Abstract
Background
Rapid diagnostic test (RDT) detecting the nonstructural 1 (NS1) antigen is increasingly used for dengue diagnosis in endemic and nonendemic settings, but its clinical utility has not been studied in travel clinic practice.
Methods
From August 2012 to July 2016, travelers returning from the tropics with fever were evaluated in the Institute of Tropical Medicine (Antwerp, Belgium) with the routine use of NS1 antigen RDT that provided results within 1 hour. We determined the diagnostic performance, assessed the management of patients with a positive RDT result, and compared it with that of historical cases of dengue diagnosed from 2000 to 2006, when only antibody detection assays were available.
Results
Of 335 travelers evaluated for fever, 54 (16%) were diagnosed with dengue, including 1 severe case. Nonstructural 1 antigen RDT was performed in 308 patients. It was truly positive in 43 of 52 tested dengue cases and falsely positive in only 1 of the 256 nondengue cases; therefore, sensitivity was 82.7% (95% confidence interval [CI], 74.4%–93.0%) and specificity was 99.6% (95% CI, 98.8%–100%). Only 3 (7%) of the 43 febrile travelers “immediately” diagnosed by RDT were admitted, and only 2 (5%) were given empirical antibacterial treatment, without adverse outcome. Admission and antibiotic prescription rates were significantly higher in the historical cases (n = 43) diagnosed by antibody detection (33%, P = .006 and 26%, P = .014, respectively), although the frequency of severe dengue was similar.
Conclusions
In our practice, the diagnostic performance of NS1 antigen RDT substantially contributed in withholding unnecessary hospitalization and antibiotherapy in dengue patients.
Keywords: dengue, fever, NS1 antigen, rapid diagnostic test, travel
Dengue fever has become a global arboviral illness associated with substantial morbidity and mortality, with an estimated 100 million cases occurring worldwide annually [1]. Dengue is nowadays one of the leading etiologies of fever in travelers returning from any tropical area [2]. Currently, no single test is perfectly accurate for diagnosing dengue virus (DENV) infection [3]. Serological assays detecting immunoglobulin (Ig)M or IgG have well identified pitfalls, including nonreactivity in the early course of the disease and cross-reactivity with other flavivirus infections and vaccination. Interpretation can be difficult in subsequent dengue infections, and turnaround times of the laboratory results may be prolonged because of batch testing in clinical practice as well as the need for follow-up sample to demonstrate seroconversion. Detection of DENV ribonucleic acid in serum by real-time, reverse-transcriptase polymerase chain reaction (RT-PCR) is limited to the rather short period of viremia, although the diagnostic window can be extended by testing urine [4]. In addition, because RT-PCR is resource intensive, it is not available in most routine laboratories and samples are usually tested in batch. Lateral-flow, immunochromatography-based rapid diagnostic tests (RDTs) detecting the nonstructural 1 (NS1) dengue antigen are increasingly used in endemic and nonendemic settings because they may provide a rapid (near) point-of-care result. High specificity, approaching 100%, is usually reported for NS1 antigen-based assays [5–8]. In contrast, sensitivity may vary from 40% to 80% according to the study locations and evaluated kits, with higher values reported in the first days of disease and in case of primary infection [9–11]. Therefore, many experts recommend to use RDTs combining NS1 antigen and IgM detection to increase the sensitivity in field settings [12, 13]. In travelers, a retrospective laboratory evaluation of an NS1 antigen-detecting, enzyme-linked immunosorbent assay showed a sensitivity of 90% within 3 days after fever onset, which declined to 70% in the subsequent days; reported specificity was greater than 90% [14]. In this study, we determined the operational performance of an NS1 antigen RDT for the routine diagnosis of dengue in international travelers presenting with fever. In addition, we assessed the current management of patients with a positive RDT result and compared it with that of a historical cohort of dengue cases from a time when only (non-RDT) antibody detection assays were available.
METHODS
This study was conducted in the outpatient travel clinic of the Institute of Tropical Medicine, Antwerp (ITMA) and in its inpatient ward located in the University Hospital of Antwerp, Belgium. The ITMA is the national reference clinical and laboratory center for infectious and tropical diseases, and its travel clinic attends to an average of 6000 to 7000 patients a year for posttravel screening and care. It is also a clinical research center, which, for more than 15 years, has focused on the diagnostic evaluation and clinical management of febrile illness after a stay in the tropics. After a large prospective study (conducted from 2000 to 2006) explored the etiology and outcome of travel-related fever [15, 16], an active clinical surveillance of tropical diseases, including among others malaria, dengue, and emerging arboviroses, was established from 2006 onwards, with a presumed consent to systematically collect data on patient symptoms, diagnosis, and outcome.
In our travel clinic, the diagnosis of dengue relied exclusively on antibody detection until 2011. Several serological assays have been successively used and are detailed elsewhere [17]. In brief, dengue was confirmed in case of documented antibody seroconversion or increase of IgG ratios in paired sera. Dengue was considered as probable in case IgM antibodies were detected (in a single serum) in combination with at least 1 of the following 3 predictors of dengue: rash, leukopenia (white blood cell count <4000/µL), and thrombocytopenia (platelet count <150000/µL), and no alternative diagnosis [16]. Currently, the antibody detection assays in use are Dengue Virus IgM Capture Dx Select and Dengue Virus IgG Dx Select (Focus Diagnostica, Cypress, CA). Cutoff ratio values for positivity are 1.0 and 1.0 for IgM and IgG, respectively. Reported sensitivity and specificity for IgM detection were 98.6% and 79.9%, respectively, in an independent evaluation compared with reference antibody detection testing [18]. From 2011 onwards, real-time DENV RT-PCR was available upon request in our travel center (described in detail in Ref. [4]). Finally, in August 2012, an NS1 antigen RDT (SD Bioline, Standard Diagnostics, Korea) was also introduced in our diagnostic workflow and could be requested in case of fever present for less than 7 days and with no obvious clinical focus. Results were available within 1 hour, if needed in an emergency for rapid decisions.
For the first part of this study on diagnostic performance, we reviewed all results of the dengue diagnostic workup in travelers evaluated in our center for ongoing fever (defined as temperature above 37.8°C within 24 hours before inclusion) from August 2012 to July 2016. Patients with fever resolved before >24 hours were purposively not included because they represent a rather different challenge in clinical practice. We then determined the sensitivity and specificity of the NS1 antigen RDT against a composite standard case definition of dengue, consisting of either the demonstration of DENV by PCR in an acute-phase sample or serological antibody detection results consistent with a confirmed or a probable case of dengue (see respective definitions here above). Dengue infection was considered as secondary when the ratio of IgG to IgM was above 1 in the acute serum sample [19].
For the second part of the study, we investigated the baseline characteristics, presenting features, and clinical management of the group of febrile travelers found with a positive NS1 antigen RDT result (also called the “contemporary NS1 antigen group”) and compared them with a historical cohort of patients diagnosed with dengue fever from 2000 to 2006, when only non-RDT antibody detection assays were available (also called the “historical antibody detection group”). Warning signs and complications were defined according to the World Health Organization (WHO) classification revised in 2009 [20]. The main comparative endpoints for both groups were the hospitalization rate and proportion of empirical antibiotic treatment.
RESULTS
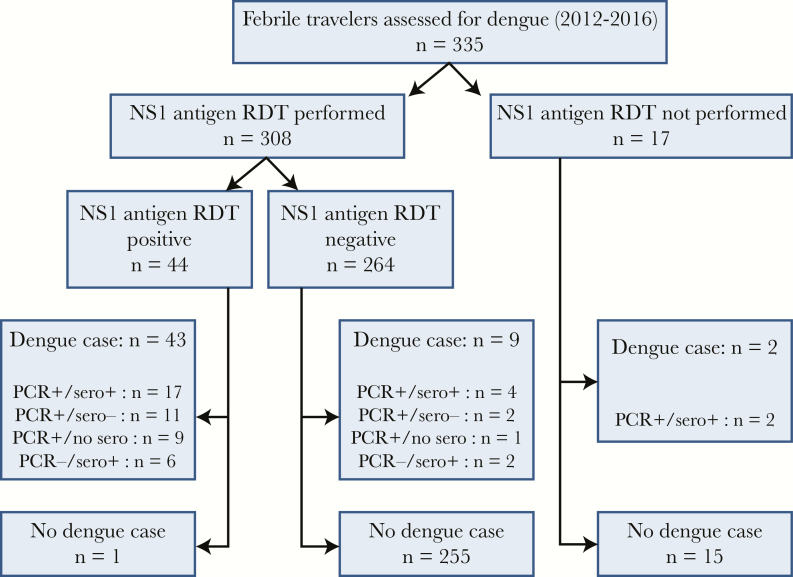
From August 2012 to July 2016, a diagnostic dengue workup was performed in 335 international travelers evaluated for fever of less than 7 days and no obvious clinical cause, and dengue was diagnosed in 54 of them (16%), including 1 severe case presenting with cerebellitis [20]. Secondary infection was confirmed in 11 cases (20.3%). As shown in Figure 1, the NS1 antigen RDT was not requested in 17 patients (5%), most often during the first months of implementation of the test, and 2 patients were diagnosed with dengue, by both PCR and antibody detection only. Of the remaining 308 patients subjected to the NS1 antigen RDT, 44 were positive and 43 of them were confirmed as dengue case either by PCR (n = 37) or antibody detection only (n = 6). Only 1 case was finally considered as a false positive, corresponding to a visitor from Burkina Faso, who had a documented malaria and in whom dengue could not be confirmed by PCR or paired serology. In the remaining 264 febrile patients with negative NS1 antigen RDT result (Figure 1), 9 were finally diagnosed with dengue (7 by PCR and 2 by antibody detection only, both latter cases seen at the 7th day of their illness).
Figure 1.
Diagnostic flow chart of febrile travelers evaluated for dengue diagnosis (n = 335). Polymerase chain reaction (PCR) “sero+” means either documented antibody seroconversion in paired sera or single-serum antibody detection (see case definition); “sero-” means no antibody detection or seroconversion was observed; “no sero” means no antibody detection result was available. NS1, nonstructual 1; RDT, rapid diagnostic test.
As shown in Table 1, in our clinical setting, sensitivity of NS1 antigen RDT was determined at 82.7% (95% confidence interval [CI], 74.4%–93.0%), corresponding to 43 true positives of 52 tested dengue cases, and specificity was determined at 99.6% (95% CI, 98.8%–100%), resulting from 255 true negatives of 256 nondengue cases. This gave a very high positive likelihood ratio (>100), and a positive predictive value (or posttest probability) >90%, considering a prevalence (or pretest probability) of dengue of approximately 15% in the febrile travelers presenting at our clinic.
Table 1.
Performance of the NS1 Antigen Rapid Diagnostic Test for the Diagnosis of Dengue in 308 Tested Travelers With Fever
| Diagnostic Under Evaluation | Result RDT | Samples Tested by NS1 Antigen RDT | Total | |
|---|---|---|---|---|
| Confirmed Dengue Case | No Dengue | |||
| NS1 antigen RDT | positive | 43 | 1 | 44 |
| negative | 9 | 255 | 264 | |
| 52 | 256 | 308 | ||
| sensitivity: 43/52 = 82.7% (95% CI, 74.4–93.0) | specificity: 255/256 = 99.6% (95% CI, 98.8–100) | |||
Abbreviations: CI, confidence interval; NS1, nonstructual 1; RDT, rapid diagnostic test.
In our setting, DENV could be demonstrated by RT-PCR in 46 of 54 dengue cases, with the following serotype distribution: DENV-1 (n = 21), DENV-2 (n = 14), DENV-3 (n = 5), and DENV-4 (n = 6). This gave a sensitivity of 85.2% (95% CI, 75.7%–94.7%) for PCR testing. Four of the 6 patients positive by NS1 antigen RDT and negative by RT-PCR were travelers evaluated at the 6th or 7th day after fever onset. In addition, 12 of the 43 (28%) patients with positive NS1 antigen RDT had negative IgM and IgG results when they initially presented for evaluation.
The clinical and laboratory features as well as management data of the 43 patients of the contemporary NS1 antigen group (who had a positive NS1 antigen RDT result and were further confirmed as dengue cases) are presented in Table 2. Most of them were young travelers returning from Southern Asia (Southeast Asia and Indian subcontinent). One third of the group was seen in second line in our clinic. Approximately 20% had no “classic” clinical or first-line laboratory predictors of dengue at presentation. Warning signs and complications were infrequent. Only 3 patients (7%) had to be admitted, and only 2 (5%) patients received an empirical antibacterial treatment (Table 2). No additional patient required secondary hospitalization at a later time. The number of dengue cases not immediately detected by the NS1 antigen RDT was too small (n = 9) to allow meaningful comparisons in terms of management with the patients found with positive RDT; however, 2 of them were hospitalized (one for an empirical intravenous antibiotherapy and the other one for further investigations) and a third patient received an ambulatory antibiotic treatment. For this reason, the contemporary NS1 antigen group was compared with the historical antibody detection group of patients diagnosed with dengue between 2000 and 2006 by antibody detection only (n = 43). It appeared that baseline characteristics, travel destination, referral pattern, prior exposure to antibiotic, clinical presentation, first-line laboratory findings, proportion of secondary dengue, and disease severity were very similar in both groups (Table 2). However, the rate of immediate hospitalization and the proportion of patients given an empirical antibiotic treatment were significantly higher in the historical group (P = .006 and P = .014, respectively). Only 1 case (in the historical group) was secondarily admitted because of development of gum bleeding.
Table 2.
Clinical and Laboratory Features of the Returning Travelers Diagnosed With Dengue Fever (2000–2006 by Antibody Detection and 2012–2016 by NS1 Antigen RDT) at the Institute of Tropical Medicine, Antwerp, Belgiuma
| Features of Study Participants | “Historical Antibody Detection Group” 2000–2006 (n = 43) |
“Contemporary NS1Antigen Group” 2012–2016 (n = 43) |
P |
|---|---|---|---|
| Epidemiological Data | |||
| Male | 24 (56) | 21 (49) | NS |
| Age group 15–60 years | 42 (98) | 38 (88) | NS |
| Stay in Southern Asia (Southeast Asia and Indian subcontinent) | 33 (77) | 30 (70) | NS |
| Stay in Latin America/Caribbean | 8 (19) | 11 (26) | NS |
| Previous contact with another care provider | 16 (37) | 15 (35) | NS |
| Previous antibiotic treatment | 13 (30) | 6 (14) | NS |
| Presenting Symptoms | |||
| Fever onset before return/arrival | 22 (51) | 17 (40) | NS |
| Duration of fever before initial contact, mean in days (range) | 4.6 (1–10) | 4.1 (1–7) | NS |
| Fever ≥39°C | 23 (54) | 28 (65) | NS |
| Headache and/or myalgia | 41 (95) | 40 (93) | NS |
| Cough | 13 (30) | 11 (26) | NS |
| Vomiting and/or diarrhea | 13 (30) | 17 (40) | NS |
| Skin rash (reported or observed) | 24 (56) | 27 (63) | NS |
| Laboratory Testing | |||
| Hemoglobin level mean in g/dL (standard deviation) | 14.8 (1.3) | 14.2 (2.1) | NS |
| Leukopenia (leukocyte count below 4000/µL) | 24 (56) | 27 (63) | NS |
| Thrombocytopenia (platelet count below 150000/µL) | 25 (59) | 19 (44) | NS |
| At least 1 dengue predictor (rash OR leukopenia OR thrombocytopenia) | 37 (87) | 34 (79) | NS |
| Absence of IgM and IgG in acute-phase serum | 17 (39) | 12 (28) | NS |
| Positive RT-PCR in acute phase serum | — | 36b (84) | — |
| Secondary dengue infection | 7 (16) | 9 (21) | NS |
| Severity Parameters and Outcome | |||
| Severe denguec | 2 (5) | 1 (2) | NS |
| Presence of at least one warning signd | 2 (5) | 2 (5) | NS |
| Immediate admission | 14 (33) | 3 (7) | .006 |
| Empirical antibiotic treatment | 11 (26) | 2 (5) | .014 |
| Total fever duration >7 days | 3 (7) | 5 (12) | NS |
Abbreviations: CI, confidence interval; DENV, dengue virus; Ig, immunoglobulin; NS1, nonstructual 1; NS, not significant; OR, odds ratio; RDT, rapid diagnostic test; RT-PCR, reverse-transcriptase polymerase chain reaction.
All results are expressed to the numbers of available data (%). NS1 antigen RDT denotes nonstructural 1 rapid diagnostic test.
Including the following: DENV-1, n = 16; DENV-2, n = 12; DENV-3, n = 5; and DENV-4, n = 3.
In 2000–2006, dengue shock syndrome/dengue hemorrhagic fever (n = 1) and meningitis (n = 1); in 2012–2016, cerebellitis (n = 1).
In 2000–2006, gum bleeding (n = 2); in 2012–2016, gum bleeding (n = 1) and slight hematemesis (n = 1).
DISCUSSION
In our reference travel clinic, we observed that the introduction of a NS1 antigen RDT in routine care allowed a correct and immediate diagnosis in approximately 80% of travelers presenting with dengue fever, with an extremely low rate of false-positive results. In addition, 28% of the travelers diagnosed with dengue fever by NS1 antigen RDT would have been missed with a single antibody detection testing at presentation. In this group of febrile travelers with a positive NS1 antigen RDT result, the rate of hospital admission and proportion of patients given unnecessary antibiotics were very low with no adverse outcome. They were significantly lower than the hospitalization and antibiotic prescription rates observed in a previous cohort of dengue cases diagnosed when only antibody detection assays were available.
This study has several obvious limitations. First, the evaluation of the performance of the NS1 antigen RDT was not designed to obtain perfectly accurate sensitivity and specificity values as in a phase 2 or 3 diagnostic study that would have required many more positive cases. However, it was purposed to assess its diagnostic yield and utility in the daily practice of a travel clinic setting. Second, the impact of NS1 antigen RDTs on the case management of dengue has not yet been fully studied in the nonendemic setting, and an observational comparison with historical cases, even if well documented, is not ideal to fully demonstrate its clinical added-value. However, setting up a randomized control trial was ethically difficult to justify, because experience in endemic countries suggested that an immediate benefit could be expected for the participants of the intervention arm [9]. In addition, conducting a multicenter study to obtain a sufficient sample size would have made it extremely complex to investigate the impact on management in very diverse clinical practices. Third, comparison of both study groups is hampered by the unknown proportion of true dengue cases who have been missed in either period, because no diagnostic strategy can fully capture all cases. Moreover, we could not investigate the pattern of serotype distribution in the historical cohort, but this unlikely had an important influence on the study endpoints. Finally, and probably most importantly, the lower rate of admission and antibiotic prescription during the second period may be partly due to several unmeasured and difficult-to-quantify factors such as the increasing experience of attending physicians over the years, the changes in WHO guidance for dengue management (in 2009), or some differences in the care-seeking trajectory. However, it must be stressed that both study groups were very similar, including for proportion of secondary infection and disease severity. In addition, the medical staff was already highly experienced in the first 2000–2006 period, as somehow reflected by the rather low use of empirical antibiotics at that time in febrile travelers with uncertain diagnosis.
In this study, close to the real-life practice, the sensitivity of the NS1 antigen RDT was in line with previous laboratory studies on accuracy of NS1 antigen-based assays in travelers [14, 21, 22]. In fact, it was equivalent to that of DENV detection by PCR, but the NS1 antigen RDT even allowed capturing some PCR-negative cases mainly at the end of the viremic phase. The rather high sensitivity of the NS1 antigen RDT alone compared with that observed in some studies conducted in endemic areas [11, 12] is likely due to the predominance of early presentation (ongoing fever was an entry criteria) and primary infection (approximately 80%) in this study. However, a negative NS1 antigen RDT result does not completely exclude dengue, and clinicians have to remain aware that diagnosis cannot always be immediately made. In such situations, an antibody detection assay has to be combined, because this approach has the highest diagnostic yield [23]. On the other hand, and maybe more importantly, due to its excellent specificity, the strong confirming power of NS1 antigen RDT allowed to accurately identify true dengue patients with a high degree of certainty. In travel clinics, where the “waiting room” probability of dengue in febrile patients ranges from 5% to 15% according to the continent of exposure [2, 15], a positive NS1 antigen RDT result would provide a posttest probability beyond 90%, particularly if any dengue predictor is also present [16]. In the absence of signs of severity, using a NS1 antigen RDT resulted in avoiding hospital admission and antibiotic exposure in the vast majority of dengue patients, although some of them felt subjectively very ill at presentation. The difference with “historical” rates of hospitalization and antibiotic prescription in our setting suggests that, at least in part, clinical care may be improved by judiciously using this new diagnostic tool. In addition, although not specifically studied here, prompt diagnosis also allowed for an immediate targeted assessment and counseling regarding the specific dengue warning signs and complications. Moreover, it may have decreased patients’ psychological stress due to diagnostic uncertainty and limited the investigations that are often required in the workup of travel-related fever. Finally, in nonendemic regions where competent vectors exist, increasing and accelerating dengue diagnosis could also reduce the risk of secondary transmission. The development of similar antigen-based RDTs, including in multiplex format, targeting other arboviral infections that geographically and clinically overlap with dengue would represent a major advance in the challenging management of tropical fever [24].
CONCLUSIONS
In conclusion, the rational use of NS1 antigen RDTs should be promoted in facilities taking care of sick travelers returning from the tropics because, similar to other relevant disease-specific RDTs, it appears to also have a beneficial impact on the clinical management of fever in this population.
Acknowledgments
This study was performed within the reference activities of the Department of Clinical Sciences of the Institute of Tropical Medicine, Antwerp.
Financial support. The National Reference Center for Arboviruses of the Institute of Tropical Medicine is partially supported by the Belgian Ministry of Social Affairs through a fund within the Health Insurance System.
Potential conflicts of interest. L. C. holds an innovation mandate from the Agency for Innovation by Science and Technology from the Flemish Government. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leder K, Torresi J, Libman MD, et al. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med 2013; 158:456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med 2012; 366:1423–32. [DOI] [PubMed] [Google Scholar]
- 4. Van den Bossche D, Cnops L, Van Esbroeck M. Recovery of dengue virus from urine samples by real-time RT-PCR. Eur J Clin Microbiol Infect Dis 2015; 34:1361–7. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed NH, Broor S. Comparison of NS1 antigen detection ELISA, real time RT-PCR and virus isolation for rapid diagnosis of dengue infection in acute phase. J Vector Borne Dis 2014; 51:194–9. [PubMed] [Google Scholar]
- 6. Chaterji S, Allen JC, Jr, Chow A, et al. Evaluation of the NS1 rapid test and the WHO dengue classification schemes for use as bedside diagnosis of acute dengue fever in adults. Am J Trop Med Hyg 2011; 84:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. da Costa VG, Marques-Silva AC, Moreli ML. A meta-analysis of the diagnostic accuracy of two commercial NS1 antigen ELISA tests for early dengue virus detection. PLoS One 2014; 9:e94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzman MG, Jaenisch T, Gaczkowski R, et al. Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis 2010; 4:pii:e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andries AC, Duong V, Ngan C, et al. Field evaluation and impact on clinical management of a rapid diagnostic kit that detects dengue NS1, IgM and IgG. PLoS Negl Trop Dis 2012; 6:e1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang CH, Kuo LL, Yang KD, et al. Laboratory diagnostics of dengue fever: an emphasis on the role of commercial dengue virus nonstructural protein 1 antigen rapid test. J Microbiol Immunol Infect 2013; 46:358–65. [DOI] [PubMed] [Google Scholar]
- 11. Hunsperger EA, Yoksan S, Buchy P, et al. Evaluation of commercially available diagnostic tests for the detection of dengue virus NS1 antigen and anti-dengue virus IgM antibody. PLoS Negl Trop Dis 2014; 8:e3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blacksell SD, Jarman RG, Bailey MS, et al. Evaluation of six commercial point-of-care tests for diagnosis of acute dengue infections: the need for combining NS1 antigen and IgM/IgG antibody detection to achieve acceptable levels of accuracy. Clin Vaccine Immunol 2011; 18:2095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fry SR, Meyer M, Semple MG, et al. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Negl Trop Dis 2011; 5:e1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuchs I, Bin H, Schlezinger S, Schwartz E. NS1 antigen testing for the diagnosis of dengue in returned Israeli travelers. J Med Virol 2014; 86:2005–10. [DOI] [PubMed] [Google Scholar]
- 15. Bottieau E, Clerinx J, Schrooten W, et al. Etiology and outcome of fever after a stay in the tropics. Arch Intern Med 2006; 166:1642–8. [DOI] [PubMed] [Google Scholar]
- 16. Bottieau E, Clerinx J, Van den Enden E, et al. Fever after a stay in the tropics: diagnostic predictors of the leading tropical conditions. Medicine (Baltimore) 2007; 86:18–25. [DOI] [PubMed] [Google Scholar]
- 17. Verschueren J, Cnops L, Van Esbroeck M. Twelve years of dengue surveillance in Belgian travellers and significant increases in the number of cases in 2010 and 2013. Clin Microbiol Infect 2015; 21:867–72. [DOI] [PubMed] [Google Scholar]
- 18. Hunsperger EA, Yoksan S, Buchy P, et al. Evaluation of commercially available anti-dengue virus immunoglobulin M tests. Emerg Infect Dis 2009; 15:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Changal KH, Raina AH, Raina A, et al. Differentiating secondary from primary dengue using IgG to IgM ratio in early dengue: an observational hospital based clinico-serological study from North India. BMC Infect Dis 2016; 16:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New edition Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 21. Huhtamo E, Hasu E, Uzcátegui NY, et al. Early diagnosis of dengue in travelers: comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J Clin Virol 2010; 47:49–53. [DOI] [PubMed] [Google Scholar]
- 22. Moi ML, Omatsu T, Tajima S, et al. Detection of dengue virus nonstructural protein 1 (NS1) by using ELISA as a useful laboratory diagnostic method for dengue virus infection of international travelers. J Travel Med 2013; 20:185–93. [DOI] [PubMed] [Google Scholar]
- 23. Hunsperger EA, Muñoz-Jordán J, Beltran M, et al. Performance of dengue diagnostic tests in a single-specimen diagnostic algorithm. J Infect Dis 2016; 214:836–44. [DOI] [PubMed] [Google Scholar]
- 24. Okabayashi T, Sasaki T, Masrinoul P, et al. Detection of chikungunya virus antigen by a novel rapid immunochromatographic test. J Clin Microbiol 2015; 53:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]