Abstract
Background
The incidence of recurrent herpes zoster (HZ) and the relationship between initial and recurrent HZ are not clear.
Methods
The Miyazaki Dermatologist Society has surveyed ~5000 patients with HZ annually since 1997. A questionnaire regarding HZ and its recurrence was completed by the dermatologists.
Results
A total of 34 877 patients with HZ were registered at 43 clinics between June 2009 and November 2015. Among 16 784 patients seen at 10 of the 43 clinics, 1076 patients (6.41%) experienced recurrence. Herpes zoster was more frequent in female than in male patients (5.27 vs 4.25 in 1000 person-years, P < .001), as was HZ recurrence (7.63% vs 4.73%, P < .001). Two and three recurrences were observed in 49 and 3 patients, respectively. Recurrence in the same dermatome was observed in 16.3% of patients, and more frequently this occurred in the left side (P = .027). The number of HZ-experienced persons increased with age, and one third of the population had experienced HZ by the age of 80.
Conclusions
Recurrent HZ was observed in 6.41% of patients, with a higher incidence in women. Moreover, HZ experience reduced the HZ incidence to 31.7% of the incidence in the HZ-naive population.
Keywords: epidemiology, herpes zoster, recurrence, varicella-zoster virus
Varicella-zoster virus (VZV) infection causes varicella and results in latent infection in the sensory ganglia. We previously reported that reactivation of VZV caused herpes zoster (HZ) in all age groups, especially in the elderly, at rates of 3–8 per 1000 person-years in a study of 48 388 patients with HZ [1]. The major complication of HZ is chronic pain (postherpetic neuralgia [PHN]); the pain is related to peripheral nerve injury and activation of brain-derived neurotrophic factor by the anti- immediate early protein 62 antibody [2]. Herpes zoster and PHN are major health concerns in the elderly. Zostavax reduces the incidences of HZ and PHN to approximately one half and one third, respectively [3], and the efficacy of the glycoprotein E vaccine against HZ was found to be 97.2% [4].
The lifetime incidence of HZ recurrence in immunocompetent persons is estimated to be between 1% and 6%, and recurrence typically occurs many years after the initial episode [5–9]. Of the 1669 persons who experienced an episode of HZ during the 6 years of one study, 95 cases were recurrences [5]. In a shingles prevention study, 2 of 19 247 vaccine placebo recipients had another episode of HZ within 3 years of the initial episode [3]. In surveys of patients with HZ, recurrence has been reported to occur in 9 of 192 [6], 18 of 1112 [7], 2 of 206 [10], 5 of 184 [11], 4 of >400 [12], 26 of 339 [13], and 31 of 590 [14] patients. Thus, although many studies have estimated the recurrence of HZ, and recurrence seems to be low but common [5, 15], the exact incidence and clinical features of HZ recurrence in the general population are not clear.
Universal vaccination of varicella vaccination started in October 2014 in Japan, and varicella vaccine was licensed for the use of HZ prevention in April 2017. In a 6.5-year survey from June 2009 to November 2015, a total of 34 877 patients with HZ were monitored at 43 dermatology clinics in Miyazaki Prefecture, Japan without the effect of varicella vaccine for HZ prevention. In 10 of the 43 clinics that participated in the recurrence study, 1076 of 16 784 patients were observed to have a recurrence of HZ. The study of HZ recurrence in the general population helps further elucidate the incidence, duration of immunity, sex preference, age, underlying diseases, and dermatomes affected in the epidemiology of HZ.
METHODS
Collection of Patients and Study Population
All new medically documented episodes of HZ occurring from June 2009 to November 2015 in a total of 43 clinics, which included 36 dermatology clinics and the dermatology departments of 7 flagship general hospitals belonging to the Miyazaki Dermatologist Society (members of the Miyazaki Dermatologist Society are listed in the Appendix), were evaluated. A questionnaire about the age and sex of patients with HZ was completed by dermatologists in the 43 dermatology clinics, and the number of patients with HZ in this survey was used to estimate the total number of patients with recurrent HZ in the prefecture.
Ten among the 43 clinics agreed to monitor the recurrence of HZ and completed a questionnaire about underlying diseases, timing of the previous HZ episode, and the dermatomes affected, in addition to the age and sex of patients with recurrent HZ. The dermatomes were determined by using illustrations of the cutaneous fields of peripheral nerves [16]. Data about previous episodes of HZ were accepted only if those episodes were confirmed by clinical data or the presence or site of scarring. A summary of questionnaires from each clinic was collected monthly by the Toyama Dermatology Clinic. The study was approved by the ethics committee of the University of Toyama. This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry, number UMIN000008738.
The mean population of Miyazaki Prefecture was 1.12 million in 2009–2015 [17]. This study was a voluntary surveillance of HZ based on patient care by the Miyazaki Dermatologist Society, maintained without financial support since 1997 [1], and the direct immunofluorescent antibody (DFA) test used for confirmation of HZ was covered by health insurance. The coverage rate of HZ in Miyazaki Prefecture was estimated by identifying the total number of patients with HZ who were treated with famciclovir (3346) among all patients who visited the dermatology clinics between January 2012 and January 2013 (3920; 85.36%), because famciclovir was licensed only for the treatment of HZ in this period. This indicated that the coverage rate of patients with HZ in dermatology clinics was 85% in this study. Thus, the catchment area is different from those of other countries in that patients with HZ in Japan primarily visit dermatologists instead of general practitioners. Cases of PHN, dermatologic diseases causing erythema or vesicles resembling HZ, and suspected cases without confirmation with the DFA test were excluded. A history of varicella was confirmed in children and young patients for whom this information was available.
Diagnosis of Herpes Zoster by Polymearse Chain Reaction
The 2630 swab specimens taken from 2438 cases confirmed as HZ and 192 suspected cases were tested for the presence of VZV deoxyribonucleic acid (DNA) from June to September 2013 and February to April 2014. Deoxyribonucleic acid from the samples was isolated by using High Pure Viral Nucleic Acid kit version 18 (Roche Diagnostics GmbH, Mannheim, Germany), and the DNA fragments of VZV, herpes simplex virus (HSV), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were subjected to polymerase chain reaction (PCR) amplification using VZV primers (5’-tccgacatgcagtcaatttcaacgtc-3’and 5’-ggtcgggtagacgctaccactcgttt-3’), HSV primers (5’-agggagtggcgcagctgcttc-3’ and 5’-aagccatacccgcttctacaaggc-3’), and GAPDH primers (5’-tgtgctcccactcctgatttc-3’and 5’-cctagtcccagggctttgatt-3’) [18].
Analysis of Data
The HZ-naive population of age 0–9 years was calculated by subtracting the number of patients with HZ of age 0–9 years. Subsequently, the ratio of the HZ-naive population to the total population at age 0–9 years was used to estimate the HZ-naive population at age 10–19 years. The incidence of HZ in HZ-naive and HZ-experienced populations was deduced from (1) the number of patients with initial and recurrent HZ and (2) the HZ-naive and HZ-experienced populations of each age (Supplementary Table 1). Statistical comparison was performed using IBM SPSS Statistics version 23 (IBM, Armonk, NY).
RESULTS
Diagnosis of Herpes Zoster
A total of 34 877 patients with HZ were diagnosed clinically, and 2398 patients among them were diagnosed by PCR. The correct diagnosis of HZ was confirmed through PCR testing of the swab specimens from patients, from June to September 2013 and from February to April 2014. A total of 2630 swab specimens were taken from 2438 cases with clinical diagnosis of HZ and 192 suspected cases, and the number of VZV-positive samples was 2398 (98.36%) in 2438 GAPDH-positive samples and 2398 (97.32%) in a total of 2464 samples from patients with a clinical diagnosis of HZ (Supplementary Table 2). Suspected patients included those who were being tested to confirm or exclude HZ, and 124 cases among 192 were diagnosed as HZ. The correct clinical diagnosis rate of HZ by the dermatologist was approximately 98% by PCR testing.
Age-Dependent Incidence of the Initial Episode and Recurrence of Herpes Zoster
A total of 34 877 patients with HZ were registered at 43 dermatologic clinics covering 1.12 million persons in Miyazaki Prefecture from June 2009 to November 2015, and the annual mean numbers of cases were 2230 in male and 3135 in female patients. Figure 1 shows the age- and gender-dependent population of Miyazaki Prefecture (Figure 1A), 5365 HZ patients with clinical diagnosis as the mean of 6.5 years survey (Figure 1B), 2398 HZ patients with PCR-diagnosis (Figure 1C), and 1076 recurrent HZ patients (Figure 1D). Age- and gender-dependent distributions of HZ patients with clinical and PCR diagnosis were similar, and this supports the clinical diagnosis of HZ by the dermatologist.
Figure 1.
Population data related to herpes zoster (HZ) in Miyazaki Prefecture. Horizontal and vertical axes indicate the age and number of patients, respectively. (A) The total population of Miyazaki Prefecture was 1.12 million, including 525 317 men and 594 595 women, based on census data from 2009 to 2015. (B) The 34 877 patients with HZ from June 2009 to November 2015. Of the 34 877 patients with clinical diagnosis of HZ, the mean number of patients with HZ per year increased at ≥50 years, with women being more affected than men in that age group. (C) The age- and gender-dependent distribution of 2438 polymerase chain reaction (PCR)-diagnosed patients with HZ. The distribution of patients with PCR-diagnosed HZ was similar to that of patients with clinical diagnosis of HZ shown in B. (D) The age- and gender-dependent distribution of 1076 patients with recurrent HZ among a total of 16 784 patients with HZ. The number of patients with recurrent HZ per year increased with age, and the ratio of female patients with recurrent HZ was much more than that of HZ shown in B. Male and female populations are shown in blue and red columns, respectively.
The incidence of HZ was determined by the population of Miyazaki Prefecture as the denominator. The mean incidence of HZ was 4.79 per 1000 person-years in the total population and was significantly higher in female (5.27) than in male (4.25) patients (P < .001). The age-dependent incidence of HZ in male and female patients increased at the age of 50 years and older. The incidence of HZ in women increased when they were in their 50s and 60s, compared with men whose peak incidence occurred when they were in their 70s, and was 8.44 and 8.89 (mean 8.69) per 1000 person-years in the male and female populations, respectively (Figure 2A). The incidence of recurrent HZ was 0.31 per 1000 person-years, as shown by the dashed lines.
Figure 2.
The age-dependent herpes zoster (HZ) incidences per 1000 person-years and HZ-naive and HZ-experienced population in Miyazaki Prefecture and the population of Miyazaki Prefecture was used as the denominator. (A) The age-dependent incidences per 1000 person-years of the total, male, and female patients are shown by black, blue, and red lines, respectively, and the incidence (per 1000 person-years) of HZ (solid line) and HZ recurrence ([Rec] dashed line) increased at age 50 years and older, with female patients being more affected than male patients in that age group. (B) Herpes zoster-naive and HZ-experienced populations are shown as open and closed columns, respectively. For each age group, the proportion (%) of HZ-naive population is indicated below the age group. The ratio of HZ-experienced persons increased with age, and the HZ-experienced population exceeded one third before the age of 80 years. (C) The age-dependent incidences of initial HZ in the HZ-naive population and recurrent HZ in the HZ-experienced population are shown as solid and dashed lines, respectively. The incidence of recurrent HZ was low but showed a small peak at the age of 60–69 years, with 2.31 per 1000 person-years. The incidence of initial HZ increased with age up to 11.5 per 1000 person-years at the age of 80–89 years.
Of the 16 784 patients observed at the 10 clinics, 1076 (6.41%) experienced a recurrence of HZ, with 49 and 3 patients experiencing 3 and 4 episodes, respectively. Recurrence was noted in 765 (7.79%) of 9821 female patients, which was significantly more frequent than that noted in 311 (4.47%) of 6963 male patients (P < .001).
The number of HZ-experienced persons increased with age in every age group, and one third of the population had experienced HZ before the age of 80 years (Figure 2B). Because the incidence of recurrence is calculated based on the population of Miyazaki Prefecture as the denominator, total 1076 recurrent patients were monitored in 10 of 43 clinics in the survey. The recurrent HZ cases in the prefecture were estimated by multiplying 2.08 (34 877 of 16 784, 43 clinics/10 clinics) to the number of recurrent cases in 10 clinics that participated in the study on HZ recurrence. The comparative age-dependent incidences of initial HZ in an HZ-naive population and recurrent HZ in an HZ-experienced population are shown in Figure 2C. The incidence of initial HZ episodes increased in patients in their 50s and reached the peak at the ages of 80–89 years, with 11.54 per 1000 person-years in contrast to 1.73 in the HZ-experienced population. The mean incidence (5.45 per 1000 person-years) of initial episodes in the HZ-naive population was significantly higher than that (1.73 per 1000 person-years) of recurrences in the HZ-experienced population (P < .001). Alternatively, this indicated the lower risk of HZ in an HZ-experienced population than in an HZ-naive population, especially in patients >50 years old (odds ratio for recurrence, 0.176; 95% confidence interval [CI], .170–.196).
Interval between the Initial and Recurrent Episodes
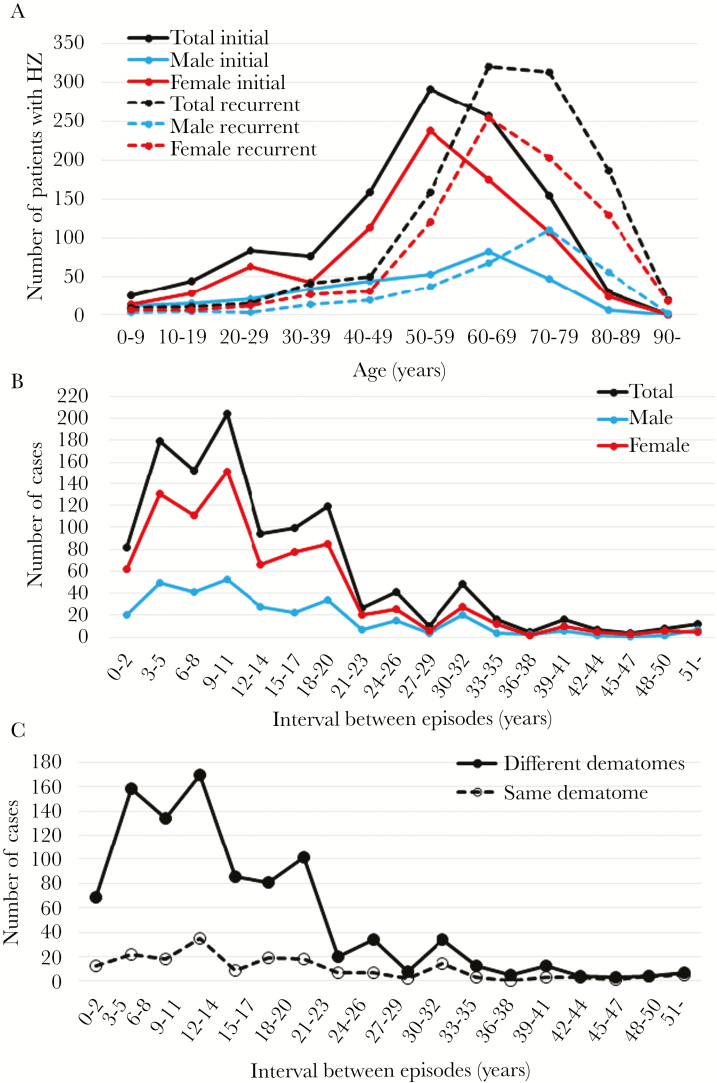
The age of 6 patients with the initial episode was not clear among 1076 patients, and they were excluded from the analysis; episodes before the recurrence in 52 patients with 3 and 4 episodes were included in the initial episodes. Initial and recurrent episodes of HZ occurred in every age group, with the peak age of initial episodes being 50–59 years, and that of recurrent episodes being 60–79 years (Figure 3A). The peak age at which male patients experienced initial and recurrent HZ episodes was 10 years older than that of female patients.
Figure 3.
The ages at the initial and recurrent episodes of herpes zoster (HZ), and the interval between episodes. The figures show the profile of the ages and gender of initial and recurrent HZ and their interval. The difference in the peak age between male and female are noted, and the median and mean intervals were 10 and 14 years, respectively. (A) The ages at the initial (solid line) and recurrent (dashed line) HZ episodes of the total, male, and female patients are expressed as black, blue, and red lines. The episode before the recurrent episode was included as the initial episode in patients with 2 and 3 recurrences. The difference in the peak age between male and female are noted. (B) The cases are shown according to the sex-dependent interval length between the initial (prior) and recurrent episodes in the total, male, and female patients as black, blue, and red lines, respectively. (C) The intervals of the number of patients who experienced recurrences in different dermatomes and in the same dermatome are shown by the black solid line and black dashed line, respectively.
The intervals between the prior and recurrent episodes among 1125 cases ranged from 2 months to 73 years with a mean period of 13.71 ± 10.96 years, peaked at 3–11 years, and then decreased gradually with time (Figure 3B). The profiles of the interval of recurrence showed similar tendencies in male and female patients. The intervals in different dermatomes showed an interval-dependent decrease similar to that of all cases; however, the frequency of recurrence in the same dermatome was not dependent on the interval between episodes (Figure 3C). Episodes within 8 years occurred more frequently in a different dermatome (361 of 942, 38.3%) than in the same dermatome (53 of 183, 29.0%) (P = .016), indicating that recurrence was more likely to occur in a different dermatome than in the same dermatome during that period.
Underlying Diseases and Recurrence
Among 1076 patients with recurrent HZ, 516 patients had no underlying disease, whereas the remainder had 1 or more diseases. The underlying diseases in patients with recurrent HZ were mostly consistent with diseases that are generally observed in the elderly (Table 1). Of the 49 patients with 3 HZ episodes, 8 experienced it twice in the same dermatome and once in a separate dermatome, and 1 experienced it in the same dermatome 3 times. Three patients had 4 episodes without an underlying disease; however, 1 patient had a history of frequent renal stones.
Table 1.
Underlying Diseases in Patients with Recurrent Herpes Zostera
| Underlying Disease | Total Number | Three Episodes |
|---|---|---|
| Hypertension | 242 | 10 |
| Diabetes mellitus | 88 | 2 |
| Hyperlipidemia | 74 | 1 |
| Heart diseases | 53 | |
| Rheumatoid arthritis | 29 | 3 |
| Thyroid diseases | 28 | 2 |
| Stroke | 21 | |
| Asthma | 20 | |
| Prostate hypertrophy | 17 | |
| Pulmonary diseases | 16 | |
| Osteoporosis | 13 | |
| Liver diseases | 12 | 1 |
| Gout | 10 | |
| Gastric ulcer | 8 | |
| Hyperuricemia | 8 | 1 |
| Lymphoma | 7 | 1 |
| Glaucoma | 7 | |
| Prostate cancer | 6 | |
| Atopic dermatitis | 6 | |
| Breast cancer | 5 | 1 |
| Systemic lupus erythematosus | 5 | 1 |
| Reflux esophagitis | 5 | |
| Gastric cancer | 4 | 1 |
| HTLV-1 carrier | 4 | |
| Brain tumor | 4 | |
| Colon cancer | 4 | |
| Allergic rhinitis | 3 | |
| Hemodialysis | 3 | 1 |
| Adult T-cell leukemia | 3 | |
| Depression | 3 | |
| Bladder cancer | 3 | |
| Sarcoidosis | 3 | 1 |
Abbreviations: HTLV, human T-lymphotropic virus.
aThe table shows the underlying diseases observed in 560 of 1076 patients with recurrent herpes zoster. A disease was included if it occurred in 3 or more patients. The underlying diseases observed in 29 of 49 patients with 3 episodes are listed. One of the 3 patients with 4 episodes had experienced urolithiasis, and the others had no underlying diseases.
Distribution of Dermatomes in Recurrence
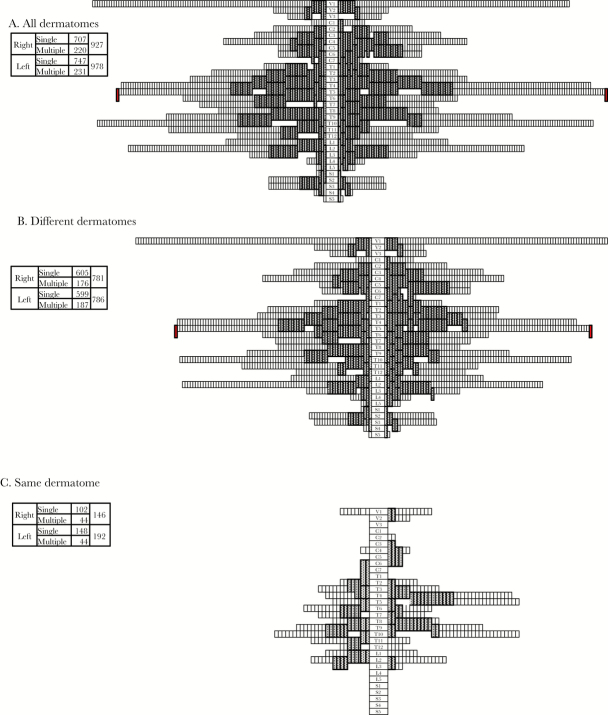
In the 1076 patients, a total of 2207 episodes were noted. The exact dermatome was noted in 1905 episodes. Figure 4A shows the dermatome distribution of the 1905 episodes. The dermatomal distribution ranged from head to foot, and it frequently involved the thoracic nerves and the first branch of the trigeminal nerve. The right and left dermatomes were involved in 927 and 978 cases, respectively. Of the 1905 recurrent episodes, 1454 (76.3%) were noted to involve a single dermatome and 451 (23.7%) involved multiple dermatomes; recurrence in the same dermatome was observed in 16.3% of patients. One otherwise healthy patient experienced bilateral noncontiguous HZ (HZ duplex bilateralis) at Th5 and Th6 at the age of 27 years, as marked in red (Figure 4A), and recurrence at the right Th3 at the age of 74 years. This was the only case with HZ duplex bilateralis among the 1076 patients.
Figure 4.
A total of 1905 cases in 1076 patients with recurrent herpes zoster (HZ) occurred both in different dermatomes and in the same dermatome. There was no difference between left and right in the distribution even between men and women, but the left dermatomes were significantly more affected than right dermatomes in patients with recurrence in the same dermatome. The (1) right and left dermatomes and (2) a single dermatome and multiple dermatomes are summarized in the box. Segmental innervations of dermatomes are indicated as trigeminal (V), cervical (C), thoracic (T), lumbar (L), and sacral (S) nerves, and single and multiple dermatomes are separated by white and dark boxes, respectively. (A) The distribution of a total of 1905 dermatomes is shown, and 1 otherwise healthy patient who experienced HZ duplex bilateralis at Th5 and Th6 at the age of 27 years is indicated in red. The distribution of dermatomes in patients with HZ in different dermatomes (B) and in the same dermatome (C) is shown. Recurrence in the same dermatome showed a significant preference for the left side in the comparison of dermatomal distribution in patients with recurrence (odds ratio, 1.31; 95% confidence interval, 1.03–1.66; P = .027, by the χ2 test).
The right (781) and left (786) dermatomes were similarly affected in patients who experienced recurrence in different dermatomes (Figure 4B), whereas left (192) dermatomes were more affected than right (146) dermatomes in patients with recurrence in the same dermatome (Figure 4C), indicating a significant preference for the left dermatomes in those with recurrence in the same dermatome (odds ratio, 1.31; 95% CI, 1.03–1.66; P = .027, by the χ2 test). The distribution did not differ in the initial and recurrent episodes or according to sex (data not shown).
DISCUSSION
Herpes zoster was carefully diagnosed by dermatology specialists in Miyazaki Prefecture. The correct diagnosis of HZ can be difficult, as shown by the HZ prevention study of Oxman et al [3], in which 152 of the suspected 1308 cases (11.6%) were not based on PCR assay results. Among 230 patients diagnosed as having HZ by family practitioners in the United Kingdom, 204 were confirmed with DFA and/or PCR, and 26 (17%) were incorrectly diagnosed as HZ [19]. The most reliable incidences of HZ with laboratory confirmation of even mild skin manifestations are 10.79 and 11.50 in patients in their 60s and 70s, respectively, per 1000 person-years in an HZ prevention study [3], and 10.8 and 9.4 in those in their 60s and 70s and older, respectively, per 1000 person-years in an HZ subunit vaccine study [4]. The HZ incidence in our study was similar to that in the other studies considering the coverage of 85% of HZ patients.
The extent to which HZ patients in Miyazaki Prefecture visit a dermatologist was important in this epidemiological study. The coverage of patients with HZ was 85% in the present surveillance system in Miyazaki Prefecture [1]. Use of the DFA test for confirmation of HZ was covered by health insurance, and the utilization of DFA test for patients with suspected HZ may differ among dermatologists. The information collected on the surveillance of patients with HZ included age and sex; for recurrent HZ, the age and sex, dermatome of the initial and recurrent episodes, and underlying diseases of patients were collected. Herpes zoster in patients with serious underlying diseases is treated in subspecialty clinics and in the dermatology departments of the same flagship hospitals, and thus patients with HZ were covered in this surveillance. Diagnosis of HZ not confirmed with PCR and medication information in treatment of underlying diseases was lacking in this surveillance. Despite the limitations, the correct diagnosis rate by using PCR was approximately 98%, and this is the largest study thus far to elucidate the epidemiology and recurrence of HZ.
Relationship of recurrent HZ with underlying diseases was examined, because the incidence of HZ is increased in patients [20] with malignancy, immunological diseases, human immunodeficiency virus (HIV) infection, diabetes mellitus [21], and treatment with tumor necrosis factor-α inhibitors [22, 23]. Our population included patients with some of these high-risk diseases. Twenty-eight patients had thyroid diseases, which may be unexpected among underlying diseases; however, information on the prevalence of thyroid diseases is not available in Miyazaki Prefecture. Therefore, it is difficult to evaluate the relationship between recurrence and thyroid diseases or other diseases. Twenty-four persons with HIV infection were registered in Miyazaki Prefecture from 2009 to 2015 [24], and thus HIV infection may not have affected the incidence of HZ in this analysis.
We examined whether recurrence was recrudescence in the same dermatome or 2 single onset cases occurred within 1 year or due to underlying diseases. Recurrence within 1 year was observed in 9 patients, including 3 patients with rheumatoid arthritis, diabetes mellitus, or atopic dermatitis, and 6 otherwise healthy persons. One had a recurrence in the same dermatome, and the other 8 had a recurrence in different dermatomes. This indicated that the recurrence of HZ was not a recrudescence of the same sensory ganglia but was independent of the initial episode. Recurrence within 8 years in the same dermatome was less frequent than in a different dermatome. Pathological changes in sensory ganglia after HZ are characterized by fibrosis and loss of ganglion cells, axons, and myelin [25] and might contribute to the decrease in the incidence of HZ in the same dermatome.
When comparing with the previous reports on the distribution of the dermatome in 803 cases, the dermatomes of the trigeminal nerve and thoracic nerves were frequently involved as reported previously [6, 13, 25, 26]. A single dermatome was involved in 75% of 803 episodes including recurrent episodes in this study. Involvement of a single dermatome was reported for 66% of 2063 patients with HZ [26] and 49% of 208 patients with PHN [27]. The preference for the left side (124 in 208) [27] was observed similarly in recurrences in the same dermatome. The reason for the left-side prevalence is not clear; however, similar observations on left-side asymmetry have been reported in muscle involvements such as poliomyelitis of the upper limb [28], diaphragm paralysis [29], and neonatal hip instability [30].
CONCLUSIONS
The epidemiological study of HZ in Miyazaki Prefecture collected the largest number of cases in a general population including the epidemiology of HZ recurrence that has been characterized to date. The recurrence of HZ was approximately 6% in the general population, and the incidence of HZ was 1.69 and 5.80 per 1000 person-years in HZ-experienced and HZ-naive populations, respectively. Thus, this is the first study to elucidate the incidence and clinical features of recurrent HZ in the largest epidemiological study worldwide.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank Drs. Richard Whitley, Mark Prichard, and Nathan Price for critically reading the manuscript and for their helpful scientific advice. We also thank Katherine Ono for editing the manuscript.
Financial support. This work was conducted at the university’s expense without other financial support.
Potential conflicts of interest. K. S. reports receiving research grants, honoraria, and consultancy fees from Toyama Chemical Co. Ltd, Maruho Co. Ltd, Japan Blood Products Organization, Yakult Honsha Co. Ltd, Original Image Co., OTA Inc., and Alfresa Co.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
The 43 members of the Miyazaki Dermatologist Society in Miyazaki Prefecture participated in this study.
From 10 dermatology clinics that participated in monitoring the recurrence of herpes zoster (HZ): N. Kawana, MD (Nobeoka City); K. Horinouchi, MD (Hyuga City); H. Narita, MD (Miyazaki City); T. Tazaki, MD (Miyazaki City); Y. Aoki, MD (Miyazaki City); A. Tajiri, MD (Miyazaki City); K. Sakai, MD (Miyakonojo City); S. Narahara, MD (Miyakonojo City); T. Kitamura, MD (Miyakonojo City); N. Toyama, MD (Nichinan City).
From the other 33 dermatology clinics and departments of dermatology in general hospitals: M. Kohashi, MD (Kunitomi Town, Higashi-Morokata County); Y. Kuwahara, MD (Kobayashi City); N. Takasaki, MD (Kobayashi City); Y. Ishi, MD (Miyakonojo City); I. Anekawa, MD (Miyakonojo City). Miyakonojo Medical Center Hospital: F. Nakayama, MD (Miyakonojo City); N. Teruya, MD (Nichinan City); K. Era, MD (Nichinan City); K. Okamura, MD (Nobeoka City); H. Nagamine, MD (Hyuga City). Chiyada Hospital: Y. Oda, MD (Hyuga City); Y. Kurogi, MD (Takanabe Town, Koyu County); K. Ohtuka, MD (Saito City); M. Yoshiyama, MD (Saito City); S. Nakano, MD (Miyazaki City); T. Nishida, MD (Miyazaki City); M. Hiejima, MD (Miyazaki City); K. Nakayama, MD (Miyazaki City); H. Hatisuka, MD (Miyazaki City); M. Kurokawa, MD (Miyazaki City); R. Kaneda, MD (Miyazaki City); H. Kikuchi, MD (Miyazaki City); K. Higashi, MD (Miyazaki City); S. Tada, MD (Miyazaki City); E. Muroi, MD (Miyazaki City). Nanbu Hospital: S. Tateyama, MD (Miyazaki City). Dermatology Departments—University of Miyazaki Hospital: M. Setoyama, MD, M. Amono, MD (Miyazaki City). Miyazaki Prefectural Miyazaki Hospital: H. Koketsu, MD, N. Chosa, MD, K. Mochida, MD, T. Inoue, MD (Miyazaki City). Koga General Hospital: S. Tumori, MD (Miyazaki City). Miyazaki Prefectural Nobeoka Hospital: F. Nakayama, MD, Y. Ishii, MD (Nobeoka City). Kyoritu Hospital: E. Sakaguchi, MD (Nobeoka City). Miyazaki Prefectural Nichinan Hospital: A. Kashima, MD, H. Koketsu, MD (Nichinan City). Aisenkai Nichinan Hospital: K. Yamaguchi, MD (Nichinan City).
References
- 1. Toyama N, Shiraki K. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48 388 herpes zoster cases in Miyazaki prefecture. J Med Virol 2009; 81:2053–8. [DOI] [PubMed] [Google Scholar]
- 2. Hama Y, Shiraki K, Yoshida Y, et al. Antibody to varicella-zoster virus immediate-early protein 62 augments allodynia in zoster via brain-derived neurotrophic factor. J Virol 2010; 84:1616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 4. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 5. Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc 2011; 86:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 1965; 58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 7. Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med 1995; 155:1605–9. [PubMed] [Google Scholar]
- 8. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis 2007; 44:S1–26. [DOI] [PubMed] [Google Scholar]
- 9. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burgoon CF, Jr, Burgoon JS, Baldridge GD. The natural history of herpes zoster. J Am Med Assoc 1957; 164:265–9. [DOI] [PubMed] [Google Scholar]
- 11. Seiler HE. A study of herpes zoster particularly in its relationship to chickenpox. J Hyg 1949; 47:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Head H, Campbell AW, Kennedy PG. The pathology of herpes zoster and its bearing on sensory localisation. Rev Med Virol 1997; 7:131–43. [DOI] [PubMed] [Google Scholar]
- 13. Molin L. Aspects of the natural history of herpes zoster. A follow-up investigation of outpatient material. Acta Derm Venereol 1969; 49:569–83. [PubMed] [Google Scholar]
- 14. Ragozzino MW, Melton LJ, 3rd, Kurland LT, et al. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982; 61:310–6. [DOI] [PubMed] [Google Scholar]
- 15. Tseng HF, Chi M, Smith N, et al. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis 2012; 206:190–6. [DOI] [PubMed] [Google Scholar]
- 16. Adams RD. Dermatology in General Medicine: Textbook and Atlas. 3rd ed (Thomas B. Fitzpatrick AZE Klaus Wolff Irwin M. Freedberg K. Frank Austen, ed). New York, New York: McGraw-Hill, 1987. [Google Scholar]
- 17. Miyazaki-Prefecture. Population and Number of Households in Miyazaki Prefecture Available at: http://www.pref.miyazaki.lg.jp/contents/org/honbu/toukei/jinko-setai/kako2.html Accessed 8 March 2016.
- 18. Tanaka T, Kogawa K, Sasa H, et al. Rapid and simultaneous detection of 6 types of human herpes virus (herpes simplex virus, varicella-zoster virus, Epstein-Barr virus, cytomegalovirus, human herpes virus 6A/B, and human herpes virus 7) by multiplex PCR assay. Biomed Res 2009; 30:279–85. [DOI] [PubMed] [Google Scholar]
- 19. Scott FT, Leedham-Green ME, Barrett-Muir WY, et al. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virol 2003; 70:S24–30. [DOI] [PubMed] [Google Scholar]
- 20. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30. [PubMed] [Google Scholar]
- 21. Heymann AD, Chodick G, Karpati T, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection 2008; 36:226–30. [DOI] [PubMed] [Google Scholar]
- 22. Strangfeld A, Listing J, Herzer P, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA 2009; 301:737–44. [DOI] [PubMed] [Google Scholar]
- 23. Winthrop KL, Baddley JW, Chen L, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA 2013; 309:887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ministry of Health LaWASC. Annual AIDS Incidence Report. Available at: http://api-net.jfap.or.jp/status/2010/10nenpo/nenpo_menu.htm Accessed 10 February 2016. [Google Scholar]
- 25. Watson CP, Deck JH, Morshead C, et al. Post-herpetic neuralgia: further post-mortem studies of cases with and without pain. Pain 1991; 44:105–17. [DOI] [PubMed] [Google Scholar]
- 26. Meister W, Neiss A, Gross G, et al. Demography, symptomatology, and course of disease in ambulatory zoster patients. A physician-based survey in Germany. Intervirology 1998; 41:272–7. [DOI] [PubMed] [Google Scholar]
- 27. Watson CP, Evans RJ, Watt VR, Birkett N. Post-herpetic neuralgia: 208 cases. Pain 1988; 35:289–97. [DOI] [PubMed] [Google Scholar]
- 28. Kumar K, Kapahtia NK. The pattern of muscle involvement in poliomyelitis of the upper limb. Int Orthop 1986; 10:11–5. [DOI] [PubMed] [Google Scholar]
- 29. Elefteriades J, Singh M, Tang P, et al. Unilateral diaphragm paralysis: etiology, impact, and natural history. J Cardiovasc Surg (Torino) 2008; 49:289–95. [PubMed] [Google Scholar]
- 30. Andersson JE. Neonatal hip instability: results and experiences from ten years of screening with the anterior-dynamic ultrasound method. Acta Paediatr 2002; 91:926–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.