Abstract
DNA damage and other forms of replication stress can cause replication forks to stall. Replication stress response proteins stabilize and resolve stalled forks by mechanisms that include fork remodeling to facilitate repair or bypass of damaged templates. Several enzymes including SMARCAL1, HLTF, and ZRANB3 catalyze these reactions. SMARCAL1 and HLTF utilize structurally distinct accessory domains attached to an ATPase motor domain to facilitate DNA binding and catalysis of fork remodeling reactions. Here we describe a substrate recognition domain within ZRANB3 that is needed for it to recognize forked DNA structures, hydrolyze ATP, catalyze fork remodeling, and act as a structure-specific endonuclease. Thus, substrate recognition domains are a common feature of fork remodeling, SNF2-family, DNA-dependent ATPases, and our study provides further mechanistic understanding of how these enzymes maintain genome integrity during DNA replication.
Keywords: DNA, DNA-binding protein, DNA damage response, DNA repair, DNA replication, endonuclease, protein domain
Introduction
Genomic replication is a highly challenging task. The DNA replication machinery must precisely duplicate billions of base pairs while tolerating a multitude of obstacles including damaged DNA, collisions with transcriptional machineries, unusual DNA structures, and other difficult to replicate sequences (1). Many of these obstacles stall replication forks and activate replication stress responses that stabilize and restart persistently stalled forks. These mechanisms include fork remodeling to regress replication forks into a chicken foot DNA structure (2, 3). Fork regression may facilitate DNA repair or template switching to bypass the obstruction (3).
Several members of the SNF2 family of DNA-dependent ATPases including SMARCAL1 (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A-like protein 1),3 HLTF (helicase-like transcription factor) and ZRANB3 (zinc finger Ran-binding domain containing 3) are replication stress response proteins that catalyze fork remodeling including fork regression (4–6). The replication stress response is essential to complete replication accurately. Therefore, defects in this response cause human disease (1). For example, bi-allelic loss of function mutations in SMARCAL1 cause Schimke-Immunoosseous Dysplasia (SIOD) (7). SIOD is a developmental disorder characterized by growth defects, immune-deficiency, and renal failure. Recent studies also suggest that SIOD may be a cancer predisposition syndrome (8–10). HLTF is silenced in colorectal cancer and ZRANB3 is mutated in endometrial cancers suggesting that both may be tumor suppressors (11, 12).
SMARCAL1 localizes to stalled replication forks through an interaction with RPA (13–16). The RPA interaction also regulates SMARCAL1 enzymatic activity to ensure that it regresses only stalled forks (17, 18). Although HLTF is present at replication forks, it is unclear if it is recruited through a protein-protein interaction or simply by its structure-specific DNA binding activity (19). ZRANB3 is recruited by binding to poly-ubiquitinated PCNA (5).
The enzymatic activities of SMARCAL1 and HLTF are dependent on a SNF2 ATPase motor domain and a substrate recognition domain (SRD) that is thought to mediate binding to specific structures at stalled replication forks. The SRD of SMARCAL1 is its HARP2 domain, which is required for SMARCAL1 binding to branched DNA structures as well as DNA-dependent ATPase and fork regression activities (4, 20). The HARP domain is structurally related to the damage recognition domain of the XPB helicase and the mismatch recognition domain of MutS (20). The SRD in HLTF is its HIRAN domain, which is unrelated in sequence and structure to the HARP domain and interacts with the exposed 3′ end of small DNA flaps (19–21). The HIRAN domain is also important for HLTF mediated fork regression activity (19, 22). In both SMARCAL1 and HLTF, mutations in the HARP or HIRAN domains interfere with their ability to bind DNA (4, 19–21).
Yuan et al. reported that ZRANB3 contains a domain similar in sequence to the HARP domains of SMARCAL1 (23). However, they reported that deletion of this putative SRD domain inactivates its strand annealing activity without interfering with DNA binding or DNA-dependent ATPase activity (23). Given the apparent differences in the reported activities of the SMARCAL1 HARP and ZRANB3 HARP-like domains, we revisited the requirements for ZRANB3 to bind DNA, hydrolyze ATP and catalyze fork remodeling. We define a ZRANB3 SRD that is essential for all three functions and define the ZRANB3 minimal enzymatic unit for fork remodeling as containing only the SNF2 ATPase domain and its SRD.
Experimental Procedures
Recombinant DNA Cloning
All ZRANB3 expression vectors and amino acid numberings are based on ZRANB3 isoform 2. The 1–501∼720–869 minimal enzymatic unit contains a (GGGGS)3 linker (24). All vectors and oligonucleotide sequences used for PCR and mutagenesis are available upon request.
Recombinant Protein Expression and Purification
FLAG-ZRANB3 proteins (WT (wild type), Δ712–818, K163D (mutation in walker A motif in ATPase domain that impairs ATP hydrolysis), 1–501, 1–650, 1–869, ΔNZF, ΔHNH, ΔAPIM, Δ651–720, Δ712–794, Δ795–859, L760A/D761A/I762A (MT1), W790A/S791A/S792A (MT2)) were purified from HEK293T cells as previously described (4) with the following modifications: cells were lysed for 40 min on ice in lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm DTT, 0.2 mm PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 0.1% Triton X-100, 0.1 mm EDTA). Cleared cell lysate was incubated with FLAG-M2 beads (Sigma F2426, EZ View Red Anti-FLAG M2 Affinity gel) for 4 h at 4 °C. Beads were washed twice with lysis buffer, once with LiCl buffer (10 mm HEPES, pH 7.9, 0.3 m LiCl, 20% glycerol, 0.2 mm EDTA, 0.1% Triton X-100, 1 mm DTT, 0.2 mm PMSF, 1.5 mm MgCl2, 5 μg/ml aprotinin, 5 μg/ml leupeptin), and twice with the elution buffer (20 mm HEPES, pH 7.9, 0.1 m KCl, 1.5 mm MgCl2, 0.2 mm EDTA, 20% glycerol, 0.01% Nonidet P-40, 1 mm DTT, 0.2 mm PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin). Proteins were eluted using 300 μg/ml FLAG peptide (F3290 Sigma). FLAG-ZRANB3 protein from insect cells was purified using the same procedure as FLAG-SMARCAL1 (25).
GST-720–869 and GST-720–869 L760A/D761A/I762A (MT1) were purified from ArcticExpress Escherichia coli (Agilent Technologies). Cells were grown at 37 °C and upon reaching an OD600, protein expression was induced with 1 mm IPTG and grown at 16 °C overnight. The cell pellet was solubilized in lysis buffer (25 mm Tris, pH 8.0, 50 mm NaCl, 0.1 mm EDTA, 5% glycerol, 1 mm DTT, 0.1 mm PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin) and lysed by sonication. Triton X-100 was added to reach a final concentration of 1% and the lysate was incubated on ice for 30 min. Following high-speed centrifugation, the lysate was incubated with GST beads for 4 h at 4 °C. Afterward, the beads were washed three times with lysis buffer containing 1% Triton X-100. Protein was eluted with elution buffer (75 mm Tris, pH 8, 15 mm glutathione, 0.1 mg/ml leupeptin), and dialyzed overnight at 4 °C (dialysis buffer: 20 mm Tris, pH 8.0, 1 mm DTT, 0.1 mm EDTA, 20% glycerol, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 0.2 mm PMSF). Dialyzed samples were applied to a heparin column and eluted with increasing concentration of KCl (50 mm, 75 mm, 150 mm, 300 mm). Fractions containing the desired protein were combined and concentrated using a Millipore 10,000 MWCO protein concentration filter.
Alignment
ZRANB3 protein sequence alignments were performed using Clustal Omega. The HARP1 and HARP2 sequences were obtained from the boundaries identified previously (4, 20). Secondary structure prediction was performed using POLYVIEW, PSIPRED, and JNETPRED prediction software (26–28).
DNA Substrate Assembly and Purification
The single-stranded (30nt), double-stranded (30nt), splayed arm, replication fork, fork regression, and fork restoration DNA substrates used for ATPase, DNA binding, and fork remodeling reactions were assembled and purified as described previously (4, 17, 18).
ATPase Assay
ATPase assays were conducted as previously described (25).
DNA Binding Assay
DNA binding was conducted largely as previously described (25). Briefly, protein was incubated with 10 nm of 32P-labeled DNA substrate at 30 °C for 30 min. Following incubation, 15% Ficol was added to a final concentration of 2.5%. Samples were resolved on a 5% or 8% polyacrylamide gel in 1×TBE (100 mm Tris, 90 mm boric acid, 2 mm EDTA) at 40 V for 180 min at 4 °C.
Nuclease Assay
Nuclease assays were conducted as previously described (29). Briefly, protein was incubated with 10 nm of 32P-labeled splayed arm substrate for 30 min at 30 °C in nuclease buffer (50 mm Tris, pH 7.5, 50 mm NaCl, 5 mm MgCl2, 2 mm ATP, 0.1 mg/ml BSA). The products were resolved on a 10% polyacrylamide gel in 1×TBE at 80 V for 80 min at room temperature.
Fork Remodeling Assay
Fork regression and fork restoration reactions were conducted as previously described (17, 18). For the fork regression reaction, 3 nm of protein was incubated with 3 nm of labeled leading strand gap regression substrate for increasing amount of time at 37 °C. For the fork restoration reaction, increasing amount of protein was incubated with 3 nm of the lagging strand gap restoration substrate for 30 min at room temperature. The products were resolved on an 8% polyacrylamide gel in 1×TBE at 80 V for 80 min at room temperature.
Results
Region 720–869 Is Highly Conserved and Necessary for ZRANB3 ATPase Activity
ZRANB3 is a DNA-dependent ATPase in the same SNF2 family as SMARCAL1 and HLTF (30). SMARCAL1 and ZRANB3 are both annealing helicases that re-anneal complementary DNA strands (25, 31) and catalyze replication fork remodeling reactions (4, 5). The SMARCAL1 HARP2 domain is required for SMARCAL1 to bind DNA, hydrolyze ATP, anneal DNA, and remodel replication forks (4, 20).
A previous study concluded that a region encompassing amino acids 712–818 in ZRANB3 contains a HARP-like domain that is required for ZRANB3 annealing helicase activity (23). However, unlike the SMARCAL1 HARP domain, the HARP-like domain was reported to be dispensable for DNA binding and ATPase activity. Fork remodeling was not tested. Due to the striking functional differences between the HARP and HARP-like domains, we revisited whether the HARP-like domain of ZRANB3 really shares similar functional properties to the SMARCAL1 HARP domains. We purified wild type (WT) and Δ712–818 ZRANB3 (Fig. 1A) and tested their ability to bind a splayed arm DNA substrate and hydrolyze ATP. In contrast to the previously published findings, purified Δ712–818 ZRANB3, which lacks the HARP-like domain, failed to bind a splayed arm DNA substrate (Fig. 1B). It also lacked DNA-stimulated ATPase activity (Fig. 1C). In contrast, WT ZRANB3 displayed both DNA-binding and DNA-dependent ATPase activity.
FIGURE 1.
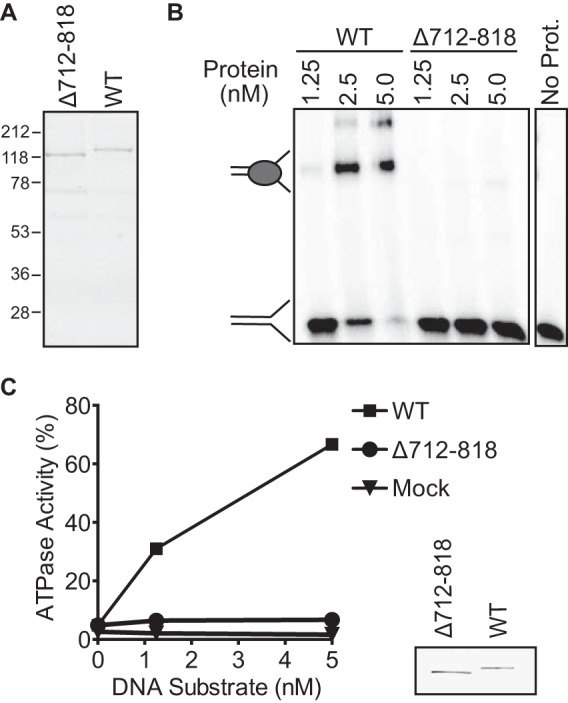
Δ712–818 ZRANB3 does not bind DNA and cannot hydrolyze ATP. A, Coomassie-stained SDS-PAGE gel of purified wild-type (WT) and Δ712–818 ZRANB3. B, Δ712–818 ZRANB3 and WT ZRANB3 were incubated with a splayed arm DNA substrate. To assess DNA binding, samples were resolved on a polyacrylamide gel and visualized by autoradiography. A representative experiment is shown. C, Δ712–818 ZRANB3 and WT ZRANB3 were incubated with a splayed arm substrate, and ATPase activity was measured. The mean and standard deviation from three experiments are shown. In most cases the standard deviation is smaller than the symbol size. The inset is an anti-FLAG immunoblot of the purified proteins.
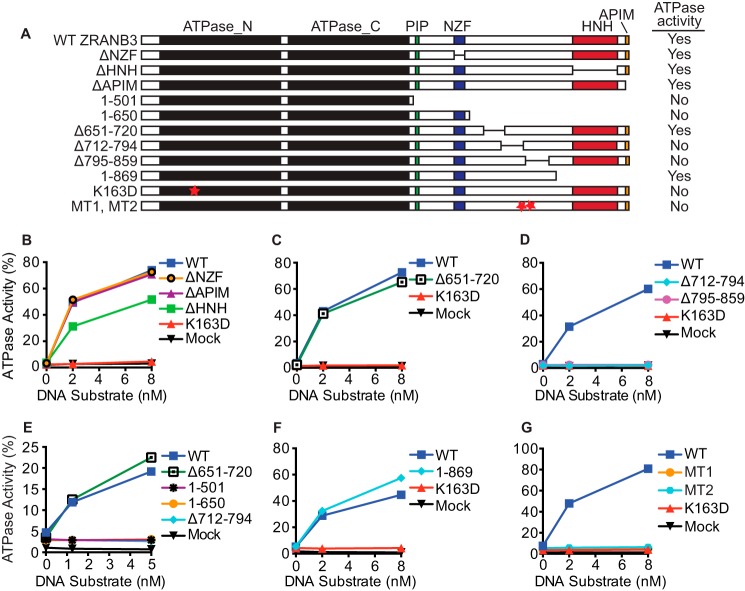
It is unclear whether deleting amino acids 712–818 generates a protein that is properly folded. Since important amino acids in the SMARCAL1 HARP domain have already been identified (4, 20), we attempted to generate a sequence alignment between the SMARCAL1 HARP domains and the ZRANB3 HARP-like domain to identify critical amino acids for mutagenesis. However, we were unable to find sufficient sequence similarity to generate a useful alignment. Therefore, using evolutionarily conserved regions of ZRANB3 as a guide, we designed and tested various deletion, truncation, and point mutants to determine regions within the protein that are necessary for DNA-dependent ATPase activity (Fig. 2A). Deletion of the NZF and APIM motifs, which bind polyubiquitinated PCNA (5), did not impair ATPase activity (Fig. 2B). Deletion of the HNH nuclease domain caused a modest but reproducible decrease in activity (Fig. 2B). Deletion of amino acids 651–720 also yielded an active enzyme (Fig. 2C). In contrast, ZRANB3 Δ712–794 and ZRANB3 Δ795–859 were both inactive (Fig. 2D).
FIGURE 2.
Amino acids 721–869 are necessary for ZRANB3 DNA-dependent ATPase activity. A, schematic and summary of results for the various deletion, truncation, and point mutants. Purified (B) ΔNZF, ΔHNH, ΔAPIM motif deletion mutants; C, Δ651–720 deletion mutant; D, Δ712–794 and Δ795–859 deletion mutants; E, 1–501 and 1–650 truncation mutants; F, 1–869 truncation mutant; and G, triple mutants L760A/D761A/I762A (MT1) and W790A/S791A/S792A (MT2) were incubated with a splayed arm DNA substrate and ATPase activity was measured. A representative experiment (of at least two replicates) is shown for each mutant.
Likewise, C-terminal deletion constructs containing only the ATPase domain (ZRANB3 1–501) or the ATPase domain, PIP and NZF motifs (ZRANB3 1–650) were also inactive (Fig. 2E). However, a protein consisting of amino acids 1–869 was as active as the wild type protein (Fig. 2F). In all cases, the active proteins required DNA for ATP hydrolysis. Thus, the ZRANB3 ATPase domain requires an accessory domain that likely includes amino acids 721–869 for activity.
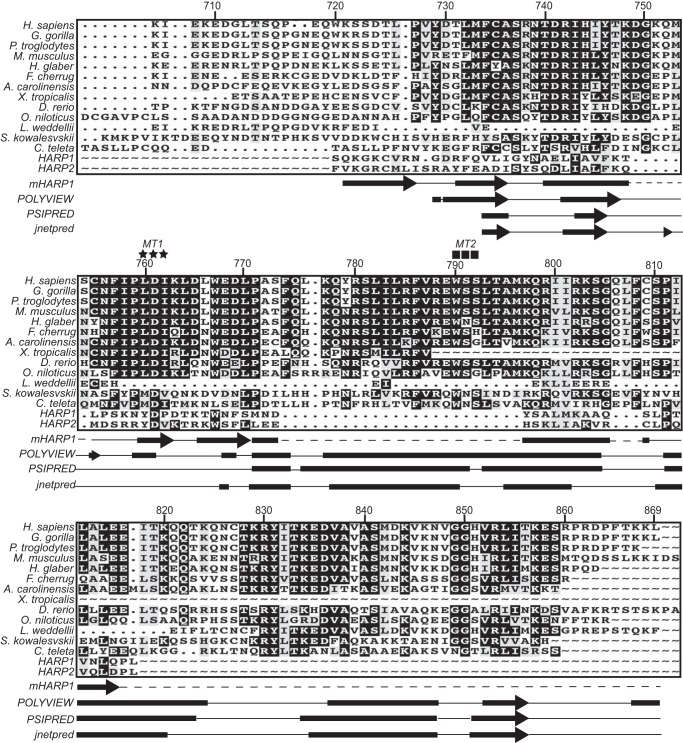
Amino acids 720–869 contain most, but not all, of the residues previously described to make-up the HARP-like domain. It is relatively highly evolutionarily conserved compared with flanking regions of ZRANB3 (Fig. 3). However, our sequence alignment failed to detect significant similarity with the HARP domains of SMARCAL1. We also compared the known secondary structure of the HARP domain to the predicted secondary structure of this ZRANB3 region. While there is some similarity, the ZRANB3 domain contains a large insertion that is predicted to be α-helical. Mutations in highly conserved amino acids within this helix and in other highly conserved amino acids in this region (MT1: L760A/D761A/I762A and MT2: W790A/S791A/S792A) inactivate the protein (Fig. 2G). These data confirm that this region is necessary for DNA-dependent ATPase activity. Based on this data as well as additional information (see below) we designate amino acids 720–869 of ZRANB3 as a substrate recognition domain (SRD).
FIGURE 3.
ZRANB3 amino acids 720–869 are highly conserved but have minimal similarity to the HARP domains of SMARCAL1. Amino acid sequence numbering corresponds to human ZRANB3 isoform 2. Secondary structure of mouse HARP1 (based on PDB ID 4O66), and predicted secondary structure of ZRANB3 generated from POLYVIEW, PSIPRED, and JNETPRED are depicted. MTI (L760A/D761A/I762A) residues are indicated as stars and MT2 (W790A/S791A/S792A) residues are indicated as squares.
The ZRANB3 SRD Is Sufficient to Impart DNA Binding, ATPase, and Fork Remodeling Activities to the ATPase Domain
Since the ZRANB3 ATPase domain by itself is not active, we tested whether addition of the SRD via a flexible linker (Fig. 4A) is sufficient to impart DNA-dependent activity. Indeed, ZRANB3 1–501∼720–869 is active in the presence of DNA although its activity is modestly decreased compared with wild-type ZRANB3 (Fig. 4, B and C). Consistent with its DNA-dependent ATPase activity, ZRANB3 1–501∼720–869 is capable of binding complex DNA substrates that mimic a replication fork (Fig. 4D). Similar to WT ZRANB3, ZRANB3 1–501∼720–869 also catalyzes fork regression and fork restoration reactions, whereas an ATPase-deficient mutant (K163D) is inactive in these assays (Fig. 4, E and F).
FIGURE 4.
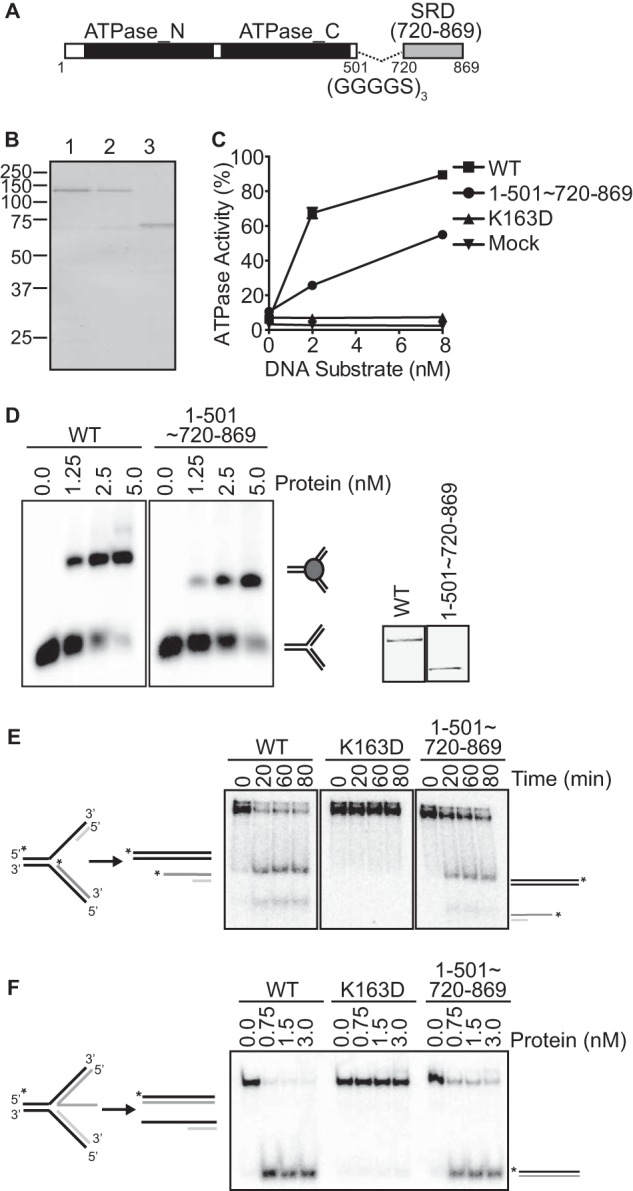
ZRANB3 1–501∼720–869 represents a minimal enzymatic unit that retains DNA binding, ATPase, and fork remodeling activities. A, schematic of ZRANB3 1–501∼720–869. B, Coomassie-stained SDS-PAGE gel of WT (lane 1), K163D (lane 2), and 1–501∼720–869 (lane 3) ZRANB3. C, ATPase activity of ZRANB3 1–501∼720–869 to a splayed arm DNA substrate. The mean and standard deviation for three experiments are shown. In most cases the standard deviation is smaller than the symbol size. D, DNA binding activity of ZRANB3 1–501∼720–869 to a replication fork mimicking DNA substrate. Samples were resolved on a polyacrylamide gel and visualized by autoradiography. The inset is an anti-FLAG immunoblot of the purified proteins. E, purified WT ZRANB3, K163D and ZRANB3 1–501∼720–869 were incubated with a model stalled fork DNA substrate for increasing times. Reaction products were separated by gel electrophoresis and visualized by autoradiography. F, increasing amounts of purified WT ZRANB3, K163D and ZRANB3 1–501∼720–869 were incubated with a model fork restoration substrate for 30 min at room temperature. Reaction products were separated by gel electrophoresis and visualized by autoradiography.
Incorporation of the MT1 mutations into this minimal enzymatic unit (1–501∼720–869-MT1) inactivates its ATPase, DNA binding, and fork remodeling activities (Fig. 5). Thus, we conclude that amino acids 720–869 of ZRANB3 encode a SRD that is necessary and sufficient to impart DNA binding, ATPase and in vitro fork remodeling activities onto the ZRANB3 motor domain. Furthermore, this analysis defines the minimal enzymatic unit of ZRANB3 capable of catalyzing fork remodeling as containing amino acids 1–501 and 720–869.
FIGURE 5.
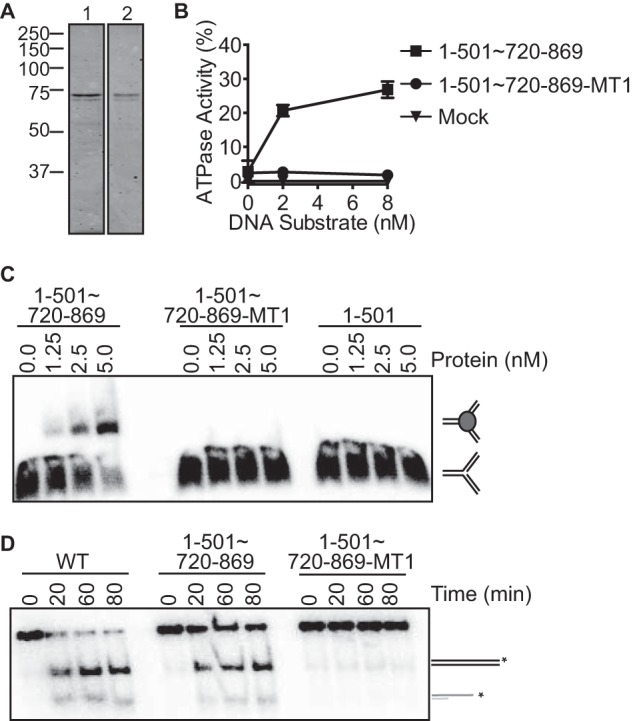
The substrate recognition domain is necessary and sufficient to impart DNA binding, ATPase, and fork remodeling activities to the ZRANB3 ATPase domain. A, Coomassie-stained SDS-PAGE gel of purified 1–501∼720–869 (lane 1) and 1–501∼720–869 containing MT1 mutations (lane 2). B, ATPase activity of ZRANB3 1–501∼720–869 and 1–501∼720–869-MT1. The mean and standard deviation for three experiments are shown. C, DNA binding of 1–501∼720–869, 1–501∼720–869-MT1, and 1–501 to a replication fork mimicking DNA substrate. Products were separated by gel electrophoresis and visualized by autoradiography. D, fork regression activity of 1–501∼720–869 and 1–501∼720–869-MT1 to a model stalled fork DNA substrate for increasing times. Reaction products were separated by gel electrophoresis and visualized by autoradiography.
The ZRANB3 SRD Is Required for Structure-specific Endonuclease Activity
In addition to catalyzing fork remodeling reactions, ZRANB3 was reported to act as an ATP-dependent, structure-specific endonuclease that nicks the duplex DNA of a splayed arm substrate (29). Endonuclease activity required both the HNH and ATPase domains (29). Thus, we hypothesized that the SRD domain of ZRANB3 may also be required for its nuclease activity. Indeed, mutations in the SRD inactivate nuclease activity (Fig. 6).
FIGURE 6.
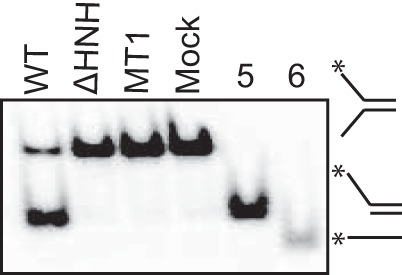
The substrate recognition domain is necessary for ZRANB3 nuclease activity. Nuclease assay of WT, MT1, and ΔHNH ZRANB3 proteins to a splayed arm substrate. Overhang DNA (lane 5) and ssDNA (lane 6) were used as size markers. Products were separated by gel electrophoresis and visualized by autoradiography.
The ZRANB3 SRD Binds DNA
To test if amino acids 720–869 in ZRANB3 actually contain a DNA binding domain, we purified recombinant GST-720–869 from E. coli (Fig. 7A). Like full-length ZRANB3, GST-720–869 is not capable of binding either single-stranded or double-stranded DNA (Fig. 7B). However it can bind a splayed arm substrate, albeit with reduced affinity compared with full-length ZRANB3 (Fig. 7, C and D). Incorporating the MT1 mutations into either GST-720–869 or full-length ZRANB3 greatly reduced their ability to bind the splayed arm DNA substrate (Fig. 7, C and D).
FIGURE 7.
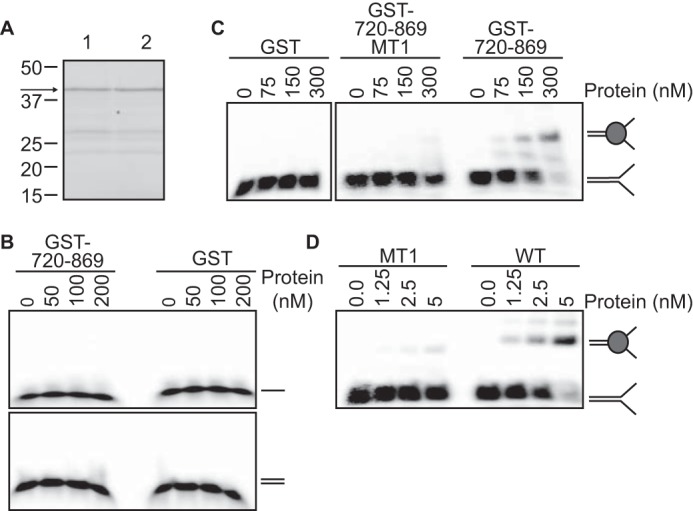
ZRANB3 amino acids 720–869 encode a structure-specific DNA binding domain. A, Coomassie-stained SDS-PAGE gel of GST-720–869-MT1 (lane 1) and GST-720–869 (lane 2). B and C, DNA binding of GST-720–869, GST-720–869-MT1, and GST proteins to (B) single or double-stranded DNA or a (C) splayed arm DNA substrate was assessed by a electrophoretic mobility shift assay. D, DNA binding assay of full-length WT and MT1 proteins to a splayed arm DNA substrate. Representative experiments are shown.
Overall these results indicate that ZRANB3 amino acids 720–869 contains a domain that is both necessary and sufficient to impart substrate-selective DNA binding and enzymatic activity to the ZRANB3 ATPase domain. Thus, it acts as a SRD similar to the HARP domain of SMARCAL1 and the HIRAN domain of HLTF.
Discussion
In this study, we identified a structure recognition domain (SRD) in ZRANB3 that binds branched DNA substrates and confers DNA-dependent ATPase and fork remodeling activity to its SNF2-type motor domain. The SRD is also required for structure-specific endonuclease activity. A minimal enzymatic unit, containing only the SRD and the SNF2 ATPase domains, retains similar fork remodeling activities as the full-length protein. Thus, these data suggest that ZRANB3 shares a similar mechanism of action as SMARCAL1 and HLTF and support the idea that structure recognition domains impart fork remodeling activities onto the motor domains of these proteins.
Why cells express several different fork remodeling proteins that catalyze similar reactions in vitro is unknown. The exact DNA structure that is formed at a stalled replication fork in a cell is also unknown. Presumably, the stalled fork adopts a multitude of structures dependent upon the nature of the obstacle. This heterogeneity may warrant the need for several fork remodeling enzymes with substrate recognition domains that interact and bind to different DNA forms present at a stalled and/or regressed fork.
The high-resolution structures of the SRDs of HLTF and SMARCAL1 (HIRAN and HARP domains respectively) have been determined by x-ray crystallography (19–21). The HIRAN domain structure includes DNA, and explains its binding preference for duplex DNA with a short 3′ single-stranded DNA overhang (19). The HARP domain structure did not include DNA, but it resembles domains in other proteins that bind distorted DNA structures (20). SMARCAL1 prefers to bind DNA structures that contain at least five nucleotides of ssDNA (4), and point mutants in the HARP2 domain impair the ability of the SMARCAL1 catalytic domain to bind branched DNA structures (4, 20). Whether the HARP domain recognizes the fork junction itself is unknown. Our data indicate that the ZRANB3 SRD does bind forked DNA on its own although with significantly lower binding affinity than when it is attached to the motor domain. Most likely the SRD recognizes the fork structure and the motor binds the duplex DNA. As it hydrolyzes ATP, it can act to displace the nascent strands while re-annealing the parental strands. The SRD may act at the junction to facilitate this reaction.
Our data are inconsistent with the results from Yuan et al. (23). While the SRD we identified overlaps their HARP-like domain, we find that ZRANB3 is unable to bind forked DNA structures without this region and also lacks DNA-dependent ATPase activity. Also, we found that mutations in the SRD inactivated ZRANB3 endonuclease activity as would be predicted if the SRD were required for DNA binding. We do not know why Yuan and colleagues were able to observe both DNA binding and ATPase activity in their mutant protein; however, we note that other mammalian DNA-dependent ATPases could have contaminated their protein purifications.
This study extends our understanding of how ZRANB3 operates as a fork remodeling enzyme and determines the necessary components to carry out its enzymatic activities. Future high-resolution structural analyses of the ZRANB3 and SMARCAL1 proteins bound to DNA will be useful to better understand how their SRDs provide specificity to their fork remodeling activities.
Author Contributions
A. B. N. and D. C. designed the study and interpreted results. A. B. N. performed most experiments and A. C. M. and B. F. E. assisted with the DNA binding studies of the ZRANB3 SRD. A. B. N. and D. C. wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Miaw-Sheue Tsai for the production of baculovirus-infected insect cells to purify ZRANB3 proteins.
This work was supported by National Institutes of Health Grants R01CA102729-11A1S1, R01CA160432, and P01CA092584 (to D. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- SMARCAL1
- SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A-like protein 1
- dsDNA
- double-stranded DNA
- HARP
- HepA-related protein
- HIRAN
- HIP116 Rad5p N-terminal
- HLTF
- helicase-like transcription factor
- MT1 mutants
- L760A/D761A/I762A
- MT2 mutants
- W790A/S791A/S792A
- PCNA
- proliferating cell nuclear antigen
- RPA
- replication protein A
- SIOD
- Schimke-Immunoosseous Dysplasia
- SRD
- substrate recognition domain
- ssDNA
- single-stranded DNA
- ZRANB3
- zinc finger Ran-binding domain containing 3.
References
- 1.Zeman M. K., and Cimprich K. A. (2014) Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petermann E., and Helleday T. (2010) Pathways of mammalian replication fork restart. Nat. Rev. Mol. Cell Biol. 11, 683–687 [DOI] [PubMed] [Google Scholar]
- 3.Neelsen K. J., and Lopes M. (2015) Replication fork reversal in eukaryotes: from dead end to dynamic response. Nat. Rev. Mol. Cell Biol. 16, 207–220 [DOI] [PubMed] [Google Scholar]
- 4.Bétous R., Mason A. C., Rambo R. P., Bansbach C. E., Badu-Nkansah A., Sirbu B. M., Eichman B. F., and Cortez D. (2012) SMARCAL1 catalyzes fork regression and Holliday junction migration to maintain genome stability during DNA replication. Genes Dev. 26, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccia A., Nimonkar A. V., Hu Y., Hajdu I., Achar Y. J., Izhar L., Petit S. A., Adamson B., Yoon J. C., Kowalczykowski S. C., Livingston D. M., Haracska L., and Elledge S. J. (2012) The ZRANB3 translocase associates with poly-ubiquitinated PCNA to promote fork restart and limit recombination after replication stress. Mol. Cell 47, 396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blastyák A., Hajdú I., Unk I., and Haracska L. (2010) Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA. Mol. Cell Biol. 30, 684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boerkoel C. F., Takashima H., John J., Yan J., Stankiewicz P., Rosenbarker L., André J. L., Bogdanovic R., Burguet A., Cockfield S., Cordeiro I., Fründ S., Illies F., Joseph M., Kaitila I., Lama G., Loirat C., McLeod D. R., Milford D. V., Petty E. M., Rodrigo F., Saraiva J. M., Schmidt B., Smith G. C., Spranger J., Stein A., Thiele H., Tizard J., Weksberg R., Lupski J. R., and Stockton D.W. (2002) Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno-osseous dysplasia. Nat. Genet. 30, 215–220 [DOI] [PubMed] [Google Scholar]
- 8.Baradaran-Heravi A., Raams A., Lubieniecka J., Cho K. S., DeHaai K. A., Basiratnia M., Mari P.-O., Xue Y., Rauth M., Olney A. H., Shago M., Choi K., Weksberg R. A., Nowaczyk M. J. M., Wang W., Jaspers N. G. J., and Boerkoel C. F. (2012) SMARCAL1 deficiency predisposes to non-Hodgkin lymphoma and hypersensitivity to genotoxic agents in vivo. Am. J. Med. Genet. A 158A, 2204–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll C., Hunley T. E., Guo Y., and Cortez D. (2015) A novel splice site mutation in SMARCAL1 results in aberrant exon definition in a child with schimke immunoosseous dysplasia. Am. J. Med. Genet. 167, 2260–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll C., Badu-Nkansah A., Hunley T., Baradaran-Heravi A., Cortez D., and Frangoul H. (2013) Schimke Immunoosseous Dysplasia associated with undifferentiated carcinoma and a novel SMARCAL1 mutation in a child. Pediatric Blood Cancer 60, E88-E90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moinova H. R., Chen W.-D., Shen L., Smiraglia D., Olechnowicz J., Ravi L., Kasturi L., Myeroff L., Plass C., Parsons R., Minna J., Willson J. K. V., Green S. B., Issa J.-P., and Markowitz S. D. (2002) HLTF gene silencing in human colon cancer. Proc. Natl. Acad. Sci. U.S.A. 99, 4562–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence M. S., Stojanov P., Mermel C. H., Robinson J. T., Garraway L. A., Golub T. R, Meyerson M., Gabriel S. B., Lander E. S., and Getz G. (2014) Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansbach C. E., Bétous R., Lovejoy C. A., Glick G. G., and Cortez D. (2009) The annealing helicase SMARCAL1 maintains genome integrity at stalled replication forks. Genes Dev. 23, 2405–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postow L., Woo E. M., Chait B. T., and Funabiki H. (2009) Identification of SMARCAL1 as a component of the DNA damage response. J. Biol. Chem. 284, 35951–35961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciccia A., Bredemeyer A. L., Sowa M. E., Terret M.-E., Jallepalli P. V., Harper J. W., and Elledge S. J. (2009) The SIOD disorder protein SMARCAL1 is an RPA-interacting protein involved in replication fork restart. Genes Dev. 23, 2415–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan J., Ghosal G., and Chen J. (2009) The annealing helicase HARP protects stalled replication forks. Genes Dev. 23, 2394–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat K. P., Bétous R., and Cortez D. (2015) High-affinity DNA-binding domains of replication protein A (RPA) direct SMARCAL1-dependent replication fork remodeling. J. Biol. Chem. 290, 4110–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bétous R., Couch F. B., Mason A. C., Eichman B. F., Manosas M., and Cortez D. (2013) Substrate-selective repair and restart of replication forks by DNA translocases. Cell Rep. 3, 1958–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kile A. C., Chavez D. A., Bacal J., Eldirany S., Korzhnev D. M., Bezsonova I., Eichman B. F., and Cimprich K. A. (2015) HLTF's ancient HIRAN domain binds 3′ DNA ends to drive replication fork reversal. Mol. Cell 58, 1090–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason A. C., Rambo R. P., Greer B., Pritchett M., Tainer J. A., Cortez D., and Eichman B. F. (2014) A structure-specific nucleic acid-binding domain conserved among DNA repair proteins. Proc. Natl. Acad. Sci. U.S.A. 111, 7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hishiki A., Hara K., Ikegaya Y., Yokoyama H., Shimizu T., Sato M., and Hashimoto H. (2015) Structure of a Novel DNA-binding domain of helicase-like transcription factor (HLTF) and its functional implication in DNA damage tolerance. J. Biol. Chem. 290, 13215–13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achar Y. J., Balogh D., Neculai D., Juhasz S., Morocz M., Gali H., Dhe-Paganon S., Venclovas Č., and Haracska L. (2015) Human HLTF mediates postreplication repair by its HIRAN domain-dependent replication fork remodelling. Nucleic Acids Res. 43, 10277–10291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan J., G. G., and Chen J. (2012) The HARP-like domain-containing protein AH2/ZRANB3 binds to PCNA and participates in cellular response to replication stress. Mol. Cell 10, 410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X., Zaro J. L., and Shen W.-C. (2013) Fusion protein linkers: property, design and functionality. Adv. Drug Deliv. Rev. 65, 1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusufzai T., and Kadonaga J. T. (2008) HARP is an ATP-driven annealing helicase. Science 322, 748–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porollo A. A., Adamczak R., and Meller J. (2004) POLYVIEW: a flexible visualization tool for structural and functional annotations of proteins. Bioinformatics 20, 2460–2462 [DOI] [PubMed] [Google Scholar]
- 27.Buchan D. W. A., Minneci F., Nugent T. C. O., Bryson K., and Jones D. T. (2013) Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 41, W349-W357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole C., Barber J. D., and Barton G. J. (2008) The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36, W197-W201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston R., Peeters H., and Ahel D. (2012) ZRANB3 is a structure-specific ATP-dependent endonuclease involved in replication stress response. Genes Dev. 26, 1558–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaus A., Martin D. M. A., Barton G. J., and Owen-Hughes T. (2006) Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 34, 2887–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yusufzai T., and Kadonaga J. T. (2010) Annealing helicase 2 (AH2), a DNA-rewinding motor with an HNH motif. Proc. Natl. Acad. Sci. U.S.A. 107, 20970–20973 [DOI] [PMC free article] [PubMed] [Google Scholar]