Abstract
Sex differences in the immune response and in infectious disease susceptibility have been well described, although the mechanisms underlying these differences remain incompletely understood. We evaluated the frequency of cord blood CD4 T cell subsets in a highly malaria-exposed birth cohort of mother-infant pairs in Uganda by sex. We found that frequencies of cord blood regulatory T cell ([Treg] CD4+CD25+FoxP3+CD127lo/−) differed by infant sex, with significantly lower frequencies of Tregs in female than in male neonates (P = .006). When stratified by in utero malaria exposure status, this difference was observed in the exposed, but not in the unexposed infants.
Keywords: immunity, malaria, sex, T regulatory cells, vaccines
In both adults and children, there is an abundant precedent for sex disparities in the immune response to infections [1, 2] and to vaccinations [3]. In general, females exhibit more robust innate and adaptive immunity than males. Females develop higher postvaccination antibody titers, clear antigen more quickly, and exhibit lower levels of viremia during chronic viral infections, but they are more susceptible to autoimmune and inflammatory disease and have higher rates of postvaccination adverse effects including local and systemic reactions [3]. Several mechanisms have been shown to contribute to these differences, including the effects of sex steroid hormones, as well as intrinsic genetic differences arising from X chromosome-encoded genes [3]. Disparities in the immune response and manifestations of infection are evident in prepubertal children [2], suggesting that sexual differentiation in the immune response may begin during infancy or gestation.
It is noteworthy to mention that, in a recent phase III trial of the RTS,S/AS01 malaria vaccine, RTS,S/AS01 vaccination was associated with higher all-cause mortality in female but not male children compared with the control arm [4]. This difference was highly statistically significant and was observed in both age groups in which the vaccine trials were conducted. Furthermore, there was a tendency towards an increased risk of fatal malaria in females but not males [4]. As the authors noted, there is precedent for increased vaccine-associated mortality in females: after introduction of the high-dose measles vaccine, a doubling of all-cause mortality among girls (but not boys) led to eventual withdrawal of the vaccine from the market [5]. The potential immunologic basis for sex differences in postvaccination mortality after RTS,S/AS01 was not explored.
Malaria is known to trigger numerous immunoregulatory pathways, including induction of regulatory T cell (Treg) populations [6]. Clinical manifestations of malaria infection are likely to result from an imbalance of inflammatory and regulatory aspects of the immune response. For instance, malaria infection in a naive host results in peripheral induction and expansion of suppressive FoxP3+ Tregs [6], and these Tregs have been shown to impact subsequent T-cell effector responses. After acute malaria, Treg induction may limit the magnitude of subsequent Th1 response. Lower levels of FoxP3 messenger ribonucleic acid during acute malaria are associated with higher malaria-specific interferon (IFN)-γ responses to repeated malaria antigen exposure during follow-up [7]. Although sex differences in the prevalence, severity, and pathophysiology of numerous infectious diseases have been described [1, 2], including malaria-related mortality [8], there are few published studies examining potential sex differences in effector and regulatory immune responses to malaria, which is a leading cause of childhood deaths in Africa.
We sought to investigate sex differences in T-cell differentiation among infants in a highly malaria-endemic setting in Eastern Uganda, where the majority of pregnancies are complicated by placental malaria. Using cord blood samples from a birth cohort of pregnant mothers and their infants, we evaluated the frequency of total, regulatory, and activated CD4 cells in cord blood by sex.
METHODS
Study Populations
Cord blood samples were collected from infants born to mothers enrolled in a clinical trial of prenatal malaria chemoprevention conducted in Tororo, Uganda, an area of high malaria endemicity. Clinical trial outcomes are described in a prior publication [9]. In brief, between June 2014 and October 2014, 300 human immunodeficiency virus negative mother-infant pairs were enrolled between 12 and 20 weeks of gestation. Informed consent was obtained from the parent or guardian of all study participants. The study protocol was approved by the institutional review boards of the Uganda National Council of Science and Technology, the University of California, San Francisco, and Makerere University. We collected cord blood from 166 participants from which sufficient cord blood mononuclear cells (CBMCs) were available. Participants received routine medical care at the study clinic, and mothers were evaluated monthly throughout pregnancy for Plasmodium parasitemia by peripheral blood via loop-mediated isothermal amplification (LAMP), which detects Plasmodium deoxyribonucleic acid (DNA). During febrile episodes, mothers were evaluated with blood microscopy and, if positive, treated per local guidelines for clinical malaria, as previously described [9]. At the time of delivery, maternal peripheral blood, placental blood, and cord blood were tested for parasitemia by both LAMP and microscopy. Placental tissue was processed for histopathologic evidence of malaria infection, determined by standardized placental malaria histopathology criteria as previously described [9].
Cord Blood Mononuclear Cells Collection
At the time of delivery, whole cord blood was collected in umbilical cord blood collection kits (Pall Medical). Cord blood mononuclear cells were isolated by Ficoll-histopaque density centrifugation (GE Life Sciences) and cryopreserved in liquid nitrogen.
Flow Cytometry Immunophenotyping
Cord blood mononuclear cells were thawed, aliquoted at 1 × 106 cells, and surface stained using standard protocols with the following antibodies: APC/Cy7-conjugated CD3, PerCP-conjugated CD4, BV421-conjugated CD25, BV650-conjugated CD127 (BioLegend). Cells were then fixed and permeabilized with FoxP3 transcription factor staining buffer set (eBioscience). After washing, cells were then intracellularly stained with PE-conjugated FoxP3 (eBioscience). BV510-conjugated CD8, CD14, CD19 (BioLegend), and LIVE/DEAD aqua amine (Invitrogen) were used as exclusion markers to minimize nonspecific binding. Flow cytometry data were collected on an LSRII 4-laser flow cytometer with FACSDiva software. Color compensations were performed using compensation beads. An isotype control was used to define negative and positive populations for FoxP3 and CD25. Cellular profiles were gated on live CD3+CD4+ lymphocytes. Fresh cord blood CD4 T cells were enumerated from 50 μL of whole cord blood stained with antibodies in BD TruCount tubes or with 20 μL of CountBright counting beads (ThermoFisher Scientific) on 152 available cord blood samples. Cells were incubated for 20 minutes, and 900 μL of BD FACS lysis solution was added for 15 minutes. CD4 T cell staining was performed using PerCP-conjugated CD3, APC-conjugated CD4 antibodies. Cells were immediately analyzed on an Accuri C6 cytometer. Absolute CD4 counts were recorded per microliter of whole cord blood. To normalize the frequency of Tregs and activated CD4 T cells from cryopreserved CBMCs to absolute rates of CD4 per microliter of fresh whole cord blood, we calculated absolute CD4 subset counts (absolute subset count = (subset frequency) × (absolute CD4 count per μL of whole cord blood)).
Statistical Analysis
Cell frequencies were compared between sexes using Wilcoxon rank-sum testing. Associations between sex and malaria exposure outcomes were compared using χ2 testing. Statistical analyses were completed using PRISM 7.0 (GraphPad). Two-sided P values were calculated for all test statistics, and P < .05 was considered significant.
RESULTS
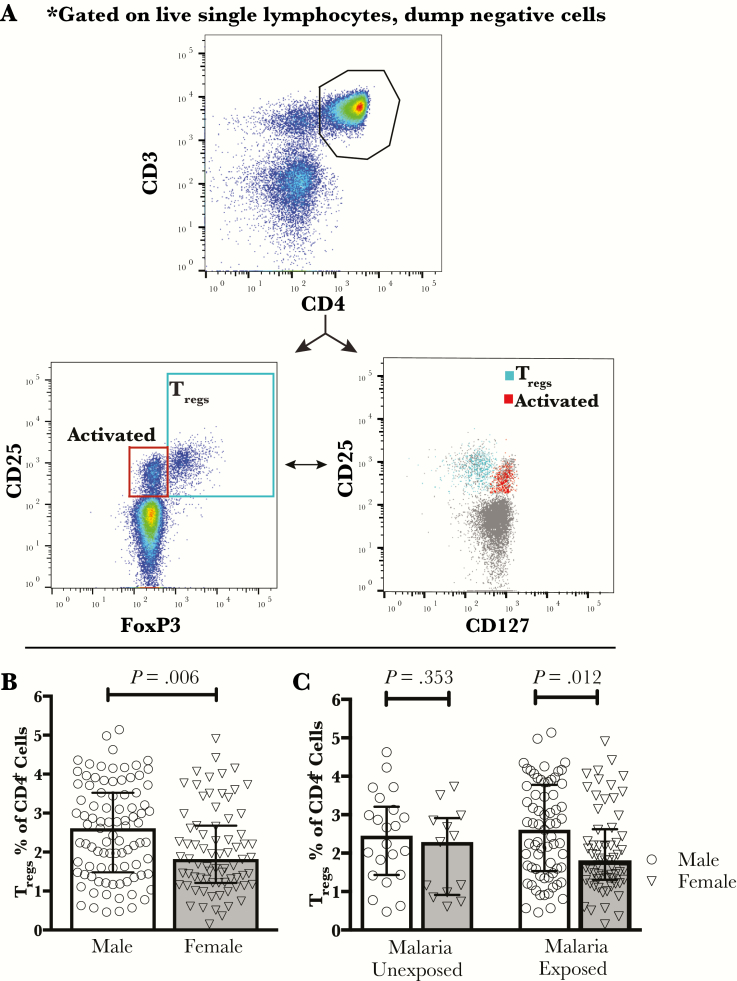
We evaluated the frequency of total, regulatory (Tregs; CD25+ FoxP3+ CD127lo/−), and activated (CD25+FoxP3−CD127hi) CD4 T cells (Figure 1A) in the cord blood of 166 infants born to mothers enrolled in a randomized clinical trial of prenatal malaria chemoprevention. Pregnancy-associated malaria was common, with 79% of infants exhibiting evidence of in utero malaria exposure by either maternal, cord, or placental microscopy or P falciparum DNA testing, and/or positive placental histopathology for malaria infection. Rates of in utero malaria exposure did not differ between male and female infants (75.8% vs 82.7%, P = .282) (Table 1). However, we found that frequencies of Tregs in cord blood differed significantly by infant sex, with female neonates having lower frequencies of Tregs than males (1.82% vs 2.61%, P = .006) (Figure 1B; Table 2). In addition, absolute Treg counts, calculated by normalizing subset frequency to CD4 counts per volume of whole blood, were also lower in females than in males (24.4 vs 35.8 cells/μL whole cord blood, P = .012) (Table 2).
Figure 1.
Lower frequency of cord blood T regulatory cells (Tregs) in females vs males in in utero malaria-exposed infants. (A) Cord blood cells were gated on live, single lymphocytes, dump-negative T cells. Gating of CD4+CD25+ cord blood T cells revealed 2 distinct subsets, Tregs (CD25+FoxP3+CD127lo/−) and activated CD4 T cells (CD25+FoxP3−CD127hi). (B) The frequency of cord blood Tregs (CD25+FoxP3+CD127lo/−) from all infants evaluated, regardless of in utero malaria exposure, differs by sex (P = .006; Wilcoxon rank-sum testing; error bars indicate median with interquartile range; n = 166). (C) The frequency of Tregs differs by sex in the in utero malaria-exposed infants (P = .012), but not the malaria-unexposed infants (P = .353; Wilcoxon rank-sum testing; error bars indicate median with interquartile range).
Table 1.
In Utero Malaria Exposure by Infant Sex
| Malaria Exposure Category | Female | Male | |
|---|---|---|---|
| Enrollmenta | Positive n (%) | Positive n (%) | P Value |
| Maternal parasitemia by LAMP | 41 (54.7) | 48 (52.8) | .805 |
| Monthly Screening During Pregnancy | |||
| Maternal parasitemia by LAMP | 42 (56.0) | 39 (42.9) | .092 |
| Delivery | |||
| Maternal parasitemia | |||
| LAMP | 13 (17.3) | 7 (7.7) | .058 |
| Microscopy | 2 (2.67) | 2 (2.2) | .649 |
| Placenta | |||
| LAMP | 11 (14.7) | 9 (9.9) | .347 |
| Microscopy | 2 (2.67) | 2 (2.2) | .845 |
| Histopathology | 30 (40.0) | 33 (36.3) | .622 |
| Cord Blood | |||
| LAMP | 1 (1.3) | 2 (2.2) | .501 |
| Any malaria during pregnancy or delivery | 62 (82.7) | 69 (75.8) | .282 |
Abbreviations: LAMP, loop-mediated isothermal amplification.
a12 to 20 weeks gestational age.
Table 2.
Cord Blood T-Cell Subsets by Infant Sex
| T-Cell Subset | Female | Male | |
|---|---|---|---|
| Median [IQR] | Median [IQR] | P-value | |
| Frequency (%)a | |||
| Total CD4 | 74.4 [68.2–83.1] | 76.3 [68.6–80.4] | .993 |
| Tregs | 1.82 [1.2–2.7] | 2.61 [1.5–3.5] | .006 |
| Activated (CD25+ FoxP3−CD127hi) | 2.17 [1.1–3.5] | 2.39 [1.4–3.0] | .884 |
| Absolute Countsb | |||
| Total CD4 | 1356 [1252–1778] | 1527 [1056–1872] | .669 |
| Tregs | 24.4 [15.6–42.2] | 35.8 [18.3–57.8] | .012 |
| Activated (CD25+ FoxP3−CD127hi) | 31.8 [18.5–49.0] | 32.0 [18.8–49.5] | .956 |
Statistically significant P-value in bold. Abbreviations: IQR, interquartile range; Tregs, T regulatory cells.
aFrequency of parent subset.
bAbsolute count per microliter of whole cord blood.
To explore whether exposure to malaria antigens during fetal life may have contributed to differential expansion of Tregs in males and females, we examined the relationship of infant sex to Tregs following stratification for in utero malaria exposure status (positive maternal, cord, or placental microscopy or P falciparum DNA testing, and/or positive placental histopathology). A sex disparity was observed in the exposed infants with females having lower Treg frequencies than males (P = .012; n = 131), but not in the unexposed infants (P = .353, n = 35) (Figure 1C). However, the small number of infants who lacked evidence of any malaria exposure limited the power of this comparison in the unexposed subgroup.
We also compared the frequency of activated CD4 T cells (CD127hi CD25+ but lacking FoxP3 expression), because we have previously shown this population to be expanded in infants who were exposed to malaria in utero [10]. There was no sex difference in the frequency of these activated CD4 cells (P = .884) (Table 2) in the cohort as a whole or in the exposed and unexposed subgroups.
Several studies have documented that female newborns have higher CD4 percentages and that this trend persists through childhood [2, 3]. In our cohort, there was no difference in the frequency (P = .993) or absolute counts of total CD4 T cells (P = .669) by infant sex. Thus, only the FoxP3+ regulatory CD4 T-cell compartment, and not total or activated CD4 T cells, differed between male and female neonates (Table 2).
DISCUSSION
We found significantly lower frequencies of cord blood FoxP3+ regulatory CD4 T cells in female than in male infants born in a region of high malaria transmission intensity, where 79% of enrolled participants had evidence of malaria infection either during pregnancy or at parturition. To our knowledge, no prior studies have reported a difference in frequencies of Tregs in the cord blood of male and female infants, either in uncomplicated healthy pregnancy or after in utero exposure to a pathogen. It is not clear whether this difference reflects an intrinsic biologic difference between male and female neonates that is independent of pathogen exposure, or if alternatively, in utero exposure to malaria antigens drives Treg differentiation more strongly in males than females.
Several potential mechanisms might contribute to the observed sex differences in cord blood Tregs. First, differences in sex steroid hormones have direct effects on numerous innate and adaptive immune cells [3]. Although infancy and childhood have historically been regarded as a time of hormonal quiescence, some data suggest that differences in the hormonal milieu of males and females emerge in utero, with testosterone first produced by male testes as early as gestational week 10 [11]. Thus, sex-based differences in the immune response may begin even before birth. In addition to hormonal influences, genetic differences between male and female individuals may drive sexual dimorphism in the immune response. Experiments comparing transgenic XY and XX mice with a common gonadal type indicate that it is the XX sex chromosome complement, rather than female steroid hormones, that predisposes females to autoimmunity [12]. Many genes critical for immune function and immunoregulation are encoded by the X chromosome (including FoxP3, TLR7, TLR9, and IRAK), and an estimated 15% of these may escape X inactivation to some degree, making them subject to gene dosage effects [13]. Together, these factors may contribute to a bias towards less Treg differentiation and more vigorous inflammatory immune responses in females.
In light of recent reports of increased postvaccination mortality in females receiving the RTS,S/AS01 malaria subunit vaccine [4], it is clear that sex differences in the immune response to malaria require further evaluation, both in naturally exposed populations and in malaria vaccine trials. The lower frequencies of Tregs observed among female infants in this malaria-endemic setting could contribute to a more vigorous effector response and increased immunopathology after natural malaria exposure or vaccination. In murine vaccination models, Treg depletion at the time of vaccination enhances CD4 IFN-γ and interleukin-2 production upon restimulation with antigen [14]. Additional studies are needed in both malaria-exposed and unexposed populations to validate our findings of sex differences in Tregs at birth. Moreover, future field studies of malaria-specific immunity, including vaccine-induced responses, should pay careful heed to potential sex differences in regulatory and effector responses, which may shed light on the mechanisms underlying the increased mortality seen in female RTS,S/AS01 malaria vaccine recipients.
CONCLUSIONS
Our findings add to the mounting evidence indicating that females and males differ in their intrinsic immune responsiveness and susceptibility to infection, and that these differences arise during early life—even in utero. Moreover, although both biological sex and societal gender roles and behaviors have been shown to contribute to differences in infectious disease outcomes, the observation that sex disparities are evident at birth highlights the biological underpinnings of sexual dimorphism in the immune response.
Acknowledgments
We are immensely grateful to the participants and families who have consented to the study.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support. This work was funded by the following: National Institutes of Health Grants 5P01HD059454-07 (to D. V. H., M. R. K., and G. D.), 2R01AI093615-06 and 5K24AI113002-03 (to M. E. F.), and 5T32AI060530-10 (to M. P.); Merle A Sande/Pfizer Fellowship in International Infectious Diseases funded by the Infectious Diseases Society of America Education and Research Foundation; and the National Foundation for Infectious Diseases (to M. P.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis 2014; 209(Suppl 3):S120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ruel TD, Zanoni BC, Ssewanyana I, et al. Sex differences in HIV RNA level and CD4 cell percentage during childhood. Clin Infect Dis 2011; 53:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 4. Klein SL, Shann F, Moss WJ, et al. RTS,S malaria vaccine and increased mortality in girls. MBio 2016; 7:e00514–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aaby P, Jensen H, Samb B, et al. Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: reanalysis of West African studies. Lancet 2003; 361:2183–8. [DOI] [PubMed] [Google Scholar]
- 6. Walther M, Tongren JE, Andrews L, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 2005; 23:287–96. [DOI] [PubMed] [Google Scholar]
- 7. Walther M, Jeffries D, Finney OC, et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 2009; 5:e1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khanga S, Thatoi PK, Mohapatra BN, et al. Severe falciparum malaria-difference in mortality among male and nonpregnant females. J Clin Diagn Res 2014; 8:MC01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kakuru A, Jagannathan P, Muhindo MK, et al. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 2016; 374:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prahl M, Jagannathan P, McIntyre TI, et al. Timing of in utero malaria exposure influences fetal CD4 T cell regulatory versus effector differentiation. Malar J 2016; 15:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carr BR, Parker CRJ, Ohashi M, et al. Regulation of human fetal testicular secretion of testosterone: low-density lipoprotein-cholesterol and cholesterol synthesized de novo as steroid precursor. Am J Obstet Gynecol 1983; 146:241–7. [DOI] [PubMed] [Google Scholar]
- 12. Smith-Bouvier DL, Divekar AA, Sasidhar M, et al. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med 2008; 205:1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005; 434:400–4. [DOI] [PubMed] [Google Scholar]
- 14. Qin L, Jiang G, Han J, Letvin NL. Regulatory t cells modulate DNA vaccine immunogenicity at early time via functional CD4(+) T cells and antigen duration. Front Immunol 2015; 6:510. [DOI] [PMC free article] [PubMed] [Google Scholar]