Abstract
Background
Beta (β) and gamma (γ) human papillomavirus (HPV) are commonly found on the skin. Few of the β types are associated with nonmelanoma skin cancer. Little is known about transmission patterns of these HPV, specifically in the anogenital (AG) areas. The primary objective of this study was to examine the AG concordance and transmission of β and γHPV types between heterosexual couples.
Methods
Archival samples from a previously published study examining concordance of alpha HPV types between couples were tested for β and γHPV. Hand, mouth, and genital samples were obtained 5 times over a 6-week period.
Results
Of the 21 couples examined, β and γHPV were detected in AG sites in 67% and 30% of men, respectively, and 41% and 25% of women. Positive concordance for β and γHPV was 27% and 20%, respectively, which was greater than the observed concordance between noncouples (10% for βHPV and 4% for γHPV). Transmission rate of βHPV between AG areas was 15.9 (95% confidence interval [CI], 3.3–46.5) per 100 person months for men-to-women at risk and for γHPV was 6.6 (95% CI, .2–36.7). Risks for women-to-men were similar.
Conclusions
Beta and γHPV are common in the AG area, and data suggest that they can be sexually transmitted.
Keywords: beta and gamma human papillomavirus, sexual transmission
Human papillomavirus (HPV) is recognized primarily by its association with skin and anogenital warts as well as anogenital and oral cancers. These morbidities are associated with alpha (α)-genus HPV types. Recent evidence also suggests HPV as the cause of nonmelanoma skin cancers [1]. However, beta (β) HPV types have been implicated in these cancers in immunocompetent and immunosuppressed individuals [2, 3]. Beta HPV is also detected in actinic keratosis, a known precancerous skin lesion that may progress to squamous cell cancer, specifically in organ transplant patients [4, 5]. Although βHPV types are considered commensal organisms acquired shortly after birth, studies have shown that immunosuppressed individuals have βHPV loads 100-fold higher than immunocompetent persons, underscoring the importance of immune control [4]. In addition, mechanistic studies highlight the transforming properties E6 and E7 oncoproteins from some βHPV types in in vitro and in vivo experimental models [6, 7].
In immunocompetent individuals, hair follicles are thought to be the natural reservoir [8]. Approximately 84%–91% of eyebrow hairs will be positive for βHPV, of which, on average, 4–6 different types can be detected [9]. However, one recent study found β and gamma (γ) HPV also quite abundant in the oral cavity [10]. In the few studies examining anogenital samples for presence of βHPV, 54%–59% of male anogenital samples and 2% of cervical samples were positive [10–12].
The presence of βHPV in the genital area suggests that βHPV may be transmitted between couples similar to what is seen for αHPV types. We showed in a previous study that the concordance between heterosexual couples for αHPV types ranged from 64% to 95% at any one of the scheduled 5 visits [13]. Using remaining samples from our αHPV transmission study previously reported, we performed β- and γHPV testing to examine the concordance and transmission of β- and γHPV between heterosexual couples as well as the persistence of specific HPV types in individuals over repeated visits.
METHODS
We used archival samples from a previously published study that examined concordance and transmission between anogenital, oral, and hand sites of αHPV type between heterosexual couples over a 6-week period [13]. The study methods were detailed previously [13, 14]. In brief, women who were participating in the San Francisco Natural History of HPV cohort and who had an incident αHPV type detected at one of their 4-month visits were eligible [15]. If at the last visit a woman reported being in a monogamous relationship, had normal cytology, and no current evidence of genital warts, she and her partner were asked to participate. Partners also had to report current monogamy and no genital warts. Both had to be 18 years or older. Men and women were consented separately according to the Institutional Review Boards of the University of California, San Francisco and San Francisco State University.
Twenty-five couples were enrolled. Couples had 5 visits (Vs). Visit 1 was baseline. Couples were scheduled for V2 and asked to have vaginal intercourse 24 hours beforehand. After V2, couples were asked to abstain from all sexual interaction and return within 48 hours (V3) of V2. After V3, couples returned 2 weeks (V4) and 6 weeks (V5) after V2 with no restrictions on sexual behavior.
At each visit, female samples were obtained from 6 sites: intra-anal canal, vulva, vagina, cervix, tongue/buccal mucosa, and palmar surface of the dominant hand. Male samples were obtained from 7 to 8 sites: glans (including corona sulcus), shaft, inner foreskin if applicable, scrotum, perianal area, tongue/buccal mucosa, hand, and semen.
Human Papillomavirus Genotyping
Frozen samples were shipped on dry ice at the International Agency for Research on Cancer in Lyon, France. Deoxyribonucleic acid (DNA) extraction was performed using the EZ1 Advanced XL BioRobot with the EZ1 DSP Virus kit according to the manufacturer’s instructions (QIAGEN, Hilden, Germany). Human papillomavirus DNA was measured in each sample using type-specific polymerase chain reaction (PCR) bead-based multiplex genotyping (MPG) assays that combine multiplex PCR and bead-based Luminex technology (Luminex Corp., Austin, TX), as described elsewhere [11, 16–21]. The multiplex type-specific PCR method uses specific primers for the detection of 43 βHPVs (species β-1: 5, 8, 12, 14, 19, 20, 21, 24, 25, 36, 47, 93; β-2: 9, 15, 17, 22, 23, 37, 38, 80, 100, 104, 107, 110, 111, 113, 120, 122, 145, 151; β-3: 49, 75, 76, 115; β-4: 92; β-5: 96, 150) and 28 γHPVs (species γ-1: 4, 65, 95; γ-2: 48; γ-3: 50; γ-4: 156; γ-5: 60, 88; γ-6: 101, 103, 108; γ-7: 109, 123, 134, 149; γ-8: 112, 119; γ-9: 116, 129; γ-10: 121, 130, 133; γ-11: 126; γ-12: 127, 132, 148; γ-13: 128; γ-14: 131). Two primers for the amplification of the β-globin gene were included to provide a positive control for the quality of the DNA in the sample, and water controls were added at several steps as negative controls [22]. After PCR amplification, 10 µL of each reaction mixture was analyzed by MPG, as described previously [16]. The results are expressed as the median fluorescence intensity (MFI) of at least 100 beads per bead set. The cutoff was computed by adding 5 MFI to 1.1× the median background value, as described by Schmitt et al [19]. Assays were repeated twice for 85 samples that had adequate remaining sample to examine intralaboratory reproducibility—overall concordance was .90 between runs.
Statistical Analysis
Results from individual genital samples were combined to create a single variable referred to as the genital site. For women, this included results from the vulvar, vaginal, and cervical samples, and for men this included scrotal, shaft, coronal, glans, semen, and foreskin samples; the anogenital area included results from the genital and perianal/intra-anal sample results. Oral and hand were kept as separate sites. Because hair follicles are thought to be the reservoirs for βHPV types, genital samples were further categorized into those containing sites with hair follicles (vulva, scrotum, and perianal) and nonhair sites (vagina, cervix intra-anal, shaft, corona/glans, semen, and foreskin). Prevalence was calculated among all 105 visits.
Type-specific concordance between couples’ same and different anatomic sites was calculated at each visit for β- and γHPV types separately. Positive concordance was defined when at least 1 HPV type was found in common at the sites examined. Concordance was determined by gender because males and females had different rates of type-specific infections. Negative concordance occurred when each partner’s sites were negative for all HPV types.
To compare HPV concordance between couples and noncouples, we first used random sampling with replacement and generated 200 random noncouples for each visit or 1000 noncouples for all 5 visits. We then calculated percentages of positive concordance among both couples and noncouples, and P value was calculated using χ2 test. We also used χ2 test or Fisher’s exact test, wherever appropriate, to compare the concordance between different visits, between hair-follicle and nonhair follicle sites, and between male-to-female concordance and female-to-male concordance. To examine the trend of concordance over time, we used Poisson regression model with number of concordance pairs as outcome and visit as predictor. Number of HPV-positive visits (in log form) was included in models as offset (or denominator). Relative risk of concordance between visits was calculated. Transmission incidence rates were compared between anatomic sites using exact Poisson test due to small incidence numbers. To examine whether the percentage HPV positive at each visit varies for various anatomic sites, we built generalized estimating equation models (GEE) with HPV status as outcome and visit as predictor, by specifying first-order of autoregressive as within-subject covariance structure. To compare the gender difference in having positive HPVs at various anatomic sites, similar GEE models were built by adding gender as predictor. Odds ratios were calculated in each GEE model. Analyses were performed for βHPV only because the numbers of γHPV were too small for any meaningful comparisons.
A couple was considered at risk for transmission if, during a single visit, a partner (the positive partner) had 1 or more HPV types not detected in the other partner (the negative, at-risk partner). Transmission was defined to occur when 1 or more HPV types detected in the positive partner was detected at the next visit in the previously negative, at-risk partner. Transmission rates are expressed as the number of transmission events per 100 person-months, with the rate denominator equaling the total number of months the study couples were exposed to HPV between visits. Overall anogenital and hand-to-anogenital transmission rates were calculated for male-to-female and female-to-male partner with the rate numerator being cumulative anogenital to anogenital transmission events from V1 through all subsequent visits.
RESULTS
All couples completed all study visits. Three men had inadequate samples for β- and γHPV testing; consequently, samples from 21 couples were available with a total of 105 visits. Twenty couples were monogamous for the duration of the study. In the 1 non-monogamous couple, both partners reported having another partner before V5. Demographics are described in Table 1.
Table 1.
Characteristics of Participants at Baseline
| Characteristics | Women | Men |
|---|---|---|
| Age, years (SD) | 22.1 (2.8) | 25.7 (2.8) |
| Reported years of monogamy with partner, years (IQR) | 02.3 (0.8–3.5) | 1.20 (0.7–3.0) |
| Number of lifetime sexual partners, N (IQR) | 7.0 (5.0–13.0) | 13.0 (5.0–25.0) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Prevalence of Beta Human Papillomavirus
Sixty seven percent of male and 41% of female anogenital samples were βHPV positive. Table 2 demonstrates the prevalence of βHPV at each anatomic site by gender. Beta HPV was most commonly detected in the hand in both men and women. The next most common sites among men were the shaft and the scrotum. Semen samples had the lowest rate. Among women, after hand samples, the next most common site was the vulva and oral sites had the lowest rate. Men were more likely to have oral and anogenital βHPV than women (odds ratio [OR] = 2.8, 95% confidence interval [CI] = 1–8.1 and OR = 2.7, 95% CI = 1.2–5.8, respectively). No difference was seen for hands (P = .65) When examining sites with and without hair follicles, 53% of men and 21% of women were positive from a site with hair follicles, and 56.2% and 32.4% of men and women, respectively, were positive from a nonhair follicle site. The percentage positive at each visit did not vary significantly for all the anatomic sites except for some variations observed in the scrotum and shaft (Table 2).
Table 2.
Percentage of Samples Positive for Beta HPV Types by Anatomic Site
| Men | Women | ||||
|---|---|---|---|---|---|
| Total of Positive Samples Among All 105 Visits, N (%) |
Range of % Positive Sample Among Visits 1–5 | Total of Positive Samples Among All 105 Visits, N (%) |
Range of % Positive Samples Among Visits 1–5 | ||
| Hand | 72 (69) | 57–81b | Hand | 69 (66) | 62–71b |
| Shaft | 52 (50) | 29–57c | Vulva | 22 (21) | 14–29b |
| Scrotum | 43 (41) | 29–52d | Vagina | 15 (14) | 5–24b |
| Glans | 30 (29) | 19–38b | CVL | 15 (14) | 5–19b |
| Perianal | 22 (21) | 14–29b | Anal | 15 (14) | 5–24b |
| Oral | 16 (15) | 10–24b | Oral | 6 (6) | 0–14e |
| Foreskina | 9 (41) | 0–67e | Anogenitalg | 43 (41) | 33–57b |
| Semen | 2 (2) | 0–5e | |||
| Anogenitalg | 70 (67) | 38–81f | |||
Abbreviations: HPV, human papillomavirus; CVL, cervical vaginal lavage; V, visit.
aFour men were uncircumcised.
bNo differences were seen between visits.
cHigher rates were seen at V2 (P < .05), V4 (P < .05), and V5 (P < .05) compared with V1.
dHigher rates were seen at V3 compared with V1 (P < .05).
eToo few samples positive for comparison.
fV1 had lower rates than V2, V3, and V4 (Ps < .05).
gAnogenital excludes oral and hand sites.
Nineteen βHPV types were found in men and 19 were found in women (Supplemental Figures 1 and 2). Sixteen and 13 types were seen in the anogenital area of men and women, respectively, with 12 types found in both. Among women, HPV38 was the most common type among all sites. In comparison, in men, HPV38 was most common in the hand, glans, and in the shaft, but type 21 was most common in the scrotum and oral samples, and type 22 was most common in the anal samples. If a type was found at multiple sites (3 or more), the most common βHPV types found were similar for males and females (types 38, 21, 17, 22, 9).
Prevalence of Gamma Human Papillomavirus
Thirty percent of male and 25% of female anogenital samples were γHPV positive. Gamma HPV was most commonly detected in the hand and anogenital areas in both men and women. The most common anogenital sites among men were the scrotum and shaft with oral, semen, and foreskin samples having the lowest rates. Among women, the vulva, vagina, and cervix had similar rates. Oral detection was uncommon. Table 3 shows the detection rates by site and gender. Men were more likely to have hand γHPV than women (OR = 2.6, 95% CI = 1.03–6.5). No difference was seen for oral and anogenital sites (P > .3).
Table 3.
Percentge of Samples Positive for Gamma HPV Types by Anatomic Site
| Men | Women | ||||
|---|---|---|---|---|---|
| Total of Positive Samples Among All 105 Visits N (%) |
Range of % Positive Sample Among Visits 1–5 | Total of Positive Samples Among All 105 Visits N (%) |
Range of % Positive Samples Among Visits 1–5 | ||
| Hand | 36 (34) | 24–43 | Hand | 18 (17) | 10–19 |
| Shaft | 16 (15) | 5–24 | Vulva | 16 (15) | 14–19 |
| Scrotum | 17 (16) | 10–24 | Vagina | 16 (15) | 10–19 |
| Glans | 11 (10) | 5–19 | CVL | 12 (11) | 5–14 |
| Perianal | 14 (13) | 10–14 | Anal | 8 (8) | 0–14 |
| Oral | 9 (9) | 0–14 | Oral | 5 (5) | 0–19 |
| Foreskina | 0 | 0 | Anogenitalb | 26 (25) | 19–38 |
| Semen | 0 | 0 | |||
| Anogenitalb | 31 (30) | 28–33 | |||
Abbreviations: HPV, human papillomavirus; CVL, cervical vaginal lavage.
aFour men were uncircumcised.
bAnogenital excludes oral and hand sites.
Ten different HPV γ types were found in men and 9 (same types except for one type) were found in women (Supplemental Figures 3 and 4). Among women, HPV 156, 128, and 121 were the most common types in the anogenital area. Whereas in men, HPV type 156 was most common among all sites except foreskin, which had no γHPV. If a type was found at multiple sites (3 or more), the most common γHPV types found were types 156 and 130 for males and 156 and 128 for females.
Overall Concordance for Beta and Gamma Human Papillomavirus Types
Taking into account positive (sharing at least 1 type) concordance only, the overall concordance for βHPV between couples’ anogenital sites was 27% (ranged from 17% to 35% for each visit), between hands was 39% (range 33% to 44%), and between oral samples was 10% (range 0% to 50%). For comparison, we calculated the concordance between noncouples to see whether our observations were by chance. We found that the noncouple anogenital and hand concordance was lower—8.2% had anogenital concordance (P < .0001), 17% had concordance in the hand (P < .0001), and 1.5% had concordance in the oral cavity (P = .7).
Overall positive concordance for γHPV between couples’ anogenital sites was 20% (ranged from 11% to 30% for each visit), between hands concordance was 14% (range 0% to 25%), and between oral samples concordance was 0%. If we compared concordance between noncouples, concordance was lower for anogenital and hand samples—3.5% had anogenital concordance (P = .002), 2.9% had concordance in the hand (P = .05), and 1.3% had concordance in the oral cavity (P = .4)
Female-to-Male and Male-to-Female Concordance for Beta Human Papillomavirus Types
Comparing female-to-male positive concordance, 43 of 105 visits (41%) were positive for βHPV in female anogenital samples. Of 43 samples, 22 (51%) visits showed that the male partner shared at least 1 type at one of the visits in the anogenital area. Of 62 visits with no βHPV positivity samples, 22 (35%) visits showed that the male partner also had no βHPV detected.
Comparing male-to-female concordance, 70 of 105 visits (67%) were positive for βHPV in male genital samples. Of 70 samples, 22 (31%) visits showed that the female partner shared at least 1 type. This rate was lower than female-to-male (P = .04). Of 35 visits with βHPV-negative samples, 22 visits (63%) showed that the female partner also had βHPV-negative genital samples. Positive concordance rates by visits for male to female and female to male are shown in Figure 1. Anogenital concordance rate did not differ between visits (most P > .1), except that there was a trend for female-to-male concordance to be higher at V3 compared with V1 (P = .07). Rates of hand concordance were relatively stable among all visits (all P > .1 for V1 compared with other visits). We were unable to examine concordance in oral samples because of the small sample size. Finding the man’s anogenital type in his partner’s hand and vice versa was approximately 50%. Reversing this, finding the man’s hand type in the anogenitals of the partner was approximately 25% at most visits, and finding the women’s hand type in the males’ anogenital area was approximately 50%.
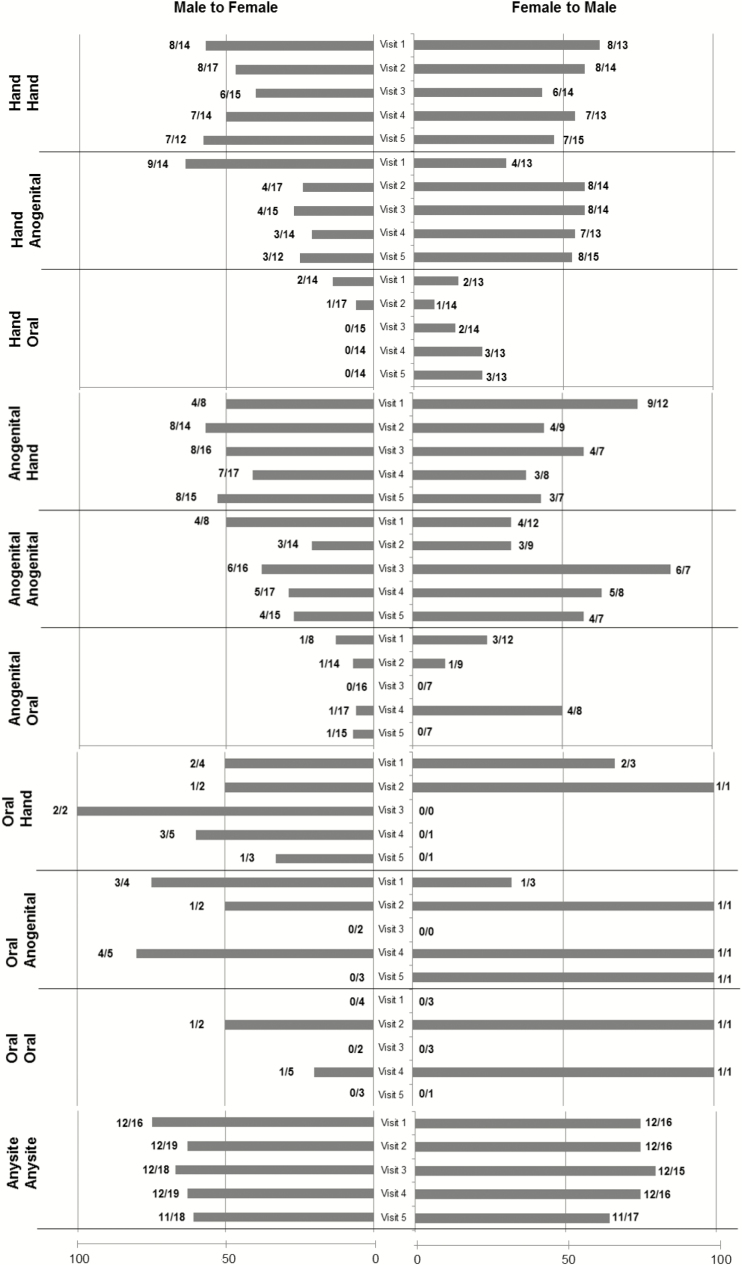
Figure 1.
Among subjects with beta human papillomavirus (βHPV) detected, positive concordance of βHPV between partners is shown at each visit by anatomic site. Because males and females partners differed in the βHPV detected at each visit, concordance is shown by gender. Bars to the right (F to M) represent percentage concordance based on the numbers at the end that represent the number of females with HPV (denominator) and the number of males having at least 1 HPV type shared with the female (numerator). Bars to the left (M to F) represent percentage concordance based on the numbers at the end of the bar that represent the number of males with HPV (denominator) and the number of females having at least 1 HPV type shared with the male (numerator).
Figure 2 shows the concordance by hair follicle sites and nonhair follicle sites between couples. The male-to-female concordance between hair follicle sites ranged between 0% and 29%, and concordance for female-to-male ranged from 0% to 50%. Nonhair follicle sites with male-to-female concordance ranged from 23% to 50%, and concordance for female-to-male ranged from 30% to 60%. Combining all visits for comparisons, concordance rate for male to female was 27% for nonhair sites compared with 11% for hair sites (P = .03), and for female-to-male concordance was 47% vs 27%, respectively (P = .14).
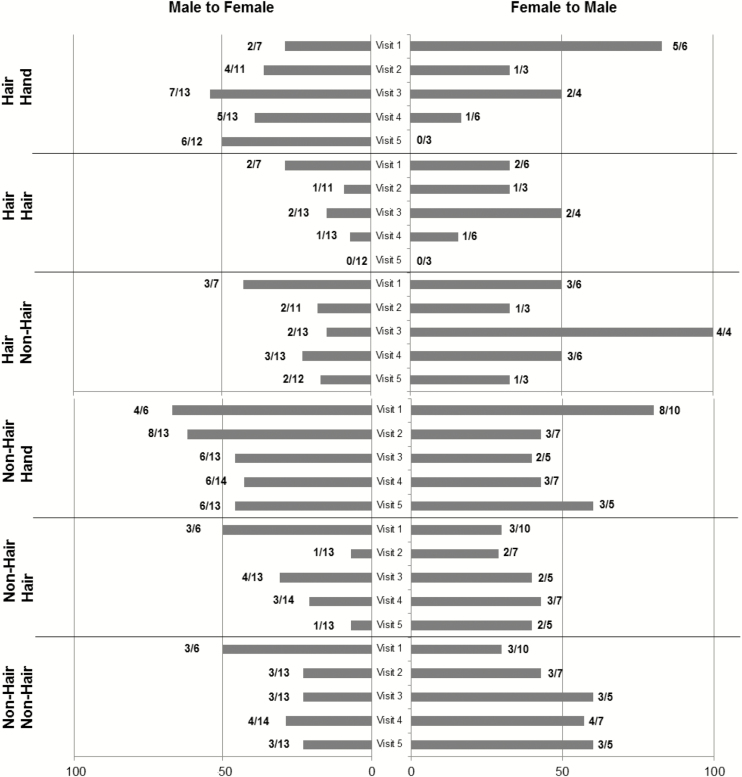
Figure 2.
Among subjects with beta human papillomavirus (βHPV) detected, the positive concordance of βHPV between partners is shown at each visit by hair follicle and nonhair follicle anatomic sites. Because males and females partners differed in the βHPV detected at each visit, concordance is shown by gender. Bars to the right (F to M) represent percentage concordance based on the numbers at the end that represent the number of females with HPV (denominator) and the number of males having at least 1 HPV type shared with the female (numerator). Bars to the left (M to F) represent percentage concordance based on the numbers at the end of the bar that represent the number of males with HPV (denominator) and the number of females having at least 1 HPV type shared with the male (numerator).
Of 97 visits in which βHPV types were detected (combining all sites) in either partner, only 4 (4%) visits matched 100% for all the types detected between partners. Of 83 visits in which βHPV was detected in one partner’s anogenital area, 2 (2.4%) visits had an identical match for all βHPV types in the other partner’s anogenital area.
Female-to-Male and Male-to-Female Concordance for Gamma Human Papillomavirus Types
Comparing female-to-male concordance, 26 of 105 visits (25%) were positive for γHPV in female anogenital samples. Of 26 samples, 9 (35%) visits showed that the male partner shared at least 1 type. Of 79 visits with no γHPV detected, 59 (75%) visits showed that the male partner also had no γHPV detected in his anogenital samples.
Comparing male-to-female concordance, 31 of 105 visits (30%) were positive for HPV in male genital samples. Of 31 samples, 9 (29%) visits showed that the female partner shared at least 1 type. Of 74 visits with no γHPV detected, 59 visits (80%) showed that the female partner also had no γHPV detected in her genital samples. Figure 3 shows the positive concordance rate between couples for each visit by anatomic site.
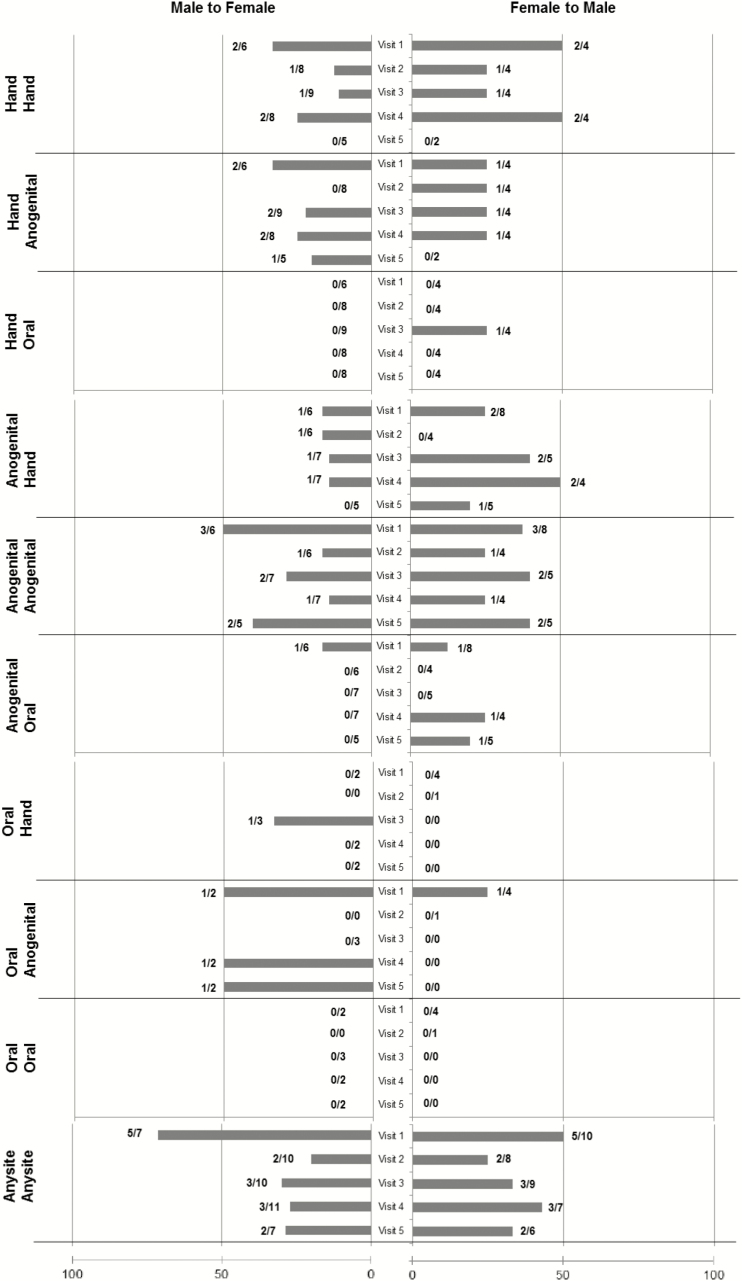
Figure 3.
Among subjects with gamma human papillomavirus (γHPV) detected, the positive concordance of γHPV between partners is shown at each visit by anatomic site. Because males and females partners differed in the γHPV detected at each visit, concordance is shown by gender. Bars to the right (F to M) represent percentage concordance based on the numbers at the end that represent the number of females with HPV (denominator) and the number of males having at least 1 HPV type shared with the female (numerator). Bars to the left (M to F) represent percentage concordance based on the numbers at the end of the bar that represent the number of males with HPV (denominator) and the number of females having at least 1 HPV type shared with the male (numerator).
For most visits, the rate of the anogenital female matching the anogenital male and vice versa was relatively similar (P > .1). Rates of hand concordance for male-to-female showed higher rates at V1 versus V2 (P = .02) and V3 (P = .05) and V4 (P = .06). No differences were seen for female-to-male hand concordance (all P > .1). Number of oral samples positive were too small for comparison. Finding the man’s anogenital type in his partner’s hand was approximately 15%, whereas finding the woman’s type in his partner’s hand ranged from 0% to 50%. Reversing this, finding the man’s hand type in the anogenitals of the partner, and vice versa, occurred in approximately 25% of the visits. As mentioned, oral infections were not commonly shared with the anogenital area or hand.
Of 62 visits in which γHPV was detected at any site in one partner, only 2 (3.2%) visits detected all the detected γHPV types somewhere in the other partner. Of 46 visits in which γHPV was detected in one partner’s anogenital area, 4 (8.7%) visits had an identical match for all γHPV types in the other partner’s anogenital area.
Rate of Transmission and Persistence
The overall transmission rate for βHPV from a man’s anogenital sites to a woman’s anogenital site was similar to women’s anogenital site to a male’s anogenital site (Table 4). The transmission rate from the women’s hand to the man’s anogenitals showed a trend to be higher than from the man’s hand to the woman’s anogenitals (P = .08).
Table 4.
Transmission Rates (per 100 Person-Month) of Beta and Gamma HPV Between Couples
| HPV Type and Direction of Transmission Between Couples | AG to AG | P * | Hand to AG | P * |
|---|---|---|---|---|
| Beta HPV man to woman | 15.9 (95% CI, 3.3–46.5) | — | 4.10 (95% CI, 0.10–22.8) | — |
| Beta HPV woman to man | 13.7 (95% CI, 3.7–35.0) | 1.0 | 24.5 (95% CI, 19.9–50.5) | .08 |
| Gamma HPV man to woman | 6.60 (95% CI, 0.2–36.7) | — | 0 | — |
| Gamma HPV woman to man | 6.70 (95% CI, 0.2–37.6) | 1.0 | 13.5 (95% CI, 0.30–75.1) | .4 |
Abbreviations: AG, anogenital; CI, confidence interval; HPV, human papillomavirus.
* P value comparing women-to-men to men-to-women transmission rate based on using exact Poisson test.
There were no differences for overall transmission rates for γHPV from a man’s anogenital sites to a women’s anogenital site or vice versa or from hand to anogenital area (P > .1) (Table 4). None of the β- or γHPV types were found persistently positive for all visits.
DISCUSSION
In this study of monogamous couples, concordance of anogenital β- and γHPV types were surprisingly high in that these HPV genera are considered commensal organisms on most squamous surfaces. The relatively high rate of concordance between couples suggests that the anogenitals represents a more common area for infectivity than previous thought and that sexual transmission is possible.
Not surprisingly, βHPV types were most commonly found in the hand. Consequently, concordance between hands was high. However, concordance between the hand and the anogenital area was equally high. Concordance between the anogenital sites was also common but had wide ranges (21% to 85%) depending on the visit, and women were less likely to have their partner’s anogenital βHPV type than men having their partner’s type. This might suggest that women were immune to certain types or that keratinized epithelium of the male genitalia is more similar to the hand epithelium and hence similar vulnerability for acquisition. In contrast, the vulvar epithelium is less keratinized and perhaps less vulnerable. We emphasize that the rate of sharing was found to be higher than chance alone (eg, noncouples), suggesting that these shared types were in fact due to skin-to-skin transmission during sexual encounters.
Although the overall prevalence of βHPV was high, in comparison to our previous published study in this same population, αHPV types were more common—approximately 84% of the samples from the men and women were positive for an αHPV type compared with two thirds of men and 41% of women samples positive for βHPV. Gamma HPV was even less common. Using this comparison, αHPV types would also be considered commensal. It is interesting to note that hair and nonhair sites in the men were similar with approximately half of the shaft and scrotum samples positive for βHPV types. For women, the vulva, a site with both hair and nonhair follicles, was lower than the male scrotum but higher than the other female genital sites.
Although oral βHPV was not common in either men or women, men were 2.5 times more likely to have a β oral HPV than women similar to the reported trend for αHPV types [23]. The rates in our population were far lower than those reported by Bottalico et al [10], who showed that 27% of adult men had βHPV detected in the oral cavity at a single visit. The difference for β types might be explained by the fact that oral rinses were used for the Bottalico et al [10] study, whereas we used tongue\buccal scrapes. Rinses have been shown to yield higher rates of αHPV types [24]. However, in comparison, only 4% had a γHPV detected similar to our findings and not explained by collection technique. The Bottalico et al [10] study also examined cervical samples from 1807 women and found 0.3% had a βHPV detected and 0.5% had a γHPV. The higher rate found in the cervix in our population may be explained by our use of cervical vaginal lavages reflecting a pool of cervical/vaginal cells, whereas the other study’s samples targeted the cervix and that the multiplex type-specific PCR-based method has a higher sensitivity than the assay used in that study [17].
The concordance data suggests that women seemed to be protected from acquiring male βHPV type. However, the observed transmission rates from anogenital to anogenital sites for βHPV were similar for males and females. This was in contrast to our observations for the αHPV types, in which females were more likely to transmit an αHPV type to the males (21.4 per 100 person-months) than the males to the females (9.21 per 100 person-months), supporting similar male vulnerability [13]. One major difference in transmission patterns observed was the absence of the spike pattern we saw with α types that occurred 24 hours after intercourse. No such spike was observed for the β- or γHPV types.
Although we hypothesized that concordance would be higher among anogenital sites with hair follicles because they are thought to be reservoirs for βHPV types, we found that sites with hair had less concordance than the nonhair sites. In part, this might be that it is more difficult to collect adequate samples from hair sites, and we did not pull hair for analysis like other studies [8].
In general, few studies are available for comparison for prevalence, and no studies examined transmission patterns. In one study of male genitalia in a US cohort, a similar high prevalence of βHPV was found in that 156 of 348 samples (44.8%) from 244 men were positive for βHPV [12]. However, their type distribution was not similar in that βHPV types 107 and 120 were the most common compared with our study, which showed that HPV38 was the most common. The difference of the results between the studies may be due to the fact that 2 different HPV detections assays were used. Of note, HPV38, together with types 4, 8, 15, 17, 20, 24, and 36, has been shown to be associated with cutaneous squamous cell cancers in immunocompetent individuals [2]. The biologic meaning of detecting HPV38 in the anogenital area is completely unknown. A study of βHPV in human immunodeficiency virus-negative men who have sex with men (MSM) reported a prevalence of 59% in the anus [11]. The higher rate is not unexpected because the number of anal intercourse partners in MSM is likely higher than reported in our heterosexual couples.
Less information is available for comparison of γHPV types likely due to the lack of any associations with known morbidity. The prevalence in the anus in MSM was 58%, similar to β types, and much higher than we found in the anus of our couples [11]. In the study by Bottalico et al [10], lower rates of cervical infection were found (4%) than those reported in our women; however, as mentioned, our cervical samples were obtained by lavages, whereas theirs were targeted to cervix only. In contrast, their oral samples had a much higher rate of 12%.
CONCLUSIONS
In summary, to our knowledge, this is the first study of sexual couples to examine concordance and transmission of β- and γHPV. Beta HPV types, and less so in γHPV, were common in the anogenital areas of young men and women. Concordance found between couples suggests that these can be transmitted sexually similar to αHPV. Further studies are needed to see whether these are commensal infections or whether they may play a role in the development of squamous cell carcinomas.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
Supplementary Material
Acknowledgments
We thank Carmen Elizabeth Chávez for manuscript preparation and David Breland and Janet Jonte for patient recruitment and specimen collection.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presentation in part. International Papillomavirus Meeting, Seattle, WA, August 20–25, 2015.
Financial support. This work was supported by the National Cancer Institute (Grant R37 CA51323; to A.-B. M.) and the American Cancer Society (Grant 92-026-12; to L. E. W.).
References
- 1. McLaughlin-Drubin ME. Human papillomaviruses and non-melanoma skin cancer. Semin Oncol 2015; 42:284–90. [DOI] [PubMed] [Google Scholar]
- 2. Chahoud J, Semaan A, Chen Y, et al. Association between beta-genus human papillomavirus and cutaneous squamous cell carcinoma in immunocompetent individuals-A meta-analysis. JAMA Dermatol 2015. doi: 10.1001/jamadermatol.2015.4530. [DOI] [PubMed] [Google Scholar]
- 3. Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet 1983; 2:422–4. [DOI] [PubMed] [Google Scholar]
- 4. Tieben LM, Berkhout RJ, Smits HL, et al. Detection of epidermodysplasia verruciformis-like human papillomavirus types in malignant and premalignant skin lesions of renal transplant recipients. Br J Dermatol 1994; 131:226–30. [DOI] [PubMed] [Google Scholar]
- 5. Weissenborn SJ, Nindl I, Purdie K, et al. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol 2005; 125:93–7. [DOI] [PubMed] [Google Scholar]
- 6. Galloway DA, Laimins LA. Human papillomaviruses: shared and distinct pathways for pathogenesis. Curr Opin Virol 2015; 14:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tommasino M. The human papillomavirus family and its role in carcinogenesis. Semin Cancer Biol 2014; 26:13–21. [DOI] [PubMed] [Google Scholar]
- 8. Weissenborn S, Neale RE, Waterboer T, et al. Beta-papillomavirus DNA loads in hair follicles of immunocompetent people and organ transplant recipients. Med Microbiol Immunol 2012; 201:117–25. [DOI] [PubMed] [Google Scholar]
- 9. de Koning MN, Weissenborn SJ, Abeni D, et al. Prevalence and associated factors of beta papillomavirus infections in individuals without cutaneous squamous cell carcinoma. J Gen Virol 2009; 90(Pt 7):1611–21. [DOI] [PubMed] [Google Scholar]
- 10. Bottalico D, Chen Z, Dunne A, et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis 2011; 204:787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Torres M, Gheit T, McKay-Chopin S, et al. Prevalence of beta and gamma human papillomaviruses in the anal canal of men who have sex with men is influenced by HIV status. J Clin Virol 2015; 67:47–51. [DOI] [PubMed] [Google Scholar]
- 12. Sichero L, Pierce Campbell CM, Ferreira S, et al. Broad HPV distribution in the genital region of men from the HPV infection in men (HIM) study. Virology 2013; 443:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Widdice L, Ma Y, Jonte J, et al. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis 2013; 207:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Widdice LE, Breland DJ, Jonte J, et al. Human papillomavirus concordance in heterosexual couples. J Adolesc Health 2010; 47:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moscicki AB, Ma Y, Farhat S, et al. Redetection of cervical human papillomavirus type 16 (HPV16) in women with a history of HPV16. J Infect Dis 2013; 208:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmitt M, Bravo IG, Snijders PJ, et al. Bead-based multiplex genotyping of human papillomaviruses. J Clin Microbiol 2006; 44:504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gheit T, Billoud G, de Koning MN, et al. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J Clin Microbiol 2007; 45:2537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruer JB, Pépin L, Gheit T, et al. Detection of alpha- and beta-human papillomavirus (HPV) in cutaneous melanoma: a matched and controlled study using specific multiplex PCR combined with DNA microarray primer extension. Exp Dermatol 2009; 18:857–62. [DOI] [PubMed] [Google Scholar]
- 19. Schmitt M, Dondog B, Waterboer T, et al. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol 2010; 48:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hampras SS, Giuliano AR, Lin HY, et al. Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLoS One 2014; 9:e104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donà MG, Gheit T, Latini A, et al. Alpha, beta and gamma human papillomaviruses in the anal canal of HIV-infected and uninfected men who have sex with men. J Infect 2015; 71:74–84. [DOI] [PubMed] [Google Scholar]
- 22. Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988; 239:487–91. [DOI] [PubMed] [Google Scholar]
- 23. Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009-2012 findings: association of sexual behaviors with higher prevalence of oral oncogenic human papillomavirus infections in U.S. men. Cancer Res 2015; 75:2468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawton G, Thomas S, Schonrock J, et al. Human papillomaviruses in normal oral mucosa: a comparison of methods for sample collection. J Oral Pathol Med 1992; 21:265–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.