Abstract
Background
There are limited data on human immunodeficiency virus (HIV) quality indicators according to model of HIV care delivery. Comparing HIV quality indicators by HIV care model could help inform best practices because patients achieving higher levels of quality indicators may have a mortality benefit.
Methods
Using the Partners HIV Cohort, we categorized 1565 patients into 3 HIV care models: infectious disease provider only (ID), generalist only (generalist), or infectious disease provider and generalist (ID plus generalist). We examined 12 HIV quality indicators used by 5 major medical and quality associations and grouped them into 4 domains: process, screening, immunization, and HIV management. We used generalized estimating equations to account for most common provider and multivariable analyses adjusted for prespecified covariates to compare composite rates of HIV quality indicator completion.
Results
We found significant differences between HIV care models, with the ID plus generalists group achieving significantly higher quality measures than the ID group in HIV management (94.4% vs 91.7%, P = .03) and higher quality measures than generalists in immunization (87.8% vs 80.6%, P = .03) in multivariable adjusted analyses. All models achieved rates that equaled or surpassed previously reported quality indicator rates. The absolute differences between groups were small and ranged from 2% to 7%.
Conclusions
Our results suggest that multiple HIV care models are effective with respect to HIV quality metrics. Factors to consider when determining HIV care model include healthcare setting, feasibility, and physician and patient preference.
Keywords: HIV, primary care models, quality indicators
The human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) epidemic has transitioned over the past 2 decades, from a focus on treatment of opportunistic infections and management of complex antiretroviral therapy (ART) regimens to a chronic disease model for care of patients with near-normal life expectancy [1–3] and an increased focus on noncommunicable disease prevention and management while maintaining high-quality HIV care [1, 2, 4].
There are multiple approaches to provide HIV care that include HIV and primary care provided by infectious disease (ID) specialist alone, by generalist alone, or by a combination model, where HIV care is provided by ID specialist and primary care is provided by a generalist. These models vary by geography, population density, and type of clinic and provider(s) [5–8]. There are no formal recommendations for preferred HIV care model.
There are limited data comparing HIV care models. Multiple papers examining generalist and specialist care have called for additional research to help identify optimal care models and roles for generalist and specialty care providers [4, 5, 9]. This topic is timely, because recent work showed a mortality benefit for patients who achieve ≥80% of HIV quality indicators [10].
We sought to compare HIV quality indicator outcomes across HIV care models. We previously found no difference across HIV care models in cardiovascular and cancer screening outcomes, standard measures of general medical care in non-HIV populations. In the current analysis, we focus on HIV quality indicators across HIV care models.
METHODS
Cohort
We used the Partners HIV cohort, derived from the Research Patient Data Registry, to define and create a database categorizing HIV patients in terms of HIV care models. The Partners HealthCare System (PHS) is associated with large, urban, academic teaching institutions. Inclusion criteria were as follows: (1) age ≥18 as of January 1, 2012; (2) HIV/AIDS as defined by ≥3 International Classification of Diseases, Ninth Revision codes for HIV (042 and all subtypes and V08) within a 5-year time frame from January 1, 2008 to December 31, 2012; and (3) active primary care in PHS defined as ≥1 visit to primary care physician or ID during 2012. This HIV definition was assessed by chart review in a randomly selected sample of 100 patients in the Partners HIV cohort, and 100% were found to be infected with HIV. Exclusion criteria included known death in 2012 or a provider outside PHS in 2011–2012. Institutional review board approval was obtained from the Partners Human Research Committee.
Human Immunodeficiency Virus Care Models
We defined 3 HIV care models: ID doctor provides HIV and primary care (“ID”), generalist provides HIV and primary care (“generalist”), and ID doctor provides HIV care while generalist provides primary care (“ID plus generalist”). Detailed HIV care model classification with validation is available in Supplemental Methods including Supplemental Table 1. In brief, the primary exposure of interest was type of HIV care model based on clinic visits to the corresponding type of provider(s) between January 1, 2011 and December 31, 2012: (1) ID, (2) generalist, or (3) ID plus generalist. Human immunodeficiency virus care model assignment underwent iterative random chart review validations (see Supplemental Methods). Validation of HIV care model classification by random 100-person final cohort chart review identified correct exposure classification in 94% of patients (see Supplemental Methods). We chose not to collapse the smaller generalist group into ID or ID plus generalists because this group was thought to represent a unique care model.
Covariates
Covariates included patient demographics, primary language (English vs non-English), CD4 and HIV viral load (most recent 2011 laboratory values), weighted Charlson comorbidity index score [11, 12], and socioeconomic status, estimated by household income using zip code-level medians from the American Community Survey [13] and transformed median household income to percentage of the state-wide median household income [14] with prespecified cut points [15].
Outcomes
We derived our quality indicators based on expanded HIV quality indicators [16–18] endorsed by 5 major medical and quality associations including the American Medical Association, National Committee for Quality Assurance, Infectious Diseases Society of America, HIV Medicine Association, and Health Resources and Services Administration. We grouped these metrics into 4 domains: processes of care, screening, immunization, and HIV management.
Processes of care included retention in care (defined as 2+ visits annually ≥60 days apart) and CD4 cell count measurement (≥2 annually vs <2). Screening included one-time chlamydia, tuberculosis (either purified protein derivative or interferon-gamma release assays), hepatitis B and C tests. Immunization included pneumococcal immunization (any formulation), annual influenza, and initial hepatitis B vaccine (excluding patients with hepatitis B infection or documented immunity). For HIV management metrics, we examined Pneumocystis jiroveci pneumonia (PCP) prophylaxis if CD4 count <200 cell/µL, appropriate current ART use (in 2012, defined as CD4 <500), and achieving viral suppression (defined as <400 copies/µL) if prescribed ART. Current ART use was determined from individual physician review of 2012 notes and medication lists. Patients without viral loads in 2012 were also confirmed by physician chart review 2012 notes to capture laboratory tests outside of PHS. Outcomes were ascertained from structured electronic medical record (EMR) data including encounters, procedure and diagnosis codes, laboratory and microbiology tests, structured health maintenance and problem lists, and both medication orders and current medication lists. Outcomes were validated by review of 100 randomly selected charts from the final cohort, comparing physician EMR review results to results derived from structured data (Supplemental Table 2).
Analysis
Analysis was conducted at the patient level. To assess differences in patient demographic and clinical characteristics across care models, we used analysis of variance and Kruskal-Wallis tests to compare normally and nonnormally distributed continuous variables, respectively, and χ2 tests or Fisher’s exact tests for categorical variables among our 3 exposure groups.
We initially examined unadjusted individual quality indicator rate of completion in each HIV care model, comparing the binary quality indicators in the 3 groups using logistic regression with generalized estimating equations (GEEs) techniques to take into account clustering by the most common provider, defined as the ID or generalist physician with the most 2012 patient encounters. We presented overall comparisons among our 3 exposure groups.
We then examined patient domain composite scores defined as percentage complete out of eligible measures as of December 31, 2012. Each patient had a minimum of 8 measures within 3 domains (process, screening, and immunization), with an additional 4 measures depending on CD4 count (PCP prophylaxis if CD4 <200 and ART use if CD4 <500), prior hepatitis B status (vaccination if no hepatitis B), and current ART status (viral suppression if on ART) for 12 total possible quality measures. We conducted both simple (unadjusted) and multivariable (adjusted) linear regression models with GEEs accounting for the most common provider for all domain outcomes. We presented overall comparisons and pairwise comparisons from the 3 exposure groups.
For the multivariable analysis, we included the following prespecified covariates in the models selected on the basis of clinical importance: age, sex, race, language (English speaking vs not), median household income, number of 2012 clinic visits, baseline viral load and CD4 counts (most recent 2011 values), and weighted Charlson score. We also performed a sensitivity analysis where we did not adjust for number of 2012 visits because number of visits was expected to be higher in the ID plus generalist model given 2 separate providers. We used SAS version 9.4 (SAS Institute Inc., Cary, NC) for all analyses. A 2-sided P ≤ .05 was considered statistically significant.
RESULTS
Our final cohort included 1565 persons (Figure 1) with HIV care model distribution of 875 ID, 90 generalist, and 675 ID plus generalist. The ID group had higher proportions of male, white, and English-speaking patients. The generalist group had higher proportions of Hispanic and non-English-speaking patients and patients with household income below the statewide median. The ID plus generalist group had higher proportions of black patients, patients with higher Charlson scores, and higher number of 2012 visits compared with ID and/or generalist providers (Table 1). There were no significant differences with respect to recent or nadir CD4 counts among groups.
Figure 1.
Cohort development. The flowchart indicates specific exclusion criteria applied in a stepwise manner to develop the final cohort for the study.
Table 1.
Characteristics of Patients by HIV Care Model
| Total, n = 1565* | ID n = 875 | Generalist n = 90 | ID Plus Generalist n = 600 | P Value† |
|---|---|---|---|---|
| Age: mean (SD) | 49.9 (9.8) | 47.1 (11.6) | 50.4 (10.1) | .06 |
| Male | 677 (77.4%) | 57 (63.3%) | 378 (63.0%) | <.0001 |
| Race: White | 490 (56.0%) | 31 (34.4%) | 254 (42.3%) | <.0001 |
| Black | 224 (25.6%) | 22 (24.4%) | 216 (36.0%) | |
| Hispanic | 121 (13.8%) | 36 (40.0%) | 100 (16.7%) | |
| Other | 40 (4.6%) | 1 (1.1%) | 30 (5.0%) | |
| English Language | 814 (93.0%) | 54 (60.0%) | 519 (86.5%) | <.0001 |
| Median household income by statewide median: <60% | 165 (19.4%) | 11 (12.4%) | 130 (22.0%) | <.0001 |
| 60%–100% | 351 (41.2%) | 58 (65.2%) | 241 (40.7%) | |
| 100%–140% | 258 (30.3%) | 9 (10.1%) | 172 (29.1%) | |
| >140% | 78 (9.2%) | 11 (12.4%) | 49 (8.3%) | |
| Median 2012 visits (IQR) | 4 (3–6) | 5 (3–6) | 6 (4–9) | <.0001 |
| Weighted Charlson: median (IQR) |
8 (7–10) | 7 (6–10) | 9 (7–11) | <.0001 |
| Recent CD4: median (IQR)‡ |
583 (384–795) | 561 (349–840) | 576.5 (390–802) | .80 |
| Nadir CD4: median (IQR)‡ |
190 (60–327) | 233 (64–322) | 213 (63–342) | .49 |
Abbreviations: ANOVA, analysis of variance; HIV, human immunodeficiency virus; ID, infectious disease; IQR, interquartile range; SD, standard deviation.
*Median household income, n = 1533; CD4 cell count, n = 1452; otherwise all data reflect N (%).
† P values testing overall differences using ANOVA, Kruskal-Wallis, Fisher’s exact, or χ2 test.
‡Missing laboratory data was 6.6%, 5.6%, and 6.3% for ID, generalist, and ID plus generalist groups.
Table 2 shows individual domain measures by HIV care models. The ID group had the lowest retention rate (96.0% vs 100% [generalist] and 99.3 [ID plus generalist]). All other individual quality indicators in screening, immunization, and HIV management were not significantly different between HIV care models.
Table 2.
Individual Quality Indicator Outcomes by HIV Care Models
| Total, n = 1565 | Outcome | ID n = 875 | Generalist n = 90 | ID Plus Generalist n = 600 | P Value* |
|---|---|---|---|---|---|
| Process | |||||
| Retention (%) | 96.0 | 100 | 99.3 | <.0001 | |
| CD4 test (%) | 82.4 | 85.6 | 81.8 | .53 | |
| Screening | |||||
| Chlamydia (%) | 79.4 | 78.9 | 79.8 | .74 | |
| Tuberculosis (%) | 80.9 | 82.2 | 81.5 | .36 | |
| Hepatitis B (%) | 94.9 | 96.7 | 94.3 | .70 | |
| Hepatitis C (%) | 95.0 | 85.6 | 95.0 | .31 | |
| Immunization | |||||
| Influenza (%) | 89.1 | 87.8 | 91.7 | .27 | |
| Pneumonia (%) | 85.7 | 82.2 | 87.5 | .16 | |
| (n = 667) | Hepatitis B (%) | 76.2 | 61.5 | 74.8 | .21 |
| HIV management | |||||
| (n = 108) | PCP prophylaxis (%) | 82.1 | 100 | 87.5 | .32 |
| (n = 538) | ART (CD4 <500) (%) | 90.3 | 84.9 | 94.2 | .15 |
| (n = 1153) | VL suppression (%) | 91.4 | 86.4 | 91.9 | .67 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; ID, infectious disease; PCP, Pneumocystis jiroveci pneumonia; SD, standard deviation; VL, viral load.
* P values testing overall differences among the 3 groups.
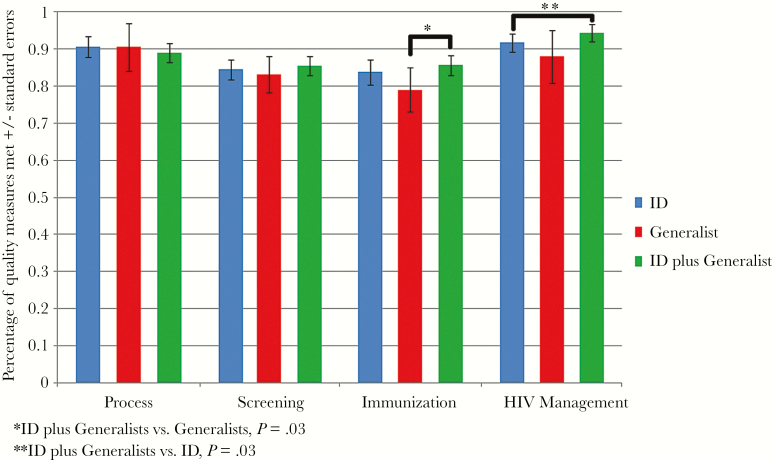
Table 3 reports the unadjusted and adjusted results of the composite score for each domain with overall comparison scores. Process and screening domain measure analyses were similar in the 3 groups with unadjusted and adjusted analyses. For the immunization domain, the overall comparison showed significant differences between groups in unadjusted (P = .04) but not adjusted analyses (P = .18) Pairwise testing for the immunization domain shows ID plus generalists achieved significantly higher composite scores than generalists in both unadjusted and adjusted models (87.8% vs 80.6%, P = .01 and 87.6% vs 79.0%, P = .03, respectively), whereas ID plus generalist vs ID and ID vs generalist both approached significance (P = .06 and P = .09, respectively), but the effects attenuated in the adjusted model. For the HIV management domain, the overall comparison was not significant in the unadjusted analyses (P = .12) but was significant in the adjusted analyses (P = .045). Pairwise testing showed that ID plus generalists had nonsignificantly higher composite scores than ID in unadjusted models (93.4% vs 91.4%, P = .10) and significantly higher scores in adjusted models (94.4% vs 91.7%, P = .03), whereas ID plus generalists were higher than generalists, approaching statistical significance in both unadjusted (93.4% vs 88.0%, P = .053) and adjusted (94.4% vs 88.0%, P = .06) models. Results of the adjusted models are depicted in Figure 2. Sensitivity analyses that did not adjust for 2012 visits had similar results (data not shown).
Table 3.
Unadjusted and Adjusted Quality Indicator Domains for HIV Care Models
| Quality Indicator Domains | Outcome | ID n = 875 | Generalist n = 90 | ID Plus Generalist n = 600 | P Value* |
|---|---|---|---|---|---|
| Process (mean ± SE) | Unadjusted | 89.2 ± 1.6 | 92.8 ± 3.5 | 90.6 ± 1.1 | .16 |
| Adjusted | 90.6 ± 1.4 | 90.4 ± 3.3 | 89.0 ± 1.3 | .59 | |
| Screening (mean ± SE) | Unadjusted | 87.5 ± 1.3 | 85.8 ± 2.4 | 87.7 ± 1.2 | .13 |
| Adjusted | 84.5 ± 1.4 | 83.2 ± 2.5 | 85.4 ± 1.3 | .62 | |
| Immunization (mean ± SE) | Unadjusted | 86.2 ± 1.5 | 80.6 ± 2.5 | 87.8 ± 1.2 | .04 |
| Adjusted | 83.7 ± 1.7 | 79.0 ± 3.0 | 86.7 ± 1.4 | .18 | |
| HIV management (mean ± SE) | Unadjusted | 91.4 ± 1.2 | 88.0 ± 3·0 | 93.4 ± 1.1 | .12 |
| Adjusted | 91.7 ± 1.2 | 88.0 ± 3.5 | 94.4 ± 1.2 | .045 |
Abbreviations: HIV, human immunodeficiency virus; ID, infectious disease; SE, standard error.
* P values testing overall differences among the 3 groups.
Figure 2.
Composite human immunodeficiency virus (HIV) care model quality metrics. The adjusted proportion of composite quality metrics by domain is shown comparing HIV care models. ID, infectious disease provider only.
DISCUSSION
We examined 4 HIV quality metric domains in a northeast US urban healthcare system and found that all HIV care models achieved high HIV quality metric scores with minimal differences. There were differences between groups in adjusted analyses with the ID plus generalists group achieving significantly higher quality measures than the ID group in HIV management and higher quality measures than generalists in immunization. However, the absolute differences between groups were small (between 2% and 7%), suggesting that multiple approaches to HIV care delivery can be effective with respect to HIV quality indicators. The small group differences observed should be interpreted in the context of cost and use and patient and/or physician preference.
Regardless of who delivered HIV and primary care, care delivery was robust and quality indicator screening and HIV management were effective, because all groups performed at or above previously reported HIV quality indicator rates [19–24]. Our study inclusion criteria (1 patient visit in 2012) may have eliminated nonengaged patients and resulted in inflated quality indicator rates. It is also possible that case management or outreach models contributed to these high numbers. Clinics differed with various initiatives and/or resources when comparing a hospital-based ID clinic to an academic community clinic moving toward patient-centered medical home status, which is why we clustered our analyses by provider to incorporate the practice environment. We conclude that the high-quality indicator rates suggests that many approaches are feasible, especially because HIV care is increasingly streamlined and because many settings are addressing HIV treatment scale up and integration of HIV and chronic disease management [1, 2, 5].
Data from the 1990s showed that generalists provided HIV care equivalent to that provided by specialists and that level of HIV experience affected both mortality and ability to incorporate new data into practice [25–31]. Studies from the mid-2000s found no differences in retention, ART prescription, or viral suppression in hospital versus community-based clinics [32] or in viral suppression by provider level factors [33]. However, further studies did show that integrated specialist and mutidisciplinary teams in the veterans affairs system and providers with larger HIV panel size in the Kaiser system for ART-naive patients achieved better viral suppression rates [33, 34]. Recent work using 2010 data showed provider experience affects viral suppression and that family providers are less likely to prescribe ART than specialists or providers in combined family and specialist models [35–37]. These contradictory data may suggest that multidisciplinary approaches or large panel size impact HIV quality metrics such as viral suppression more than the make-up of the physician HIV care team. The family practitioner data from Canada is more difficult to generalize to US HIV care models because internists are considered specialists in Canada, but they are considered generalists in this study.
In the current study, the small but significant differences between groups could be the effect of multiple providers, because the ID plus generalist group had the highest completion rates for immunizations and HIV management in adjusted analyses. The differences could also be explained by inherent differences in the patient populations, with higher comorbidity rates and visit number in the ID plus generalist group indicating a population more medically complex and engaged in care.
In a previous study, we showed no difference in noncommunicable disease (NCD) screening between HIV care models [38], and in this study we show small absolute differences in HIV screening and HIV management quality metrics. Together, these results support the role of the ID provider as the primary care provider managing both HIV and NCD screening. To date, there is a lack of data for chronic disease management by ID providers, including common disorders such as hypertension and diabetes. Prior survey data suggest ID providers are less comfortable managing these disorders [39], which likely explains why patients with more comorbidities are more likely to be in the ID plus generalist models. We plan to examine differences in NCD management between groups to help elucidate whether specific patient populations would benefit from certain HIV care models.
There are limitations generalizing our results to other populations given the variation in HIV care models geographically and by population density as well as in underresourced locations given that our study was conducted in an urban academic medical system. Our assessment of the generalist group performance was limited by a relatively small sample size. We attempted to minimize misclassification and measurement bias through validation of exposure and outcomes. Another limitation was use of 2010 guidelines for our outcomes: providers may have incorporated more recent literature into their practice and be penalized in our accounting of guidelines. For example, 2007 data indicating patients with CD4 counts between 100 and 200 and viral suppression do not need PCP prophylaxis [40] and/or the trend to collect less frequent CD4 counts. Finally, although we adjusted for factors previously linked to differences in quality metrics, we did not adjust for other factors impacting quality indicator performance such as mental health or drug use [10, 19, 22, 41], patient or physician preference, or provider panel size because the provider panel size data was limited by a single year analysis instead of a 3-year panel size as recommended by the HIV Medicine Association [42]. Strengths of the study include (1) a large healthcare system-based cohort with rich data and (2) rigorous HIV care model classification that examines a question that existing national databases cannot currently address.
CONCLUSIONS
In conclusion, we found minimal differences in HIV quality metrics between HIV care models, with performance across groups surpassing previous results reported in the literature. Small but significant differences favoring care delivered by ID and generalist providers combined over either individual specialty were observed in immunizations and HIV management, suggesting that this model may offer an incremental benefit. Whether these differences translate into improved morbidity or mortality remains unknown. Our results suggest many models of HIV care are effective for HIV-related screening and management and that healthcare system, feasibility, and patient and physician preference may guide HIV care model selection.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
Supplementary Material
Acknowledgments
Financial support. This work was funded by the Health Resources and Services Administration (grant T32 HP102; to C. M. R.), The Tyoichi Sasakawa Fellowship Fund (to C. M. R.), Harvard Catalyst Disparities Enrichment Grant (to C. M. R.), and the National Institute of Allergy and Infectious Diseases (grant K01AI73109; to V. A. T.).
Potential conflicts of interest. D. E. S. reports stock ownership from Gilead Sciences, Inc., from null, outside the submitted work.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chirch LM, Hasham M, Kuchel GA. HIV and aging: a clinical journey from Koch’s postulate to the chronic disease model and the contribution of geriatric syndromes. Curr Opin HIV AIDS 2014; 9:405–11. [DOI] [PubMed] [Google Scholar]
- 3. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyd CM, Lucas GM. Patient-centered care for people living with multimorbidity. Curr Opin HIV AIDS 2014; 9:419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu C, Selwyn PA. An epidemic in evolution: the need for new models of HIV care in the chronic disease era. J Urban Health 2011; 88:556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pellowski JA. Barriers to care for rural people living with HIV: a review of domestic research and health care models. J Assoc Nurses AIDS Care 2013; 24:422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. HealthHIV. HealthHIV’s Third Annual State of HIV Primary Care National Survey2015; https://issuu.com/healthhiv/docs/3rd_pc_survey_final. Accessed 25 September 2015.
- 8. Casalino LP, Rittenhouse DR, Gillies RR, Shortell SM. Specialist physician practices as patient-centered medical homes. N Engl J Med 2010; 362:1555–8. [DOI] [PubMed] [Google Scholar]
- 9. Smetana GW, Landon BE, Bindman AB, et al. A comparison of outcomes resulting from generalist vs specialist care for a single discrete medical condition: a systematic review and methodologic critique. Arch Intern Med 2007; 167:10–20. [DOI] [PubMed] [Google Scholar]
- 10. Korthuis PT, McGinnis KA, Kraemer KL, et al. Quality of HIV care and mortality rates in HIV-infected patients. Clin Infect Dis 2016; 62:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–9. [DOI] [PubMed] [Google Scholar]
- 12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–9. [DOI] [PubMed] [Google Scholar]
- 13. United States Census Bureau. American FactFinder: Income in the past 12 months (in 2013 inflation-adjusted dollars). 2009–2013 American community survey 5-year estimates Available at: http://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml Accessed 27 August 2015.
- 14. United States Census Bureau. Annual social and economic supplement: three-year-average median household income by state: 2011 to 2013 Available at: https://www.census.gov/hhes/www/income/data/statemedian/ Accessed 4 September 2015.
- 15. Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res 2015; 50:398–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valdiserri RO, Forsyth AD, Yakovchenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep 2013; 128:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Catumbela E, Certal V, Freitas A, et al. Definition of a core set of quality indicators for the assessment of HIV/AIDS clinical care: a systematic review. BMC Health Serv Res 2013; 13:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horberg MA, Aberg JA, Cheever LW, et al. Development of national and multiagency HIV care quality measures. Clin Infect Dis 2010; 51:732–8. [DOI] [PubMed] [Google Scholar]
- 19. Althoff KN, Rebeiro P, Brooks JT. Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis 2014; 58:1185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keller SC, Yehia BR, Momplaisir FO, et al. Assessing the overall quality of health care in persons living with HIV in an urban environment. AIDS Patient Care STDS 2014; 28:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horberg M, Hurley L, Towner W, et al. HIV quality performance measures in a large integrated health care system. AIDS Patient Care STDS 2011; 25:21–8. [DOI] [PubMed] [Google Scholar]
- 22. Backus LI, Boothroyd DB, Phillips BR, et al. National quality forum performance measures for HIV/AIDS care: the Department of Veterans Affairs’ experience. Arch Intern Med 2010; 170:1239–46. [DOI] [PubMed] [Google Scholar]
- 23. Tedaldi EM, Baker RK, Moorman AC. Hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis 2004; 38:1478–84. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention (CDC). Vital signs: HIV prevention through care and treatment--United States. MMWR Morb Mortal Wkly Rep 2011; 60:1618–23. [PubMed] [Google Scholar]
- 25. Kitahata MM, Koepsell TD, Deyo RA, et al. Physicians’ experience with the acquired immunodeficiency syndrome as a factor in patients’ survival. N Engl J Med 1996; 334:701–6. [DOI] [PubMed] [Google Scholar]
- 26. Kitahata MM, Van Rompaey SE, Shields AW. Physician experience in the care of HIV-infected persons is associated with earlier adoption of new antiretroviral therapy. J Acquir Immune Defic Syndr 2000; 24:106–14. [DOI] [PubMed] [Google Scholar]
- 27. Kitahata MM, Van Rompaey SE, Dillingham PW, et al. Primary care delivery is associated with greater physician experience and improved survival among persons with AIDS. J Gen Intern Med 2003; 18:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong WC, Luk CW, Kidd MR. Is there a role for primary care clinicians in providing shared care in HIV treatment? A systematic literature review. Sex Transm Infect 2012; 88:125–31. [DOI] [PubMed] [Google Scholar]
- 29. Page J, Weber R, Somaini B. Quality of generalist vs. specialty care for people with HIV on antiretroviral treatment: a prospective cohort study. HIV Med 2003; 4:276–86. [DOI] [PubMed] [Google Scholar]
- 30. Landon BE, Wilson IB, Wenger NS, et al. Specialty training and specialization among physicians who treat HIV/AIDS in the United States. J Gen Intern Med 2002; 17:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landon BE, Wilson IB, McInnes K, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med 2005; 165:1133–9. [DOI] [PubMed] [Google Scholar]
- 32. Schranz AJ, Brady KA, Momplaisir F, et al. Comparison of HIV outcomes for patients linked at hospital versus community-based clinics. AIDS Patient Care STDS 2015; 29:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Horberg MA, Hurley LB, Towner WJ, et al. Influence of provider experience on antiretroviral adherence and viral suppression. HIV AIDS (Auckl) 2012; 4:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoang T, Goetz MB, Yano EM, et al. The impact of integrated HIV care on patient health outcomes. Med Care 2009; 47:560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kendall CE, Manuel DG, Younger J, et al. A population-based study evaluating family physicians’ HIV experience and care of people living with HIV in Ontario. Ann Fam Med 2015; 13:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kendall CE, Taljaard M, Younger J, et al. A population-based study comparing patterns of care delivery on the quality of care for persons living with HIV in Ontario. BMJ Open 2015; 5:e007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Neill M, Karelas GD, Feller DJ, et al. The HIV workforce in New York State: does patient volume correlate with quality? Clin Infect Dis 2015; 61:1871–7. [DOI] [PubMed] [Google Scholar]
- 38. Rhodes CM, Chang Y, Regan S, et al. Preventive cardiovascular/metabolic health screening in an HIV+ cohort by type of primary care model. In: IDWeek 2015, October 10; San Diego Convention Center; Abstract number 1647. [Google Scholar]
- 39. Fultz SL, Goulet JL, Weissman S, et al. Differences between infectious diseases-certified physicians and general medicine-certified physicians in the level of comfort with providing primary care to patients. Clin Infect Dis 2005; 41:738–43. [DOI] [PubMed] [Google Scholar]
- 40. D’Egidio GE, Kravcik S, Cooper CL, et al. Pneumocystis jiroveci pneumonia prophylaxis is not required with a CD4+ T-cell count <200 cells/microl when viral replication is suppressed. AIDS 2007; 21:1711–5. [DOI] [PubMed] [Google Scholar]
- 41. Keller SC, Yehia BR, Momplaisir FO, et al. Assessing the overall quality of health care in persons living with HIV in an urban environment. AIDS Patient Care STDS 2014; 28:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.