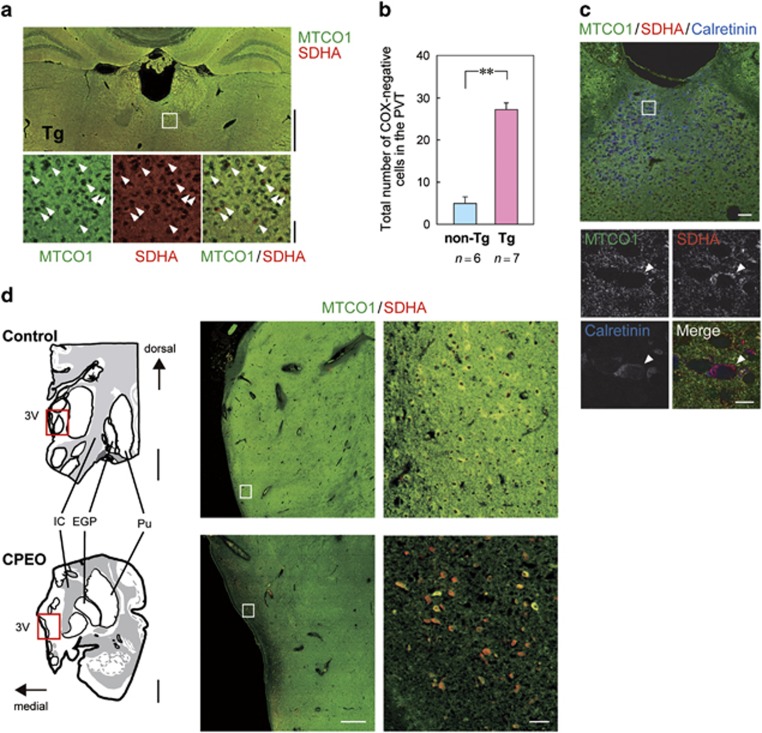
To assess mitochondrial function in the PVT at the cellular level, the enzymatic activities of two critical mitochondrial proteins, succinate dehydrogenase (SDH, mitochondrial complex II encoded by the nuclear genome) and cytochrome c oxidase (COX, complex IV containing mtDNA-encoded subunits such as MTCO1) were visualized on fresh-frozen brain sections, where the relative ratio of COX and SDH activities is indicative of accumulation of Δ-mtDNAs.28 In the PVT of mutant mice, we detected COX-negative cells that were deficient in COX activity but retained SDH activity (Figure 4d), but not in control mice. Next, we determined the level of Δ-mtDNAs in COX-negative cells (Figure 4e) and found that COX-negative cells in the PVT contained a significantly larger amount of Δ-mtDNAs (~50%) than COX-positive cells in PVT and also compared with control animals (Figure 4f), indicating that accumulation of Δ-mtDNA in a cell causes mitochondrial dysfunction at a cellular level.
To quantitatively evaluate COX-negative cells in PVT, we performed double immunofluorescent staining using anti-MTCO1 and anti-SDHA (SDH subunit A) antibodies on fixed brain sections. In the PVT, we observed significantly more COX-negative cells positive for SDHA but not MTCO1 compared with control mice (Figures 5a and b). The COX-negative cells co-stained with calretinin (Figure 5c), a marker of glutamatergic neurons in the PVT,29, 30 but not with microglial or astrocyte markers (Supplementary Figures 7a and b), suggesting the damage in excitatory neurons consistent with the expression of the CaMKIIα transgene promoter. To extend the observations to human patients, we examined the immunohistochemical COX/SDH levels in postmortem brains of two CPEO patients with comorbid mood symptoms including depression, and both contained a high level of COX-negative cells in paraventricular thalamus compared with age-matched controls (Figure 5d and Supplementary Figure 7c).
Figure 5.
Pathological hallmark in PVT of Polg1 mutant mice and CPEO patient comorbid with depression. (a) Double immunofluorescent staining of nuclear DNA-encoded protein, SDH (red) and mtDNA-encoded protein, COX (green). Higher magnification images of the indicated regions (a white rectangle; top) are shown in the bottom panels. Scale bars, 1 mm (top) and 40 μm (bottom). (b) A significantly greater number of COX-negative cells is found in the PVT of Polg1 mutant mice. We counted COX-negative cells in eight PVT-containing sections per animal. **P<0.01. (c) Triple immunofluorescent staining of the PVT of Polg1 mutant mice using anti-MTCO1 (green), anti-SDHA (red), and anti-Calretinin (blue) antibodies. Higher magnification images of the indicated region (a white rectangle; upper) are shown in the lower panels. COX-negative cells in the PVT, which are indicated by arrowheads, are co-stained with Calretinin. Scale bars, 50 μm (upper) and 10 μm (lower). (d) Double immunofluorescent staining of paraventricular thalamus of a patient with CPEO and depression, and a control subject. The postmortem brain sections (drawings in left panels) were stained using anti-MTCO1 (green) and anti-SDHA (red) antibodies. Medium magnification images of the indicated regions (red rectangles; left drawings) are shown in the middle panels, and higher magnification images of the indicated regions (white rectangles; middle) are shown in the right panels. Scale bars, 5 mm (left), 1 mm (middle), 50 μm (right). EGP, external globus pallidus; IC, internal capsule; Pu, putamen; PVT, paraventricular thalamic nucleus; SDH, succinate dehydrogenase; SDHA, SDH subunit A; 3V, third ventricle.
Causal role of the PVT in triggering depression-like episodes
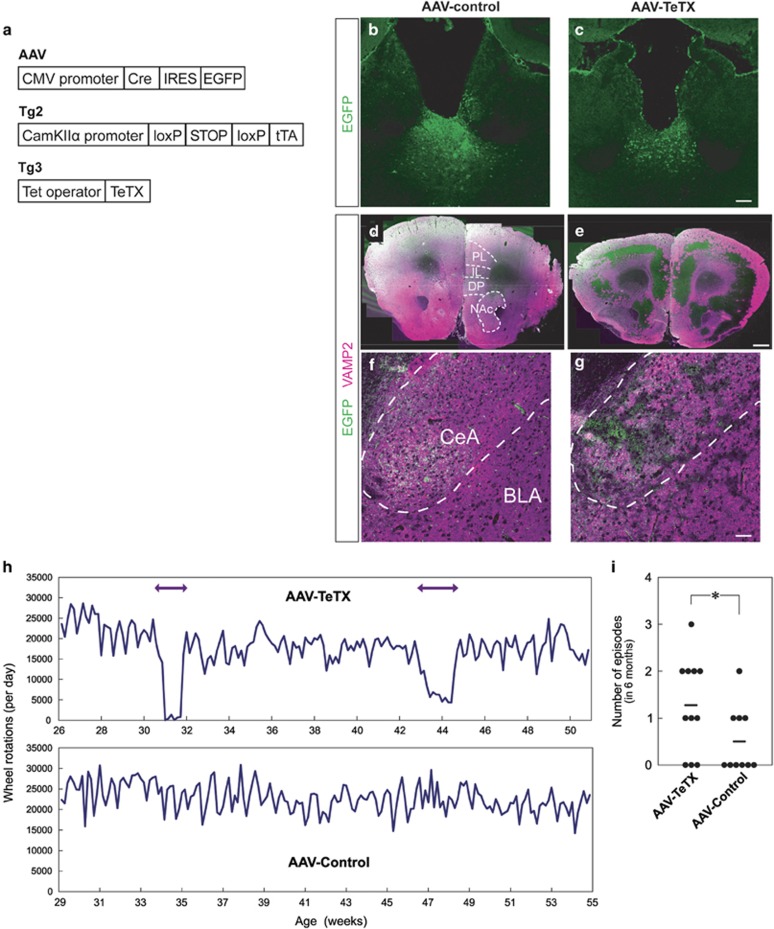
We hypothesized that defective PVT synaptic output, a probable downstream consequence of mitochondrial dysfunction in COX-negative neurons, could cause depression-like episodes. To test this, we used transgenic mice that expressed TeTX, which cleaves VAMP2 to inhibit synaptic transmission, in neurons dependent on Cre recombinase.19 Adeno-associated virus (AAV) expressing Cre and EGFP was stereotaxically injected into the PVT of the mice (Figure 6a). These mice expressing TeTX in the PVT (AAV-TeTX) showed significantly reduced VAMP2 immunoreactivity in the anterior part of the brain and amygdala (Figures 6b–g). Remarkably, when we recorded wheel-running activity over 6 months we observed the appearance of de novo hypoactivity episodes defined by the Relative Strength Index when compared with control mice (Figures 6h and i). This result indicates that PVT dysfunction is causally linked to the depression-like episodes of Polg1 mutant mice.
Figure 6.
Functional role of defective PVT output in development of hypoactivity episodes. (a) Schematic diagrams of the AAV and the transgene constructs used. AAV, Cre recombinase and EGFP are expressed in AAV infected cells. Tg2, a tetracycline transactivator (tTA) is expressed under control of CaMKIIα promoter after excision of the floxed STOP cassette by Cre. Tg3, TeTX is expressed under control of Tet-operator. (b and c) EGFP immunostaining of coronal sections at the PVT level of control (b) and TeTX-expressing mice (c) used for wheel-running analysis. Scale bars, 100 μm. (d–g) EGFP (green) and VAMP2 (magenta) immunostaining of coronal sections at the anterior part of the brains including PL, IL, DP and NAc (d and e) and the amygdala (f and g) of control (d and f) and TeTX-expressing mice (e and g). BLA, basolateral nucleus of the amygdala; CeA, central nucleus of the amygdala. Scale bars, 500 μm (d and e), 50 μm (f and g). (h) Representative recordings of wheel-running activity of a mouse expressing TeTX in the PVT (AAV-TeTX) and a control. (i) Frequency of the episodic hypoactivity. AAV-TeTX mice showed hypoactive episodes more than control mice. *P<0.05. Horizontal lines indicate average values. AAV, adeno-associated virus; CMV, cytomegalovirus; DP, dorsal peduncular; IL, infralimbic cortex; NAc, nucleus accumbens; PL, prelimbic cortex; TeTX, tetanus toxin.
Discussion
Three aspects of validity (construct, face and predictive validities) have been considered to be an acknowledged framework for the evaluation of an animal model for a human disease.1 In this study, using multi-method approaches, we demonstrated that Polg1 mutant mice showed outstanding face validity for a depression model with supporting construct and predictive validities. The mutant mice exhibited a broad spectrum of symptoms during the episodes, emotional, vegetative and psychomotor disturbances, which fulfilled five of the nine Criteria A of DSM-5 for major depressive episode (Supplementary Table 2). Another symptom in Criteria B, distress or impairment in social, work or other important areas of functioning, was also present; Polg1 mutant mice during the episodes displayed an impairment in maternal behavior (Figure 3j), one of the most important social functions of female mice. By contrast, conventional face validity for depression models has been associated with prolongation of immobility time in behavioral despair tests or a decrease of sucrose preference. Polg1 mutant mice during the episodes, however, exhibited the opposite patterns in these tests. The increased sucrose preference (Figure 3f) was possibly owing to a carbohydrate craving or an increase in appetite (Figure 3d), one of the symptoms described in the DSM-5 criteria. Sucrose preference that is confounded by appetite is not appropriate to measure anhedonia in disease models of depression. The reduced immobility time during the episodes in behavioral despair tests (Figures 3k and l) can be explained by the actual validity of these tests to measure the pharmacological effects of antidepressants, not depression itself. Although it is difficult to screen antidepressants involving the monoaminergic system by using Polg1 mutant mice, the model may instead be useful in the development of novel drugs, which are unlikely to be discovered by traditional behavioral despair tests.
We used the neuron-specific promoter to direct selective expression of proofreading-deficient POLG1 to avoid confounding effects, that is, somatic symptoms and motor dysfunction, which are caused by heterozygous mutations of POLG1 in humans9, 10, 12 and also present in mutant Polg1 knock-in mice.17 The forebrain neuron-specific mutant mice did not exhibit either somatic symptoms as we expected18 or sensorimotor dysfunction including ataxia and Parkinsonism, working memory deficits or cognitive impairment during the episodes and non-episode periods (Supplementary Figure 4a–c and Supplementary Movies 1–3). Among the psychiatric diagnoses of mitochondrial disease patients, the most common diagnosis is major depression (54%) followed by bipolar disorder (17%) and panic disorder (11%).11 Polg1 mutant mice did not show any spontaneous manic-like episodes, except for tricyclic antidepressant-induced hyperactivity.18 The mutant mice did not show altered anxiety-related behavior during the episodes in the open-field and light/dark transition tests (Supplementary Figures 4d and e), indicating that the episodes were not attributable to increased anxiety owing to panic disorder or other anxiety disorders. Nevertheless, during the episodes mutant mice were rebellious and hard to handle suggesting that behavioral responses to fearful stimuli were enhanced. This observation could potentially explain the shorter immobility time, including enhancement of struggling/survival behavior, in the forced swimming and tail suspension tests (Figures 3k and l).
The prophylactic effect of escitalopram was demonstrated (Figure 1d) for the predictive validity, whereas the curative effect on the episode was not possible because hypoactivity episodes experienced by mutant mice were of short duration (~2–3 weeks) and of relatively rare occurrence (about once in half a year). The trigger for the stochastic episodes remains unknown; however, environmental factors (for example, earthquakes and variation in humidity or pressure influenced by weather conditions even in our well-controlled animal facility) are unlikely because more than two animals seldom initiated concurrent episodes. Considering the low frequency of episodes, elucidation of the trigger of hypoactivity episodes will facilitate future preclinical studies using this model.
Major depression has complex contributions from genetic and environmental factors,4 and mitochondrial dysfunction or POLG1 mutations are not fully penetrant9, 18 and other factors may contribute. A strong risk factor for depression is being female4 and, consistent with this, only female Polg1 mutant mice exhibited spontaneous episodes. Also, the absence of episodes after ovariectomy indicates that female hormonal changes are likely to increase susceptibility. Together, the loss of proofreading activity of POLG1 and female status confer construct validity.
Our anatomical screen for Δ-mtDNAs linked to the loss of proofreading activity of POLG1, together with the TeTX inactivation experiment, suggest that the PVT is an essential brain area for mood regulation. In the epithalamus, the PVT is ideally positioned for both the neural processing of mood-related information and its transfer and regulation of downstream brain areas. The PVT receives strong afferents from the suprachiasmatic nucleus, serotonergic neurons in the dorsal raphe, and CRH and orexin neurons in hypothalamus, while PVT efferents project to core mood and reward processing regions including the nucleus accumbens, anterior cingulate cortex and amygdala.31, 32, 33 This projection pattern together with our data support a model where PVT neurons control mood by dampening mood instability in affective circuits.34 The Polg1 mutant mice that we describe may open new avenues for investigating how PVT-centered neural circuits connect to final common pathway(s) for depression and integration with neural systems linked to stress-induced models.
Acknowledgments
We thank Mizuho Ishiwata, Mizue Kametani, Fukiko Isono, Naoko Kume and Noriko Fujimori for technical assistance. We are grateful to Professor Hitoshi Takahashi (Niigata University) who provided postmortem brain samples. We thank Professor Susumu Tonegawa for providing Tg2 and Tg3 transgenic mice and valuable discussion. This work was supported by a grant for Laboratory for Molecular Dynamics of Mental Disorders, RIKEN Brain Science Institute, MEXT/JSPS KAKENHI Grants (TKas, TMK, MK-S and TKat), and a Grant-in-Aid from the Japanese Ministry of Health and Labor (TKat).
Author contributions
TKas and TKat designed the project. TKas performed the behavioral and physiological analyses of Polg1 mutant mice. AT performed quantitative mapping of Δ-mtDNA. TMK performed the experiment of neural circuit analysis of PVT. MK-S and TS performed immunofluorescent staining and activity staining experiments. TS performed LCM of COX-negative cells. AK and DK provided postmortem human brain samples. HM and KO produced AAV. TKas, AT, TMK, MK-S and TKat wrote the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
TKat receives a research grant from Takeda Pharmaceutical outside this work. The company had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. TKat received honoraria for lectures, manuscripts and/or consultancy, from Kyowa Hakko Kirin, Eli Lilly Japan K.K., Otsuka Pharmaceutical, GlaxoSmithKline K.K., Taisho Toyama Pharmaceutical, Dainippon Sumitomo Pharma, Meiji Seika Pharma, Pfizer Japan, Mochida Pharmaceutical, Shionogi, Janssen Pharmaceutical K.K., Yoshitomiyakuhin, Agilent Technologies, Astellas Pharma and Wako Pure Chemical Industries within the last 3 years. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci 2010; 13: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsher G, Costello CG. Relapse after recovery from unipolar depression: a critical review. Psychol Bull 1988; 104: 84–96. [DOI] [PubMed] [Google Scholar]
- O'Leary D, Costello F, Gormley N, Webb M. Remission onset and relapse in depression. An 18-month prospective study of course for 100 first admission patients. J Affect Disord 2000; 57: 159–171. [DOI] [PubMed] [Google Scholar]
- Flint J, Kendler KS. The genetics of major depression. Neuron 2014; 81: 484–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Blair DR, Lyttle CS, Mortensen JM, Bearden CF, Jensen AB, Khiabanian H et al. A nondegenerate code of deleterious variants in Mendelian loci contributes to complex disease risk. Cell 2013; 155: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Need AC, Petrovski S, Goldstein DB. One gene, many neuropsychiatric disorders: lessons from Mendelian diseases. Nat Neurosci 2014; 17: 773–781. [DOI] [PubMed] [Google Scholar]
- Kato T. Whole genome/exome sequencing in mood and psychotic disorders. Psychiatry Clin Neurosci 2015; 69: 65–76. [DOI] [PubMed] [Google Scholar]
- Smits BW, Fermont J, Delnooz CC, Kalkman JS, Bleijenberg G, van Engelen BG. Disease impact in chronic progressive external ophthalmoplegia: more than meets the eye. Neuromuscul Disord 2011; 21: 272–278. [DOI] [PubMed] [Google Scholar]
- Longley MJ, Graziewicz MA, Bienstock RJ, Copeland WC. Consequences of mutations in human DNA polymerase γ. Gene 2005; 354: 125–131. [DOI] [PubMed] [Google Scholar]
- Fattal O, Link J, Quinn K, Cohen BH, Franco K. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr 2007; 12: 429–438. [DOI] [PubMed] [Google Scholar]
- Suomalainen A, Majander A, Haltia M, Somer H, Lonnqvist J, Savontaus ML et al. Multiple deletions of mitochondrial DNA in several tissues of a patient with severe retarded depression and familial progressive external ophthalmoplegia. J Clin Invest 1992; 90: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Stine OC, McMahon FJ, Crowe RR. Increased levels of a mitochondrial DNA deletion in the brain of patients with bipolar disorder. Biol Psychiatry 1997; 42: 871–875. [DOI] [PubMed] [Google Scholar]
- Sequeira A, Martin MV, Rollins B, Moon EA, Bunney WE, Macciardi F et al. Mitochondrial mutations and polymorphisms in psychiatric disorders. Front Genet 2012; 3: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004; 429: 417–423. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005; 309: 481–484. [DOI] [PubMed] [Google Scholar]
- Fuke S, Kametani M, Yamada K, Kasahara T, Kubota-Sakashita M, Kujoth GC et al. Heterozygous Polg mutation causes motor dysfunction due to mtDNA deletions. Ann Clin Transl Neurol 2014; 1: 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry 2006; 11: 577–593. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 2008; 319: 1260–1264. [DOI] [PubMed] [Google Scholar]
- Engel T, Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. Chronic lithium administration to FTDP-17 tau and GSK-3β overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem 2006; 99: 1445–1455. [DOI] [PubMed] [Google Scholar]
- Suppes T, Baldessarini RJ, Faedda GL, Tohen M. Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry 1991; 48: 1082–1088. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. Psychol Med 1976; 6: 43–50. [DOI] [PubMed] [Google Scholar]
- Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry 2004; 65: 27–32. [PubMed] [Google Scholar]
- Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues Clin Neurosci 2008; 10: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Mena-Segovia J, Giordano M, Diaz JL. Induction of c-fos in nucleus accumbens in naive male Balb/c mice after wheel running. Neurosci Lett 2003; 352: 81–84. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci 2005; 8: 1445–1449. [DOI] [PubMed] [Google Scholar]
- Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Mol Cell Neurosci 2007; 36: 121–131. [DOI] [PubMed] [Google Scholar]
- Tyynismaa H, Mjosund KP, Wanrooij S, Lappalainen I, Ylikallio E, Jalanko A et al. Mutant mitochondrial helicase Twinkle causes multiple mtDNA deletions and a late-onset mitochondrial disease in mice. Proc Natl Acad Sci USA 2005; 102: 17687–17692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Scruggs JL, Young CD, Deutch AY. The distribution and origin of the calretinin-containing innervation of the nucleus accumbens of the rat. Eur J Neurosci 2000; 12: 1591–1598. [DOI] [PubMed] [Google Scholar]
- Hartig W, Riedel A, Grosche J, Edwards RH, Fremeau RT Jr, Harkany T et al. Complementary distribution of vesicular glutamate transporters 1 and 2 in the nucleus accumbens of rat: Relationship to calretinin-containing extrinsic innervation and calbindin-immunoreactive neurons. J Comp Neurol 2003; 465: 1–10. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Price JL. Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol 2009; 512: 825–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology 2010; 35: 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 2015; 519: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T. Molecular neurobiology of bipolar disorder: a disease of 'mood-stabilizing neurons'? Trends Neurosci 2008; 31: 495–503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.