Abstract
Background
Neutrophils produce and carry key components of the alternative pathway (AP) of complement, including properdin (P). The effect of chemotherapy-induced absolute neutropenia on circulating P levels and AP function has not been previously established.
Methods
We prospectively measured free P levels in serum from 27 individuals expected to develop neutropenia after administration of chemotherapy for hematological malignancies in preparation for hematopoietic stem cell transplantation and here describe the relationship between serum P levels and the neutrophil count over time.
Results
When the absolute neutrophil count (ANC) was >500 cells/mm3 pre-chemotherapy, P levels were significantly higher than P levels associated with an ANC ≤500 cells/mm3 (median values 8392 ng/mL and 6355 ng/mL, respectively; P = .001). Pairwise comparison between pre-chemotherapy P levels and P levels at initial or last documented neutropenia before recovery showed a significant decline (P < .0001). No correlation was observed between P levels during neutropenia and after recovery of neutropenia in 20 subjects for which postneutropenia samples were obtained. A small but significant (P = .02) decrease in AP hemolytic activity was noted between baseline (preneutropenia) and samples obtained at the onset of neutropenia, but only with low (6.25%) and not higher (12.5 or 25%) serum concentrations.
Conclusions
A decline in P levels and AP activity could contribute to the increased risk of infection in neutropenic patients and warrants further study.
Keywords: human, neutropenia, properdin
The complement system consists of over 30 fluid-phase proteins and enzymes and several membrane-associated molecules, which together regulate the innate immune response of the host to infections [1]. The classic and lectin complement pathways are initiated by binding of “recognition molecules” to the microbial surface, which include antibody or specific lectins (eg, mannan-binding lectin or ficolins), respectively. The alternative pathway (AP) of complement can be activated in 2 ways. In the conventional model (the C3 “tickover” model), complement C3 is spontaneously hydrolyzed and deposited on foreign surfaces; C3b deposition is then amplified by the positive feedback loop of the AP [2, 3]. A second model of AP activation is the properdin (P)-directed model, which involves recognition of activator surfaces by P, which then binds to C3b and forms a platform for AP C3 convertase assembly [4]. This second model is consistent with that proposed by Pillemer et al [5] over 60 years ago. Properdin is the only known positive regulator of the complement system and serves to stabilize the C3bBb complex, also known as AP C3 convertase [4]. Properdin is produced by neutrophils and stored in their secondary granules. Other cells that synthesize P include macrophages, monocytes, T cells, and endothelial cells [6–12]. A small amount of free P is present in human plasma at concentrations ranging from 4 to 25 µg/mL [13–15]. Activation of neutrophils stimulates the release of P from secondary granules and augments complement activation on the proximate offending target [16].
Chemotherapy-induced neutropenia renders the host at increased risk for infections; the incidence of infections increases dramatically when absolute neutrophil counts (ANCs) drop below 500 cells/mm3 [17]. Low levels of P in this setting could decrease AP activation on microbial surfaces and contribute to the increased risk of invasive infections. In addition, critically ill patients with sepsis have decreased amounts of P compared with age- and sex-matched controls [18]. We aimed to define the relationship between free P levels in serum and the absolute level of neutrophils in individuals who become neutropenic after receiving cytotoxic chemotherapy for hematological malignancy.
MATERIALS AND METHODS
Study Population
Informed written consent was obtained from all donors before enrollment into the study. The University of Massachusetts Medical School Institutional Review Board committee approved the research protocol, and the study was performed in accordance with the Declaration of Helsinki.
Subjects ≥18 years of age who were scheduled for elective chemotherapy before receiving hematopoietic stem cell transplantation (HSCT) or cord blood transplantation were prospectively recruited at the inpatient bone marrow unit at the University of Massachusetts Medical Center (Worcester, MA) from May 1, 2013 to January 31, 2014 (n = 31). The first blood sample was obtained before initiation of chemotherapy and within 24 hours of the patient’s hospitalization (Day 0). Subsequent blood samples (not more frequently than once every 48 hours) were obtained along with blood draws for routine care when the subjects became severely neutropenic with an ANC ≤500 cells/mm3. When possible, a final blood sample was obtained shortly after the patient recovered from severe neutropenia (ANC >500 cells/mm3). Individuals who received fresh frozen plasma were excluded from the study because this would affect P levels. To be eligible for analysis, each subject had to have a minimum of 2 blood samples in addition to the baseline sample. Of the 31 persons who agreed to participate in the study, 1 was excluded because the neutrophil level was <500 cells/mm3 at baseline and neutrophil counts did not recover during the course of hospitalization, and 3 were excluded because only 2 blood samples were available; thus, 27 persons were included in the final analyses.
Sample Collection
Blood samples were collected and processed within 1 h of sampling. Serum was separated from whole blood and stored at −80°C until the end of the recruitment process.
Quantification of Serum P Levels
Properdin was quantified by enzyme-linked immunosorbent assay using monoclonal anti-P antibody (Quidel, San Diego, CA) as the capture antibody to coat microtiter wells at a concentration of 0.5 µg/mL in phosphate-buffered saline (PBS) for 3 hours at 37°C, followed by 15 hours at 4°C. Microtiter wells were washed thrice with PBS-0.05% Tween 20. Serum samples were diluted 1:250 and 1:500 in PBS-0.05% Tween 20. Dilutions of purified P (Complement Technology, Inc., Tyler, TX) were used to generate a standard curve. Commercially available sera from 3 normal adults and P-depleted serum (Complement Technology, Inc.) were used as controls. Immunoglobulin G purified from polyclonal goat antihuman factor P (Complement Technology, Inc.) over protein G sepharose (Sigma-Aldrich, St. Louis, MO) was biotinylated and used as the detecting antibody at a 1:1000 dilution in PBS-0.05% Tween 20. Bound P was detected with avidin alkaline phosphatase (Sigma-Aldrich) as substrate. Absorbance was set at 405 nm in a microtiter plate spectrophotometer.
Alternative Pathway Hemolytic Assay
Functional AP complement activation was performed on paired serum samples, 1 obtained at baseline and 1 on the first available sample with an ANC ≤500 cells/mm3, using rabbit red blood cells (RBC) [19]. All sera contained 10 mM ethylene glycol tetraacetic acid (EGTA) and 10 mM of Mg2+ (Mg/EGTA-treated serum) to block the classic and lectin pathways and permit selective activation of the AP. Sera containing 10 mM ethylenediaminetetraacetic acid (EDTA) blocked all pathways and represented baseline hemolysis, and RBCs hemolyzed with sterile water represented 100% hemolysis. Serum concentrations of 6.25%, 12.5%, and 25% were tested. Reaction mixtures were incubated for 60 minutes at 37°C. Samples were centrifuged and absorbance of the supernatants was measured at 405 nm. Results are shown as the net absorbance, which was derived by subtracting baseline values (EDTA-treated serum) from absorbance obtained after lysis with Mg/EGTA-treated serum.
Statistical Analyses
Statistical analyses were conducted using either SAS version 9.3 (SAS Institute, Inc., Cary, NC) or GraphPad Prism version 7.0a. All graphs were produced with GraphPad Prism. Paired values were compared by Wilcoxon signed-rank test (GraphPad Prism).
RESULTS
Clinical Data on Subjects
Of the 27 patients included for analysis, the median age was 54 years (range, 19–81 years) and 12 (44.4%) were males. Thirteen (48%) received autologous HSCT, 13 (48%) received allogeneic HSCT, and 1 (4%) received chemotherapy without HSCT. One of the 13 allogeneic HSCT was with umbilical cord blood. Temperature >38.0°C was registered among 14 patients (52%) at 1 or more time points during the study period. Four patients (15%) had positive blood cultures during the study.
The median number of blood samples obtained from each patient for the study was 4 (range, 3–6 samples). Twenty of the 27 patients (74.1%) had a blood sample obtained after the recovery of neutrophil counts. The median duration of the neutropenic period was 8 days (range, 3–25 days). The median length of stay in the hospital until receipt of chemotherapy for the 27 enrolled patients was 6 days (range, 0–7 days).
Properdin (P) Levels Were Significantly Lower During Neutropenia Compared With the Preneutropenic State, and P and Neutrophil Levels Declined Together After Neutropenia
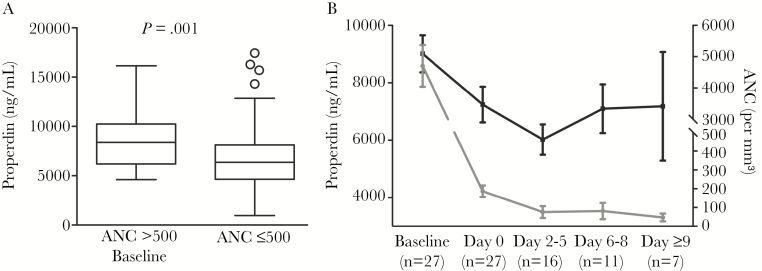
Properdin levels obtained from neutropenic and baseline, nonneutropenic serum samples are shown in Figure 1. Properdin levels in neutropenic samples (n = 61) were significantly lower than those from samples when neutrophil counts exceeded 500 cells/mm3 (n = 27) (median 6355 ng/mL vs 8392 ng/mL; P = 0.001, Mann-Whitney U test) (Figure 1A). This analysis did not include P levels obtained from samples after the recovery of neutropenia.
Figure 1.
Comparison of properdin (P) levels collected during baseline (preneutropenic) and neutropenic states. (A) Samples collected from patients with neutropenia have significantly lower P levels than during the preneutropenic state (n = 27 for absolute neutrophil count [ANC] >500, n = 61 for ANC ≤500; P = .001; Mann-Whitney U test). (B) Correlation of P levels with neutropenia. Properdin levels are low during neutropenia compared with the baseline preneutropenic state. Plot shows the mean P levels, and error bars indicate ± standard error of the mean, with n indicating the number of patients included for each time cluster.
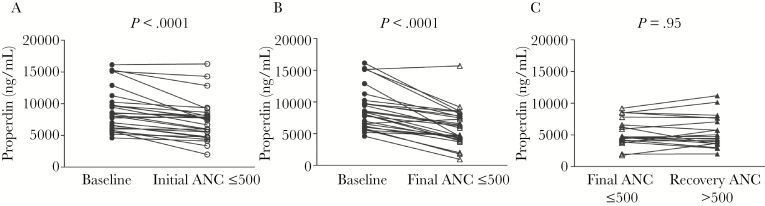
Properdin levels were then analyzed in serum samples obtained over the course of neutropenia. Baseline samples were obtained from patients before neutropenia. The day of sample collection at the first occurrence of neutropenia was designated Day 0. Subsequent samples were clustered into groups of several days, spanning Days 2–5 and Days 6–8. Seven subjects having neutropenia at Day 9 and beyond constituted the third cluster. The mean ± standard error of the mean for each point or cluster is shown in Figure 1B. Mean baseline P levels were 9010 ± 647 ng/mL, which decreased to 7292 ± 616.1 ng/mL at the onset of neutropenia (Day 0). These data show that P levels trended down with neutropenia. Comparisons between P levels obtained before neutropenia and either at the onset of neutropenia (Day 0) or at the last blood draw associated with neutropenia both showed a significant downward trend (Figure 2A and B, respectively; P < .0001 in each case).
Figure 2.
Properdin (P) levels decline between pre-chemotherapy (baseline, obtained while absolute neutrophil count [ANC] >500) and neutropenia but remain low despite recovery of neutrophil count. (A) Comparison of P levels between baseline (ANC >500, closed circles) and initial neutropenic state (ANC ≤500, open circles) (n = 27 paired subject samples; P < .0001). (B) Comparison of P levels between baseline (ANC >500, closed circles) and final neutropenic state (ANC ≤500, open triangles) (n = 27 paired subject samples; P < .0001). (C) Comparison of P levels between the final neutropenic state (ANC ≤500, open triangles) and recovery of ANC >500 (closed triangles) from the subset of patients whose recovery samples were available (n = 20 paired subject samples; P value not significant; Wilcoxon matched-pairs signed-rank test).
Properdin Levels Do Not Increase With the Recovery of Neutrophil Counts
Properdin levels were measured in a subset of patients (n = 20) from whom samples were obtained after recovery of neutrophil counts (ANC >500 cells/mm3) to determine whether P levels increased with resolution of neutropenia. In contrast to the strong association between the decline in P concentration with the occurrence of neutropenia (Figure 2A and B), recovery of neutrophil counts was not associated with a rise in serum P concentrations; mean P levels associated with the last neutropenic blood sample were 5428 ± 482.4 ng/mL compared with 5445 ± 553.6 ng/mL at time of neutrophil recovery (Figure 2C).
Effect of the Decline on Properdin Levels on Alternative Pathway Function
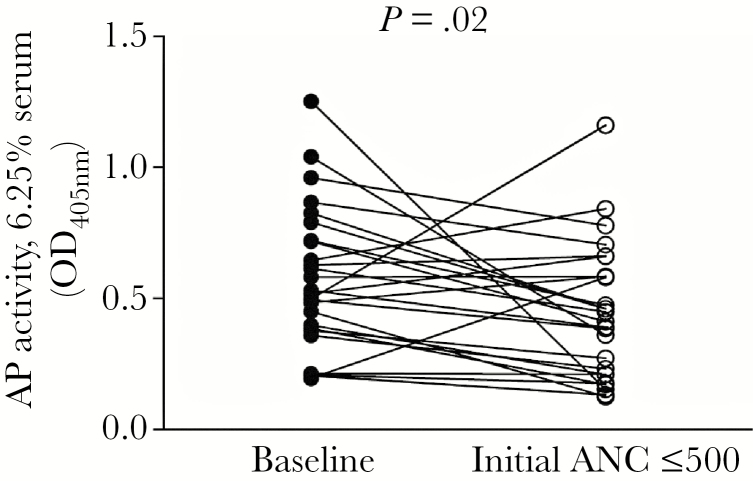
We next asked whether the decline in P levels associated with neutropenia negatively affected AP activity, as measured by lysis of rabbit RBCs by Mg/EGTA-treated serum. Hemolytic assays were performed using serum concentrations of 6.25%, 12.5%, and 25%. There was no correlation between the ANC and AP hemolytic activity of serum noted at the 2 higher concentrations of serum tested (data not shown). A small albeit statistically significant decline in AP activity with 6.25% serum was observed with the onset of neutropenia (Figure 3).
Figure 3.
Alternative pathway (AP) activity declines between baseline (preneutropenic) and the initial neutropenic state. Serum concentrations of 6.25% were tested (n = 27 paired subject samples; P = .02; Wilcoxon matched-pairs signed-rank test).
DISCUSSION
The main finding in this study was that chemotherapy-induced neutropenia is associated with a decline in serum P levels and a modest decrease in AP hemolytic activity at low serum concentrations. At baseline, patients were noted to have P levels within the typical reference range [13–15]. The onset of neutropenia was accompanied by a decline in mean P levels by ~19%. The mean P levels in subjects who remained neutropenic for an additional 2 to 5 days dropped further to ~32% of the baseline mean P levels, highlighting the importance of neutrophils in contributing to serum P levels [6]. However, P levels did not appear to drop further in persons with more prolonged neutropenia, suggesting that cells other than neutrophils contribute significantly to P synthesis. Indeed, T cells and monocytes/macrophages also synthesize P [9–11, 20] and could contribute to the pool of circulating P in the absence of neutrophils. An alternative explanation is that the half-life for P is long. However, the relatively rapid decline in P levels with neutropenia suggests a relatively short half-life of free P in serum, consistent with the rapid turnover of other complement proteins [21]. Another possible, and not mutually exclusive, explanation for low P levels is increased consumption of P secondary to binding to apoptotic cells or debris [22, 23] that may be generated during chemotherapy.
Patients receiving aggressive chemotherapy are at increased risk for systemic infections [18]. Patients with severe neutropenia are at risk for infection caused by both Gram-positive and Gram-negative bacteria [17, 24]. The positive feedback loop of the AP of complement serves to effectively amplify C3 deposition on microbes that is often initiated by the classic and/or lectin pathways. In some instances, P can act as a pattern recognition molecule, binding to the surface of select Gram-negative bacteria and activating the complement system [25]. Our data indicate that the hemolytic activity of the AP during neutropenia was relatively well preserved despite the modest drop in P levels. A slight compromise in AP activity was noted at the onset of neutropenia and only at low (6.25%) serum concentrations, which was expected because the activity of the AP is highly concentration-dependent [26]. Thus, the effects of lower P levels and the resulting decrease in C3 convertase stability are likely to be manifest at lower serum concentrations. Uchiyama et al [20] recently reported that individuals receiving interferon (IFN)-α treatment for hepatitis C had increased P levels compared with pre- or posttreatment levels. They demonstrated that treating monocytes and neutrophils with IFN-α resulted in increased P release from these cells in culture. Blocking P function in the plasma of IFN-α-treated individuals decreased the ability of monocytes to kill group A streptococci, which underscores the role of P in cell-mediated innate immune defenses.
Of note, the recovery of neutrophil counts was not associated with an increase in P levels. A possible explanation is inadequate P synthesis by the newly formed neutrophils. The lack of longer-term follow-up to determine the fate of P levels after neutrophil recovery is a limitation of our study. However, most subjects were discharged immediately upon recovery of neutrophils, which precluded obtaining further serum samples for P level determination. Recent data have underscored the importance of P in binding to neutrophils and promoting AP activation on the cell surface, which in turn enhanced neutrophil function by increasing CD11b expression and the oxidative burst [27]. Properdin plays a crucial role in the formation of platelet-granulocyte aggregates [28], which are believed to play a key role in fighting infections. Thus, in the face of lower P levels, it is possible that the newly formed neutrophils may not function optimally to ward off infections.
CONCLUSIONS
In conclusion, our data show that chemotherapy-induced neutropenia results in a significant decline in P levels, which does not normalize immediately at the time of recovery of neutrophils. Despite the drop in P serum levels, the hemolytic function of the AP is only slightly compromised at low serum concentrations. Whether lower P levels during neutropenia contribute directly to the increased risk of infections in this patient population merits further study.
Acknowledgments
We thank all the staff of the inpatient bone marrow unit at the University of Massachusetts Medical Center, without whom this study would not be possible. We also thank Melanie Trombly, University of Massachusetts Medical School, for assistance with preparation of the manuscript.
Author contributions. A. T., B. K., and S. A. performed the experiments. A. T., B. K., R. W. F., R. N., S. M. L., J. P. W., and S. R. designed the study. A. T., J. P. W., and S. R. wrote the paper. B. B. performed statistical analysis.
Potential conflicts of interest. All authors: No reported conflicts.The author has submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Botto M. C3. In: Morley BJ, Walport MJ, eds. The Complement Facts Book. London: Academic Press; 2000: pp 88–94. [Google Scholar]
- 2. Schreiber RD, Pangburn MK, Lesavre PH, Müller-Eberhard HJ. Initiation of the alternative pathway of complement: recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc Natl Acad Sci U S A 1978; 75:3948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pangburn MK, Müller-Eberhard HJ. Initiation of the alternative complement pathway due to spontaneous hydrolysis of the thioester of C3. Ann N Y Acad Sci 1983; 421:291–8. [DOI] [PubMed] [Google Scholar]
- 4. Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J Immunol 2007; 179:2600–8. [DOI] [PubMed] [Google Scholar]
- 5. Pillemer L, Blum L, Lepow IH, et al. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science 1954; 120:279–85. [DOI] [PubMed] [Google Scholar]
- 6. Wirthmueller U, Dewald B, Thelen M, et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol 1997; 158:4444–51. [PubMed] [Google Scholar]
- 7. Whaley K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med 1980; 151:501–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minta JO. Biosynthesis of complement factor P (properdin) by the human pre-monocyte cell line (U-937). Mol Immunol 1988; 25:1363–70. [DOI] [PubMed] [Google Scholar]
- 9. Schwaeble W, Dippold WG, Schäfer MK, et al. Properdin, a positive regulator of complement activation, is expressed in human T cell lines and peripheral blood T cells. J Immunol 1993; 151:2521–8. [PubMed] [Google Scholar]
- 10. Schwaeble W, Huemer HP, Möst J, et al. Expression of properdin in human monocytes. Eur J Biochem 1994; 219:759–64. [DOI] [PubMed] [Google Scholar]
- 11. Farries TC, Atkinson JP. Biosynthesis of properdin. J Immunol 1989; 142:842–7. [PubMed] [Google Scholar]
- 12. Bongrazio M, Pries AR, Zakrzewicz A. The endothelium as physiological source of properdin: role of wall shear stress. Mol Immunol 2003; 39:669–75. [DOI] [PubMed] [Google Scholar]
- 13. Fijen CA, van den Bogaard R, Schipper M, et al. Properdin deficiency: molecular basis and disease association. Mol Immunol 1999; 36:863–7. [DOI] [PubMed] [Google Scholar]
- 14. Pangburn MK. Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J Immunol 1989; 142:202–7. [PubMed] [Google Scholar]
- 15. Nolan KF, Reid KB. Properdin. Methods Enzymol 1993; 223:35–46. [DOI] [PubMed] [Google Scholar]
- 16. Walport MJ. Complement. First of two parts. N Engl J Med 2001; 344:1058–66. [DOI] [PubMed] [Google Scholar]
- 17. Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 1966; 64:328–40. [DOI] [PubMed] [Google Scholar]
- 18. Stover CM, McDonald J, Byrne S, et al. Properdin levels in human sepsis. Front Immunol 2015; 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morgan BP. Complement Methods and Protocols. Humana Press; 2000:61–71. [Google Scholar]
- 20. Uchiyama S, Keller N, Schlaepfer E, et al. Interferon α-enhanced clearance of group A Streptococcus despite neutropenia. J Infect Dis 2016; 214:321–8. [DOI] [PubMed] [Google Scholar]
- 21. Ruddy S, Carpenter CB, Chin KW, et al. Human complement metabolism: an analysis of 144 studies 1. Medicine 1975; 54:165–178. [Google Scholar]
- 22. Xu W, Berger SP, Trouw LA, et al. Properdin binds to late apoptotic and necrotic cells independently of C3b and regulates alternative pathway complement activation. J Immunol 2008; 180:7613–21. [DOI] [PubMed] [Google Scholar]
- 23. Kemper C, Mitchell LM, Zhang L, Hourcade DE. The complement protein properdin binds apoptotic T cells and promotes complement activation and phagocytosis. Proc Natl Acad Sci U S A 2008; 105:9023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis 2004; 39(Suppl 1):S25–31. [DOI] [PubMed] [Google Scholar]
- 25. Kemper C, Atkinson JP, Hourcade DE. Properdin: emerging roles of a pattern-recognition molecule. Annu Rev Immunol 2010; 28:131–55. [DOI] [PubMed] [Google Scholar]
- 26. Schreiber RD, Morrison DC, Podack ER, Müller-Eberhard HJ. Bactericidal activity of the alternative complement pathway generated from 11 isolated plasma proteins. J Exp Med 1979; 149:870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camous L, Roumenina L, Bigot S, et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 2011; 117:1340–9. [DOI] [PubMed] [Google Scholar]
- 28. Blatt AZ, Saggu G, Kulkarni KV, et al. Properdin-mediated C5a production enhances stable binding of platelets to granulocytes in human whole blood. J Immunol 2016; 196:4671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]