Abstract
Background
Antiretroviral therapy (ART) is recommended for all people living with human immunodeficiency virus (HIV), yet physician attitudes and prescribing behaviors toward members of key risk populations may limit ART access and undermine treatment as prevention strategies.
Methods
Physicians in Malaysia (N = 214) who prescribe antiretroviral therapy (ART) responded in an Internet-based survey to hypothetical clinical scenarios of HIV patients, varying by key risk population and CD4+ T-cell count, on whether they would initiate or defer ART compared with a control patient with sexually acquired HIV.
Results
The proportion of physicians who would defer ART in patients with advanced HIV (CD4 = 17 cells/μL) was significantly higher (P < .0001) for 4 key populations, including people who inject drugs ([PWID] 45.3%) or consume alcohol (42.1%), released prisoners (35.0%), and those lacking social support (26.6%), compared with a control patient (4.2%). People who inject drugs with advanced HIV (CD4 = 17 cells/μL) were 19-fold (adjusted odds ratio [AOR] = 18.9; 95% confidence interval [CI], 9.8–36.5) more likely to have ART deferred compared with the control. This effect was partially mitigated for PWID receiving methadone (AOR = 2.9; 95% CI, 1.5–5.7). At the highest CD4+ T-cell count (CD4 = 470 cells/μL), sex workers (AOR = 0.55; 95% CI, .44–.70) and patients with an HIV-uninfected sexual partner (AOR = 0.43; 95% CI, .34–.57) were significantly less likely to have ART deferred.
Conclusions
Physicians who prescribe antiretroviral therapy in Malaysia may defer ART in some key populations including PWID and released prisoners, regardless of CD4+ T-cell count, which may help to explain very low rates of ART coverage among PWID in Malaysia. Reducing HIV incidence and mortality in Malaysia, where HIV is concentrated in PWID and other key populations, requires clinician-level interventions and monitoring physician adherence to international evidence-based treatment guidelines.
Keywords: addiction, antiretroviral therapy, HIV/AIDS, physician behavior, prisoner
Global efforts to scale-up antiretroviral therapy (ART) have substantially decreased morbidity, mortality, and human immunodeficiency virus (HIV) transmission, resulting in revised international guidelines that recommend initiating ART for all persons living with HIV (PLH), regardless of CD4+ T-cell count [1]. Despite progress, HIV incidence and mortality are increasing in several countries where ART coverage is low and the epidemic is concentrated in key populations [2, 3], which are designated by the World Health Organization (WHO) as people who inject drugs (PWID), men who have sex with men (MSM), transgender women (TGW), sex workers (SW), and prisoners. Compared with the general population, key populations have higher risk of contracting and transmitting HIV, yet these populations have not benefitted equally from advances in HIV prevention and treatment because they are stigmatized or criminalized [4].
Although much of the scientific literature has primarily investigated stigmatizing attitudes of healthcare providers [5, 6], or how patients’ perceptions of HIV stigma influence their use of health services [7, 8], very few studies have assessed whether HIV-related stigma and discrimination are factors in delaying ART initiation [9–11]. Previous studies suggest that some key populations (eg, PWID) are less likely to have ART prescribed by a physician [9, 10]. In a sample of North American providers, 24.2% indicated that they would defer ART in HIV-infected PWID with a CD4+ T-cell count of 200 cells/μL [9]. Physicians may be less likely to prescribe ART if they believe that addiction or homelessness contribute to social instability and will interfere with ART adherence [10]. Yet data from systematic reviews and meta-analyses do not support these perceptions [12]. Revised international guidelines now recommend ART for PWID based on strong evidence that PWID have similar levels of adherence and rates of ART resistance compared with non-PWID [12–14]. Nevertheless, physicians’ treatment decisions remain understudied, and these decisions should be evaluated to overcome health disparities and reduce gaps in HIV treatment for key populations, who are increased risk of acquiring and transmitting HIV in many countries.
Malaysia is an upper middle-income country that, despite a decade-old policy of government-subsidized ART, remains one of the few Asian countries where HIV incidence and mortality are increasing and fewer than half (41.6%) of all clinically eligible PLH receive ART [15]. Although the HIV epidemic in Malaysia is driven primarily by PWID, less than 5% of HIV-infected PWID receives ART [16]. Because drug use and same-sex behaviors are subject to cultural and criminal sanctions in Malaysia, social stereotypes may consciously and unconsciously influence physicians’ treatment decisions and limit ART access, because currently only physicians can prescribe ART in Malaysia. Laws that criminalize members of key populations, such as PWID, MSM, TGW, and SW, contribute to their social instability through police harassment, arrest, and incarceration, and this may fuel the perception among clinicians that these patients have difficulty achieving adequate ART adherence and therefore are poor candidates for treatment [4]. Despite evidence-based guidelines in Malaysia recommending ART for all PLH with CD4+ T-cell counts ≤350 cells/μL in 2010 [17], stigma against key populations may exacerbate disparities in ART access precisely when physicians are deciding whether to initiate treatment.
Although intentions to discriminate against key populations have been identified among medical students in Malaysia [6, 18], no studies have investigated whether discrimination may contribute to physician decisions to defer ART in key populations in Malaysia. Therefore, we conducted a nation-wide survey of current ART-prescribing physicians to understand whether any patient characteristics were associated with physician decisions to defer ART in key populations.
METHODS
Study Participants
Between June and December 2013, all 425 physicians registered with Malaysia’s 2 largest professional organizations for HIV medicine, the Malaysian Society of HIV Medicine and Malaysian AIDS Council, were e-mailed an invitation to participate with a link to access the survey. The invitation assured that participation was voluntary, responses were anonymous, and that participants could win a tablet computer via lottery. Overall, 264 (62.1%) consented to participate, 8 (3.0%) were ineligible because they had not seen an HIV-infected patient within the past 12 months, and 214 (50.4%) completed the entire survey and comprised the analytic sample of ART-prescribing physicians.
Internet-Based Survey
The Internet-based survey was developed specifically for the Malaysian medical and social context by adapting a survey previously used to assess physicians’ attitudes to prescribing ART to PWID in North America [9] and expanded it to other key populations. After piloting the survey with 10 experienced Malaysian ART-prescribing physicians, a few questions were reworded to improve comprehension. To avoid leading responses, the phrase “initiate ART later” was selected to represent a provider decision to withhold or defer ART for a given scenario. Demographic and professional characteristics and information about providers’ clinical practice setting were also collected. The survey was administered using Qualtrics in English, the official language of Malaysian medical training.
Clinical Scenarios
Physicians were presented with 4 different clinical scenarios, each having patients with a different CD4+ T-cell count (17, 176, 305, 470 cells/μL). Each of the 4 scenarios presented 10 patients; 9 belonging to key populations and 1 control patient (a nonkey-population patient with heterosexually acquired HIV), for a total of 40 individual clinical scenarios. Key populations were based on the WHO definition [19] and selected through consultation with researchers and physicians in Malaysia. For each of these scenarios, physician participants were asked whether they would initiate ART “now”, to represent immediate treatment initiation based on current guidelines, or “later”. Table 1 depicts a sample clinical scenario and the choices presented to the physicians.
Table 1.
Clinical Scenario Example Presented to ART-Prescribing Physicians (N = 214) in Malaysia (June–December 2013)
| Consider the following clinical scenario: You have an HIV-infected patient who has never been on ART and is interested in initiating treatment right now. This patient is asymptomatic and has no evidence of opportunistic infections, including tuberculosis. The patient has a CD4+ T-cell count of 176 cell/μL*, which is repeated and confirmed. What would you do if this patient | ||
|---|---|---|
| Initiate ART now | Initiate ART later | |
| Lives in this area, but lives alone and has no contact with family | ⭕ | ⭕ |
| Got HIV from a partner through sexual contact | ⭕ | ⭕ |
| Is currently on MMT | ⭕ | ⭕ |
| Drinks alcohol | ⭕ | ⭕ |
| Is a sex worker | ⭕ | ⭕ |
| Is transgender | ⭕ | ⭕ |
| Has an HIV negative regular sexual partner | ⭕ | ⭕ |
| Is a man who has sex with men | ⭕ | ⭕ |
| Injects or uses drugs | ⭕ | ⭕ |
| Just got out of prison | ⭕ | ⭕ |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; MMT, methadone maintenance therapy.
*The CD4 count varied depending on the clinical scenario.
All patients were described as HIV-infected, asymptomatic, without evidence of opportunistic infections, and now requesting to initiate ART. All 10 patients were presented in random order within each clinical scenario. Clinical case scenarios were presented in order from the lowest (CD4 = 17 cells/μL) to the highest (CD4 = 470 cells/μL) CD4+ T-cell count, with lower CD4+ T-cell counts representing highest urgency for treatment. These thresholds were chosen based on 2010 WHO and Malaysian Ministry of Health guidelines that strongly recommended ART initiation for all patients with CD4 ≤350 cells/μL regardless of clinical symptoms [17]. At the time of the study, 2013 WHO guidelines had been recently released but were not yet widely adopted in Malaysia [20]. Therefore, we asked participants at the end of the survey whether they were familiar with the 2010 WHO ART guidelines and whether first-line ART is provided free of cost to Malaysian citizens.
Data Analysis
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC). For each of the 4 clinical scenarios (CD4 counts: 17, 176, 305, 470 cells/μL), 10 patient types were assessed for ART initiation now or later; the control was the patient who did not belong to any key population, ie, “Got HIV from partner through sexual contact”. Thus, each participant contributed 40 ART initiation ratings, with each being a separate record in the data file. A generalized estimating equations model was used to analyze these repeated measures using a logit link for the binary outcome and independence structure for the working correlation matrix [21]. With this approach, we regressed ART initiation on CD4 cell count, patient attributes, and their interaction, accounting for the variance-covariance structure from repeated measurements as a nuisance factor. Within each clinical scenario (ie, CD4 cell count), an adjusted odds ratio (AOR) and 95% confidence interval (CI) were calculated, representing the likelihood that a key population patient would have ART deferred compared with the control patient. To account for Bonferroni’s bound for multiple testing, we divided the standard P value of 0.05 by the 36 independent tests in our model (9 patient types, excluding the control patient, multiplied by 4 CD4 scenarios), and we adopted the adjusted P < .0014 as being statistically significant.
Ethics Statement
Ethics committees at the University of Malaya Medical Centre and Yale University approved this research.
RESULTS
Participant Characteristics
Physicians in Malaysia who prescribe antiretroviral therapy (N = 214) were on average 39.7 years old (range, 26–65), predominantly Muslim (62.1%), and female (62.6%) (Table 2). On average, physicians had been providing HIV care for over 10 years, practiced mainly in primary care settings (50.9%) and government hospitals (34.6%), cared for 18 PLH monthly, and most (80.8%) required additional patient visits before prescribing ART. Approximately half of participants (52.3%) prescribed or could reliably refer patients (72.4%) for opioid agonist therapies (OAT) such as methadone or buprenorphine maintenance therapy to treat opioid addiction. Almost all participants (99.0%) were aware that first-line ART is provided free of cost to Malaysian citizens, yet only 59.8% indicated familiarity with the 2010 WHO antiretroviral treatment guidelines.
Table 2.
Characteristics of ART-Prescribing Physicians (N = 214) in Malaysia (June–December 2013)
| Characteristics | N (%) |
|---|---|
| Mean years of age, years (SD) | 39.7 ± 8.1 |
| Sex | |
| Female | 134 (62.6) |
| Male | 80 (37.4) |
| Ethnicity | |
| Malay | 125 (58.4) |
| Non-Malay | 89 (41.6) |
| Religion | |
| Muslim | 133 (62.1) |
| Non-Muslim | 81 (37.9) |
| Medical degree completed in Malaysia | |
| Yes | 157 (73.4) |
| No | 57 (26.6) |
| Ever worked in the medical field outside Malaysia | |
| Yes | 40 (18.7) |
| No | 174 (81.3) |
| Level/Type of Training | |
| Post-baccalaureate clinical training | 77 (35.8) |
| Primary care provider | 66 (30.8) |
| Infectious disease specialist | 24 (11.2) |
| Internal medicine specialist | 20 (9.3) |
| Mean years practicing medicine ± SD | 14.3 ± 8.0 |
| Mean years providing HIV care ± SD | 11.1 ± 5.6 |
| Mean number of PLWH seen monthly ± SD | 18.4 ± 23.9 |
| Additional visits required by physician before starting ART | |
| 0 | 41 (19.2) |
| ≥1 | 173 (80.8) |
| Prescribes OAT | 112 (52.3) |
| Can refer patients to OAT sites with no wait list | |
| Yes | 155 (72.4) |
| No | 59 (27.6) |
| Familiar with 2010 WHO antiretroviral guidelines | 128 (59.8) |
| Aware the ART is provided free of cost to Malaysian citizens | 207 (96.7) |
| HIV care venue | |
| Primary care | 109 (50.9) |
| Single/multispecialty practice | 25 (11.7) |
| Hospital | 74 (34.6) |
| Ministry of Health-funded HIV care site | |
| Yes | 175 (81.8) |
| No | 39 (18.2) |
| Academic HIV care site | |
| Yes | 27 (12.6) |
| No | 187 (87.4) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; OAT, opioid agonist therapy with either methadone or buprenorphine; PLWH, people living HIV; SD, standard deviation; WHO, World Health Organization.
Physician Decisions to Defer Antiretroviral Therapy
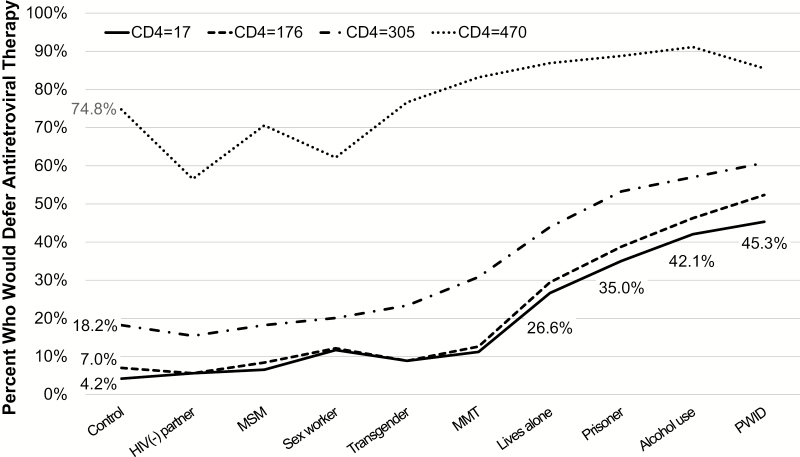
Deferral rates for patient types by CD4 levels are shown in Figure 1. The pattern differs for the highest CD4 count scenario (CD4 = 470 cells/μL) where the decision to defer was markedly higher for all patient types, with a PLH having a regular HIV-uninfected sexual partner and sex worker having the lowest likelihood of ART deferral. The other levels of CD4 showed a consistent pattern across patient types with the percentage of ART deferral decreasing as the CD4 count decreased. Our model results showed a highly significant interaction (χ2 = 94.3, P < .0001) [27], confirming a difference in patterns of deferral by CD4 count and patient type. Four key populations, including PWID, patients who consume alcohol, released prisoners, and those lacking social support, were significantly (P < .0001) more likely to have ART deferred across all 4 CD4 scenarios compared with the control patient type (Table 3).
Figure 1.
Percentage of physicians (N = 214) that would defer antiretroviral therapy by patient characteristic and CD4+ T-cell count. HIV, human immunodeficiency virus; MMT, methadone maintenance therapy; MSM, men who have sex with men; PWID, people who inject drugs.
Table 3.
Associations Between ART Deferral, CD4+ T-Cell Count, and Key-at-Risk Population Among ART-Prescribing Physicians (N = 214) in Malaysia (June–December 2013)
| Characteristic | Key-at-Risk Population* | Clinical Scenario | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4 ≤50 (CD4 = 17) AIDS-Defining Condition |
CD4 ≤200 (CD4 = 176) AIDS-Defining Condition |
CD4 ≤350 (CD4 = 305) 2010 Guidelines |
CD4 ≤500 (CD4 = 470) 2013 Guidelines |
||||||
| AOR (95% CI) | P Value | AOR (95% CI) | P Value | AOR (95% CI) |
P
Value |
AOR (95% CI) | P Value | ||
| Substance use | Injects or uses drugs | 18.9 (9.8–36.5) | <.0001 | 14.5 (8.6– 24.6) | <.0001 | 6.9 (4.8– 9.9) | <.0001 | 2.0 (1.4– 2.7) | <.0001 |
| Consumes alcohol | 16.5 (8.3– 33.1) | <.0001 | 11.4 (6.7– 19.5) | <.0001 | 5.9 (4.2– 8.3) | <.0001 | 3.4 (2.3– 5.1) | <.0001 | |
| Currently receives MMT | 2.9 (1.5– 5.7) | .0022 | 1.9 (1.2– 2.9) | .0020 | 2.0 (1.5– 2.5) | <.0001 | 1.6 (1.3– 2.1) | <.0001 | |
| Social situation | Recently released from prison | 12.3 (6.3– 24.1) | <.0001 | 8.4 (5.0– 14.0) | <.0001 | 5.1 (3.6– 7.1) | <.0001 | 2.6 (1.9– 3.7) | <.0001 |
| Lives alone and not with family | 8.3 (4.3– 16.0) | <.0001 | 5.5 (3.3– 9.1) | <.0001 | 3.5 (2.5– 4.7) | <.0001 | 2.2 (1.6– 3.1) | <.0001 | |
| Has HIV-uninfected partner | 1.4 (0.7– 2.6) | .3666 | 0.79 (0.5– 1.1) | .257 | 0.81 (0.63– 1.0) | .132 | 0.43 (0.34– 0.57) | <.0001 | |
| Sexual minority | Commercial sex worker | 3.0 (1.6– 5.8) | .0008 | 1.8 (1.1– 2.8) | .008 | 1.1 (0.84–1.5) | .414 | 0.55 (0.44–0.70) | <.0001 |
| Man who has sex with men | 1.6 (0.9– 2.9) | .1331 | 1.2 (0.76–1.9) | .405 | 1.0 (0.79–1.2) | 1.000 | 0.80 (0.66–0.98) | .037 | |
| Transgender person | 2.2 (1.2– 4.2) | .0135 | 1.2 (0.84–2.0) | .248 | 1.3 (1.1– 1.6) | .004 | 1.1 (0.50–1.3) | .0247 | |
Abbreviations: AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; AOR, adjusted odds ratio; CI, confidence interval; MMT, methadone maintenance therapy.
*Compared with a person who acquired HIV from a main sexual partner; Bonferroni-corrected P value = .0014.
At the lowest CD4 count (CD4 = 17 cells/μL), representing a patient with markedly advanced HIV, the proportion of physicians who would defer ART was highest for PWID (45.3%), followed by patients who consume alcohol (42.1%), released prisoners (35.0%), and those lacking social support (26.6%), compared with a control patient (4.2%). Differences between key populations in the odds of having ART deferred became more extreme as the level of immunologic suppression became more life threatening. Compared with the control patient, the likelihood of ART deferral was approximately 15-fold greater for PWID at CD4 = 176 cells/μL (AOR = 14.5; 95% CI, 8.6–24.6) and 19-fold higher at CD4 = 17 cells/μL (AOR = 18.9; 95% CI, 9.8–36.5). At CD4+ T-cell count ≤350 cells/μL, the likelihood of ART deferral for patients receiving methadone were smaller than for PWID, compared with controls, although the difference was not statistically significant using strictly corrected P values. More importantly, at the highest CD4+ T-cell count (CD4 = 470 cells/μL), sex workers and patients with an HIV-uninfected sexual partner were significantly less likely to have ART deferred compared with controls. At the lowest CD4+ T-cell count (CD4 = 17 cells/μL), however, sex workers were more likely to have ART deferred (AOR = 3.0; 95% CI, 1.6–5.8; P = .0008), compared with the control.
DISCUSSION
To our knowledge, this is the first study to investigate physician decisions to defer ART in WHO-designated key populations in a low- or middle-income country—specifically in Malaysia, where HIV incidence and mortality are increasing [2, 15]. These poor outcomes are related, in part, to the overall low coverage with ART in key populations, especially PWID, where coverage remains only 5% [16]. Although this study was limited to Malaysia, the HIV epidemic there is similar to those in Indonesia and several countries within Eastern Europe and Central Asia , where HIV incidence and mortality are also increasing [2, 3, 22]. Therefore, findings here should be considered of interest in global regions where HIV incidence and mortality in key populations are also increasing and HIV transmission is fueled through opioid injection [23].
Findings from this study suggest that physician prescribing behaviors and low awareness of HIV treatment guidelines are important targets for interventions to reduce Malaysia’s treatment gap [2]. Previous research with Malaysian medical students, the future prescribers of ART, showed stigmatizing attitudes and intentions to discriminate against certain key populations, including PLH generally, but also in PWID and MSM [6, 18]. This study extends these findings to ART-prescribing physicians in Malaysia who were more likely to withhold ART for 4 key populations living with HIV, including PWID, patients who consume alcohol, recently released prisoners, and persons lacking social support—even in clinical scenarios depicting patients with markedly advanced HIV disease. It is noteworthy that physicians were not more likely to defer ART in MSM, a group experiencing the highest level of stigma by medical students [18]. As the level of immune suppression became more life-threatening in these clinical scenarios, the likelihood of having ART deferred increased for patients in these 4 key populations, suggesting that even at very low CD4 thresholds, stereotypes about these highly stigmatized groups may influence physician decisions to withhold ART.
The phrase “defer ART”, which we used in the survey, was specifically selected to imply “withhold ART” and was borne out by virtue of HIV prescribers choosing to defer (or withhold) ART even for patients who had a high risk for death in the absence of ART (ie, CD4 = 17 cells/μL). We speculate that stereotypes about key populations can influence physicians’ prescribing behaviors in several ways. First, stereotypes can manifest as moral judgments and frank discrimination. Potential examples include physicians withholding ART because they believe (1) that a patient is “unworthy” of ART or (2) that ART should be prioritized for patients who are “not at fault” for their HIV infection. Treatment bias may also appear in more insidious ways, for example, when physicians withhold ART because they believe that individual or social impediments (eg, addiction or incarceration) will prevent patients from adhering to ART or benefitting from long-term therapy [9, 11]. Meta-analyses reject this notion and confirm that in the presence of adherence support, PWID can achieve similar levels of ART adherence [12] and are not more likely to develop ART resistance [14] compared with PLH who do not use drugs. Nevertheless, persistent stereotypes about the ability of specific key populations to adhere to ART—especially those who appear to have no fixed place in society because of their criminal history, drug use, and strained family support—may limit their willingness to prescribe ART to key populations [10]. This is particularly concerning in Malaysia, where nearly all incarcerated PLH are opioid-dependent PWID who face considerable postrelease challenges including stable social support [24, 25]. Although physicians often inaccurately predict adherence [26, 27], their erroneous predictions can influence ART prescription. Patients often wait for a clear signal from their clinicians about whether they should start or delay treatment [11], and patients may internalize messages from their healthcare providers that they are poor candidates for ART and therefore will not receive it. One of the main purposes of the guidelines is to standardize treatment and remove the influence of social bias, whether conscious or unconscious, on physicians’ decisions to prescribe.
Malaysia’s Ministry of Health guidelines, which are entirely aligned with contemporaneous WHO ART guidelines, specify that ART should not be withheld from ART-eligible patients with substance use disorders and that addiction treatment is not a requirement for initiating ART [20]. Nevertheless, these findings suggest that HIV prescribers in Malaysia are most likely to withhold therapy in PWID. Malaysia’s HIV epidemic is primarily concentrated among PWID, many of whom become incarcerated during their lifetime [28]. Our findings here help to explain the extremely low rates of ART coverage (5%) among PWID [16], which in turn likely contribute to increased HIV-related mortality in Malaysia [2]. Patients who consume alcohol were the second most common key population to have ART withheld. Systematic reviews have documented variable influences on the role of alcohol and ART adherence, yet most of the evidence suggests that alcohol consumption decreases ART adherence [29, 30]. Physician concerns about decreased levels of ART adherence in patients who consume alcohol may have played a role in physician decisions to defer ART for this group [31].
In this study, physicians were more likely to prescribe ART to a patient receiving methadone compared with a PWID, suggesting that at least some physicians recognize that treatment with OAT such as methadone or buprenorphine are associated with increased ART adherence and retention in care [32]. In HIV-infected patients who use opioids, methadone maintenance therapy (MMT), when dosed correctly, is associated with increased adherence to ART [33] and improved healthcare outcomes [34]. Accordingly, health services that offer MMT as part of integrated management of addiction and HIV have become the standard of care in community and criminal justice settings [35]. In Malaysia, law enforcement practices that limit access to OAT must be addressed [36], as well as lack of universal acceptance for OAT (eg, methadone) among PWID, which has been documented in community [37] and prison [24, 38] settings in Malaysia.
The finding that physicians were more likely to defer ART for recently released prisoners is of special concern given the central role of prisoners in Malaysia’s evolving HIV epidemic [15]. Although adherence to ART drops sharply after prison release [39], treating addiction and HIV at the point of release through adequate dosing of methadone [40] and directly administered ART can improve retention in care and adherence to ART [41, 42]. A recent modeling study from Ukraine suggests that methadone scale-up to 50% of PWID in prison and effective transition postrelease is the most effective strategy to reduce HIV infections in PWID [23]. Some ART-prescribing physicians in Malaysia may not yet be aware of these strategies for optimizing ART in released prisoners or PWID. Physician education, cultural competence training, and systematic quality assurance (including independent oversight of physician-prescribing practices to ensure that they meet national treatment guidelines) is needed to address differential physician prescribing behaviors in these key populations.
At the highest CD4+ T-cell count (CD4 = 470 cells/μL), both sex workers and patients with an HIV-uninfected sexual partner were significantly more likely to be prescribed ART. These findings preliminarily suggest that at least some physicians may recognize the importance of treating HIV to reduce sexual HIV transmission in these groups, but the physicians may not consider HIV treatment as prevention relevant or effective in PWID or released prisoners, despite international recommendations that both these key populations be included in global strategies that use ART to reduce HIV transmission [2, 19].
This study provides new and important evidence that characteristics of key populations are associated with physician decisions to withhold ART, yet these findings must be interpreted in the context of certain limitations. First, we studied physicians’ responses to hypothetical case scenarios, which may differ from their actual behaviors toward patients. Second, although the completed response rate (50.4%) was similar to other cross-sectional surveys of HIV physicians [10, 43] and included ART-prescribing physicians from all Malaysian territories, participants may not be representative of all ART-prescribing Malaysian physicians. Given that we recruited physicians from the 2 largest HIV provider networks in Malaysia, whose members had access to numerous HIV professional resources, our findings likely represent a best-case scenario. Third, social desirability bias may have been present, but the degree was reduced through participant anonymity and use of an Internet-based survey. In addition, randomization of case scenarios within each survey was constructed to reduce pattern recognition and bias. Notwithstanding these potential limitations, findings here suggest that some physicians are more likely to defer ART in certain key populations. Consequently, educating physicians and monitoring prescribing behaviors, to align clinical practice with national treatment guidelines, could lead to higher rates of ART coverage in key populations in Malaysia.
CONCLUSIONS
In Malaysia, physicians may be less likely to prescribe ART for PLH who inject drugs or use alcohol, were recently released from prison, or lack social support, even at extremely low CD4+ T-cell counts. Although the reasons for this are unclear, unsubstantiated concerns about ART adherence and explicit discrimination may both be factors. Interventions to educate physicians and monitor their prescribing behaviors to ensure that they conform to evidence-based treatment guidelines may increase ART use and reduce health disparities in key HIV-infected populations in Malaysia.
Acknowledgments
We gratefully acknowledge the physicians who gave their time to participate in this study, the medical and research personnel at the Centre of Excellence for Research in AIDS and University of Malaya for their commitment to the study, and Paula Dellamura and the research personnel at the Yale AIDS Program for supporting this project.
Author contributions. E. G. F. was responsible for study design, survey construction, data collection, data analysis, writing, and reviewing the manuscript. J. A. W., R. M., and A. K. were involved with study design, survey construction, data analysis, writing, and reviewing the manuscript. G. J. C., A. D. S., and H. A. P. were involved in data analysis, interpretation, and writing the manuscript. R. P. W. was involved with study design, survey construction, writing, and reviewing the manuscript. C. K. L. was involved with study design, data collection, writing, and reviewing the manuscript. F. L. A. was the Principal Investigator and was involved in study design, survey construction, data interpretation, and writing the manuscript.
Disclaimer. The funding sources played no role in study design, data collection, analysis, or interpretation, writing of the manuscript, or the decision to submit the paper for publication.
Financial support. This work was supported by the National Institute on Drug Abuse (Grants R01 DA025943 and K24 DA017072 [to F. L. A.], K01 DA038529 [to J. A. W.], and K23 DA032306 [to R. P. W.]), the University of Malaya High Impact Research Grant (E-000001-20001; to A. K.), the National Institute of Allergy and Infectious Diseases and Fogarty International Center (Grant R25 TW009338; to G. J. C.), and Yale University (Yale College Fellowship for Research in Health Studies; to E. G. F.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS. The Gap Report. Geneva: UNAIDS; 2014. [Google Scholar]
- 3. Joint United Nations Programme on HIV/AIDS. On the Fast-Track to End AIDS by 2030: Focus on Location and Population. Geneva: UNAIDS; 2015. [Google Scholar]
- 4. Grubb IR, Beckham SW, Kazatchkine M, et al. Maximizing the benefits of antiretroviral therapy for key affected populations. J Int AIDS Soc 2014; 17:19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waluyo A, Culbert GJ, Levy J, Norr KF. Understanding HIV-related stigma among Indonesian nurses. J Assoc Nurses AIDS Care 2015; 26:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Earnshaw VA, Jin H, Wickersham J, et al. Exploring intentions to discriminate against patients living with HIV/AIDS among future healthcare providers in Malaysia. Trop Med Int Health 2014; 19:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sayles JN, Wong MD, Kinsler JJ, et al. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med 2009; 24:1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahajan AP, Sayles JN, Patel VA, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS 2008; 22(Suppl 2):S67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc 2012; 15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beer L, Valverde EE, Raiford JL, et al. Clinician perspectives on delaying initiation of antiretroviral therapy for clinically eligible HIV-infected patients. J Int Assoc Provid AIDS Care 2015; 14:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christopoulos KA, Olender S, Lopez AM, et al. Retained in HIV care but not on antiretroviral treatment: a qualitative patient-provider dyadic study. PLoS Med 2015; 12:e1001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav 2010; 14:731–47. [DOI] [PubMed] [Google Scholar]
- 13. Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012; 156:817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werb D, Mills EJ, Montaner JS, Wood E. Risk of resistance to highly active antiretroviral therapy among HIV-positive injecting drug users: a meta-analysis. Lancet Infect Dis 2010; 10:464–9. [DOI] [PubMed] [Google Scholar]
- 15. Joint United Nations Programme on HIV/AIDS. HIV in Asia and the Pacific. Bangkok: UNAIDS; 2013. [PubMed] [Google Scholar]
- 16. Degenhardt L, Mathers BM, Wirtz AL, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010-2012? A review of the six highest burden countries. Int J Drug Policy 2014; 25:53–60. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 18. Jin H, Earnshaw VA, Wickersham JA, et al. An assessment of health-care students’ attitudes toward patients with or at high risk for HIV: implications for education and cultural competency. AIDS Care 2014; 26:1223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 20. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 21. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73:13–22. [Google Scholar]
- 22. Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2016. Geneva: UNAIDS; 2016. [Google Scholar]
- 23. Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet 2016; 388:1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bachireddy C, Bazazi AR, Kavasery R, et al. Attitudes toward opioid substitution therapy and pre-incarceration HIV transmission behaviors among HIV-infected prisoners in Malaysia: implications for secondary prevention. Drug Alcohol Depend 2011; 116:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Choi P, Kavasery R, Desai MM, et al. Prevalence and correlates of community re-entry challenges faced by HIV-infected male prisoners in Malaysia. Int J STD AIDS 2010; 21:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turner BJ, Hecht FM. Improving on a coin toss to predict patient adherence to medications. Ann Intern Med 2001; 134:1004–6. [DOI] [PubMed] [Google Scholar]
- 27. Murri R, Ammassari A, Trotta MP, et al. Patient-reported and physician-estimated adherence to HAART: social and clinic center-related factors are associated with discordance. J Gen Intern Med 2004; 19:1104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suleiman A. The Global AIDS Reponse Country Progress Report 2012, Malaysia. Kuala Lumpur: Section SH; 2012. [Google Scholar]
- 29. Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010; 112:178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferro EG, Weikum D, Vagenas P, Copenhaver MM, Gonzales P, Peinado J, et al. Alcohol use disorders negatively influence antiretroviral medication adherence among men who have sex with men in Peru. AIDS Care 2015; 27:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vagenas P, Azar M, Copenhaver M, et al. The impact of alcohol use and related disorders on the HIVcontinuum of care: a systematic review: alcohol and the HIV continuum of care. Curr HIV/AIDS Rep 2015; 12:421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamarulzaman A, Altice FL. Challenges in managing HIV in people who use drugs. Curr Opin Infect Dis 2015; 28:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lappalainen L, Nolan S, Dobrer S, et al. Dose-response relationship between methadone dose and adherence to antiretroviral therapy among HIV-positive people who use illicit opioids. Addiction 2015; 110:1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bachireddy C, Soule MC, Izenberg JM, et al. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend 2014; 134:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Altice FL, Kamarulzaman A, Soriano VV, et al. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet 2010; 376:367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Culbert GJ, Pillai V, Bick J, et al. Confronting the HIV, tuberculosis, addiction, and incarceration syndemic in Southeast Asia: lessons learned from Malaysia. J Neuroimmune Pharmacol 2016; 11:446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vijay A, Bazazi AR, Yee I, et al. Treatment readiness, attitudes toward, and experiences with methadone and buprenorphine maintenance therapy among people who inject drugs in Malaysia. J Subst Abuse Treat 2015; 54:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mukherjee TI, Wickersham JA, Desai MM, et al. Factors associated with interest in receiving prison-based methadone maintenance therapy in Malaysia. Drug Alcohol Depend 2016; 164:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iroh PA, Mayo H, Nijhawan AE. The HIV care cascade before, during, and after incarceration: a systematic review and data synthesis. Am J Public Health 2015; e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wickersham JA, Zahari MM, Azar MM, et al. Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend 2013; 132:378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kouyoumdjian FG, McIsaac KE, Liauw J, et al. A systematic review of randomized controlled trials of interventions to improve the health of persons during imprisonment and in the year after release. Am J Public Health 2015; 105:e13–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cunningham CO, Sohler NL, Cooperman NA, et al. Strategies to improve access to and utilization of health care services and adherence to antiretroviral therapy among HIV-infected drug users. Subst Use Misuse 2011; 46:218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arshad S, Rothberg M, Rastegar DA, et al. Survey of physician knowledge regarding antiretroviral medications in hospitalized HIV-infected patients. J Int AIDS Soc 2009; 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]