Abstract
Background
Interferon-free regimens to treat hepatitis C virus (HCV) genotype 1 are effective but costly. At this time, payers in the United States use strategies to control costs including (1) limiting treatment to those with advanced disease and (2) negotiating price discounts in exchange for exclusivity.
Methods
We used Monte Carlo simulation to investigate budgetary impact and cost effectiveness of these treatment policies and to identify strategies that balance access with cost control. Outcomes included nondiscounted 5-year payer cost per 10000 HCV-infected patients and incremental cost-effectiveness ratios.
Results
We found that the budgetary impact of HCV treatment is high, with 5-year undiscounted costs of $1.0 billion to 2.3 billion per 10000 HCV-infected patients depending on regimen choices. Among noncirrhotic patients, using the least costly interferon-free regimen leads to the lowest payer costs with negligible difference in clinical outcomes, even when the lower cost regimen is less convenient and/or effective. Among cirrhotic patients, more effective but costly regimens remain cost effective. Controlling costs by restricting treatment to those with fibrosis stage 2 or greater disease was cost ineffective for any patient type compared with treating all patients.
Conclusions
Treatment strategies using interferon-free therapies to treat all HCV-infected persons are cost effective, but short-term cost is high. Among noncirrhotic patients, using the least costly interferon-free regimen, even if it is not single tablet or once daily, is the cost-control strategy that results in best outcomes. Restricting treatment to patients with more advanced disease often results in worse outcomes than treating all patients, and it is not preferred.
Keywords: budget impact, HCV, treatment restriction
New medications to treat hepatitis C virus (HCV) are costly, with wholesale acquisition costs exceeding $1000/day in the United States [1]. Several recent cost-effectiveness analyses concur that even at such high prices, all oral sofosbuvir-based regimens to treat HCV genotype 1 (GT1) provide good value compared with the previous standard of care [2–6]. Given that there are 2–3 million HCV-infected people in the United States [7], however, the budget impact of providing new HCV treatments to all who need them could exceed the healthcare system’s ability to pay, despite attractive cost-effectiveness ratios [2–4]. As multiple interferon-free regimens enter the market, payers negotiate with pharmaceutical manufacturers covering 1 exclusive interferon-free treatment regimen in exchange for substantial price discounts, potentially trading efficacy for lower cost [8]. Many insurers further limit access to new HCV therapies by prioritizing patients with higher degrees of liver fibrosis for treatment, although there is no consensus on this threshold with thresholds ranging from fibrosis stage 2 (F2) to F4 [9]. Because these restrictions are recent inventions, data are needed to inform decision making and to shed light on the relative costs and benefits of different approaches to controlling HCV treatment costs.
In this environment—where alternative treatment options are both highly efficacious and costly—it is particularly important to understand the budgetary impact and economic value of new treatments as well as to identify approaches to expand access while controlling cost. We used the Hepatitis C Cost-Effectiveness (HEP-CE) Model [10, 11] to consider the policy questions that payers currently face when considering coverage of high-cost HCV medications in the competitive US market including the following: (1) how much to budget for every 10000 HCV-infected patients within a jurisdiction or health plan, (2) how to make cost-effective trade-offs between efficacy and cost, and (3) whether to contain costs by limiting access to only those with F2 or greater.
METHODS
Overview
The HEP-CE Model is a Monte Carlo lifetime simulation of HCV infection, screening, and treatment, summarized briefly below and in greater detail in the Supplementary Materials and published literature [10, 11]. We constructed the model and performed analyses using TreeAge Pro software (TreeAge Software, Inc., Williamstown, MA).
We assumed the health system perspective on treatment costs for HCV GT1 in patients who are engaged in care and prepared to initiate HCV therapy. We considered distinct patient types defined by GT1 subtype (1a vs 1b), fibrosis stage (noncirrhotic vs cirrhotic), treatment history (naive vs experienced), and ribonucleic acid (RNA) level for treatment-naive, noncirrhotic patients (<6 million copies vs ≥6 million copies) (Table 1). Each of these patient types was associated with a distinct menu of treatment options (Table 1) and related sustained virologic response (SVR). We did not evaluate treatment of decompensated cirrhosis.
Table 1.
Treatment Strategies Considered in a Cost-Effectiveness Analysis of Therapies for HCV Genotype 1 Infection
| Treatment History | Noncirrhotic | Cirrhotic |
|---|---|---|
| Treatment-naive | 48 weeks pegylated-interferon/ribavirin | 48 weeks pegylated-interferon/ribavirin |
| 24 weeks simeprevir/pegylated-interferon/ribavirin | 24 weeks simeprevir/pegylated-interferon/ribavirin | |
| 12 weeks sofosbuvir/pegylated-interferon/ribavirin | 12 weeks sofosbuvir/pegylated-interferon/ribavirin | |
| 8 weeks sofosbuvir/ledipasvir (HCV RNA <6 million) | 12 weeks sofosbuvir/ledipasvir | |
| 12 weeks sofosbuvir/ledipasvir (HCV RNA ≥6 million) | 12 weeks paritaprevir-ritonavir/ombitasvir/dasabuvir/ribavirin | |
| 12 weeks paritaprevir-ritonavir/ombitasvir/dasabuvir /(ribavirin)a | 24 weeks daclatasvir/sofosbuvir | |
| 12 weeks daclatasvir/sofosbuvir | ||
| Treatment-experienced | 12 weeks sofosbuvir/pegylated-interferon/ribavirin | 12 weeks sofosbuvir/pegylated-interferon/ribavirin |
| 12 weeks simeprevir/sofosbuvir | 12 weeks simeprevir/sofosbuvir | |
| 12 weeks sofosbuvir/ledipasvir | 12 weeks sofosbuvir/ledipasvir/ribavirin | |
| 24 weeks sofosbuvir/ledipasvir | 24 weeks sofosbuvir/ledipasvir | |
| 12 weeks paritaprevir-ritonavir/ombitasvir/dasabuvir/ribavirin | 12 to 24 weeks paritaprevir-ritonavir/ombitasvir/dasabuvir/ribavirinb | |
| 12 weeks daclatasvir/sofosbuvir | 24 weeks daclatasvir/sofosbuvir/ribavirin |
Abbreviations: HCV, hepatitis C virus; RNA, ribonucleic acid.
Ribavirin is included for genotype 1a and not for 1b patients.
Twelve weeks for 1b patients, 24 weeks regimen for genotype 1a patients.
We used the model to simulate clinical outcomes and costs for hypothetical cohorts of 1 million patients for each patient type and treatment strategy. Outcomes included the following: quality-adjusted life years (QALYs) and lifetime medical costs, each discounted at 3% annually [12], and the 5-year, undiscounted budgetary impact per 10000 HCV-infected patients [13]. To estimate the 5-year budget impact, we ran the model for 5 simulated years to estimate mean undiscounted cost per patient. We then multiplied that 5-year mean cost by 10000 to estimate the total budget impact. We chose a 5-year horizon because that is a time period of relevance to payers [13]. We express budget impact in terms of cost/10000 because that quantity can be scaled up or down by payers to estimate budgetary impact for their specific populations. We calculated the incremental cost-effectiveness ratio (ICER) for each strategy by dividing the additional lifetime cost by the additional QALYs gained compared with the next less expensive strategy [12]. We deemed regimens that provided lower QALYs at higher total cost than an alternative, as well as regimens that provided fewer QALYs at a higher cost per QALY gained, to be inefficient (dominated), and we excluded them from the final comparisons [12].
We next modeled a scenario in which noncirrhotic patients could be treated for HCV only when they reached METAVIR F2 or greater (F2+ only). Because of the large potential number of strategies when considering all regimens and treatment restrictions a priori, we developed an approach to incorporating the “treat F2+ only” approach. First, we identified the preferred interferon-free regimen for a given patient type assuming the “treat-all” approach. We then considered the “F2+ only” strategy only for the preferred treatment regimen. In addition, because the quality of life (QoL) of patients with early stage HCV infection is uncertain, and this value can affect the cost effectiveness of early HCV therapy, we included a scenario in which patients with early HCV had a much higher QoL than they did in the base case analysis.
Finally, we performed a series of sensitivity analyses in which we varied the efficacy and cost of one interferon-free regimen, while holding constant the efficacy and cost of all others. We report the relationships between relative efficacy, cost, and cost effectiveness using 2-way sensitivity analysis graphs [12].
We performed extensive deterministic and probabilistic sensitivity analyses. A priori parameters of interest included treatment efficacy, treatment cost, fibrosis staging test characteristics (sensitivity and specificity of correctly identifying a given stage), QoL with early HCV, mean age of the population, and QoL after SVR. We performed 2-way sensitivity analyses in which we considered various combinations of rates of fibrosis progression and QoL with early disease. We summarize sensitivity analysis results using one-way graphs and cost-effectiveness acceptability curves.
Model Structure
Hepatitis C Virus Disease Progression
The simulation includes 3 stages of liver disease: mild to moderate fibrosis, cirrhosis, and decompensated cirrhosis; the rate of progression varies among simulated patients, and the model operationalizes this variation by randomly drawing the time from the date of HCV infection to that of developing cirrhosis so that at model start an individual’s fibrosis stage is a function of their age, age of infection, and fibrosis progression (see Supplementary Appendix for details). For F2+ only strategies, we considered simulated patients to have reached F2 disease when they had accrued 50% of their total time from HCV infection to cirrhosis.
At every disease stage, HCV infection is associated with higher costs and lower QoL compared with HCV-uninfected individuals [14–16] (Table 2). When patients reach cirrhosis, they are subject to HCV-attributable mortality. The rate of HCV-attributable mortality further increases when patients reach decompensated cirrhosis, reflecting both the increasing risks posed by advanced liver disease such as hepatocellular carcinoma and the elevated mortality risk of extrahepatic conditions such as esophageal varices [17].
Table 2.
Model Inputs for a Cost-Effectiveness Analysis of Strategies for Cost Containment in Provision of Interferon-Free Therapy for HCV Genotype 1
| Variable | Base Case Value | Range Evaluated in Sensitivity Analysesa | Source(s) |
|---|---|---|---|
| Cohort Characteristics | |||
| Mean age treatment-naive (SD), years | 52 (14) | 42 (14)–62 (14) | [23] |
| Mean age treatment-experienced (SD), years | 56 (14) | 46 (14)–66 (14) | [22] |
| Proportion male (treatment-naive) | 0.59 | 0–1 | [23] |
| Proportion male (treatment-experienced) | 0.68 | 0–1 | [22] |
| Average age at HCV infection, years | 26 | 16–36 | [34] |
| HCV Disease Progression | |||
| Median time to cirrhosis from time of HCV infection, years | 25 | 10–40 | [48, 49] |
| Median time to first liver-related event after developing cirrhosis, years | 11 | 6–19 | [17] |
| Liver-related mortality with compensated cirrhosis, deaths/100 PYs | 1.39 | 0.96–1.82 | [17] |
| Liver-related mortality with decompensated cirrhosis, deaths/100 PYs | 12.00 | 8.28–15.72 | [17] |
| Reduction in liver-mortality after SVR, %b | 94 | 81–98 | [41] |
| HCV Therapy Efficacy, Treatment-Naivec | |||
| SVR probabilities for 48 weeks pegylated-interferon/ribavirin | [19] | ||
| Genotype 1 without cirrhosis | 0.44 | 0.44 (0.02) | |
| Genotype 1 with cirrhosis | 0.24 | 0.23 (0.04) | |
| SVR probabilities for 24 weeks pegylated-interferon/ribavirin/simeprevir | [20, 21] | ||
| Genotype 1a without cirrhosis | 0.78 | 0.77 (0.03) | |
| Genotype 1a with cirrhosis | 0.55 | 0.53 (0.10) | |
| Genotype 1b without cirrhosis | 0.91 | 0.89 (0.02) | |
| Genotype 1b with cirrhosis | 0.65 | 0.63 (0.10) | |
| SVR probabilities for 12 weeks pegylated-interferon/ribavirin/sofosbuvir | [32] | ||
| Genotype 1 without cirrhosis | 0.92 | 0.92 (0.02) | |
| Genotype 1 with cirrhosis | 0.80 | 0.79 (0.05) | |
| SVR probability for 8 weeks sofosbuvir/ledipasvir | [28, 50] | ||
| Genotype 1 without cirrhosis | 0.97 | 0.96 (0.02) | |
| SVR probability for 12 weeks sofosbuvir/ledipasvir | [28, 50] | ||
| Genotype 1 without cirrhosis | 0.96 | 0.96 (0.02) | |
| Genotype 1 with cirrhosis | 0.97 | 0.95 (0.04) | |
| SVR probability for 12 weeks paritaprevir-ritonavir/ombitasvir/dasabuvir ± ribavirin | [29, 31] | ||
| Genotype 1a without cirrhosis (with ribavirin) | 0.97 | 0.97 (0.02) | |
| Genotype 1a with cirrhosis (with ribavirin) | 0.92 | 0.91 (0.04) | |
| Genotype 1b without cirrhosis (without ribavirin) | 0.99 | 0.98 (0.01) | |
| Genotype 1b with cirrhosis (with ribavirin) | 0.99 | 0.97 (0.03) | |
| SVR probability for 12 or 24 weeks of daclatasvir/sofosbuvir | |||
| Genotype 1 without cirrhosis (12 weeks) | 0.99 | 0.99 (0.01) | [25] |
| Genotype 1 with cirrhosis (24 weeks) | 0.99 | 0.99 (0.01) | [27] |
| HCV Therapy Efficacy, Treatment-Experiencedc | |||
| SVR probability for 12 weeks pegylated-interferon/ribavirin/sofosbuvir | [32] | ||
| Genotype 1 without cirrhosis | 0.92 | 0.92 (0.02) | |
| Genotype 1 with cirrhosis | 0.80 | 0.78 (0.06) | |
| SVR probability for 12 or 24 weeks of sofosbuvir/ledipasvir | [22] | ||
| Genotype 1 without cirrhosis (12 weeks) | 0.95 | 0.95 (0.02) | |
| Genotype 1 with cirrhosis (24 weeks) | 0.99 | 0.97 (0.02) | |
| SVR probabilities for 12 or 24 weeks paritaprevir-ritonavir/ombitasvir/dasabuvir/ribavirin | [30, 31] | ||
| Genotype 1a without cirrhosis (12 weeks) | 0.96 | 0.96 (0.02) | |
| Genotype 1a with cirrhosis (24 weeks) | 0.95 | 0.94 (0.02) | |
| Genotype 1b without cirrhosis (12 weeks) | 0.97 | 0.96 (0.01) | |
| Genotype 1b with cirrhosis (12 weeks) | 0.97 | 0.96 (0.01) | |
| SVR probability for 12 weeks sofosbuvir/simeprevir | [24] | ||
| Genotype 1a without cirrhosis | 0.90 | 0.88 (0.10) | |
| Genotype 1a with cirrhosis | 0.91 | 0.89 (0.08) | |
| Genotype 1b without cirrhosis | 0.99 | 0.99 (0.00) | |
| Genotype 1b with cirrhosis | 0.99 | 0.97 (0.01) | |
| SVR probability for 12 weeks of sofosbuvir/ledipasvir/ribavirin | |||
| Genotype 1 with cirrhosis | 0.96 | 0.94 (0.02) | [51] |
| SVR probability for 12 or 24 weeks of daclatasvir/sofosbuvir ± ribavirin | |||
| Genotype 1 without cirrhosis (12 weeks without ribavirin) | 0.99 | 0.99 (0.01) | [25] |
| Genotype 1 with cirrhosis (24 weeks with ribavirin) | 0.98 | 0.97 (0.01) | [27] |
| Fibrosis staging (for F2+ only strategy) | [26] | ||
| FibroScan sensitivity to detect F2 or greater | 0.48 | 0.48–1 | |
| FibroScan specificity to detect F2 or greater | 0.93 | 0.93–1 | |
| Costs | |||
| Non-HCV-related medical costs, $ per month | |||
| Background medical costs (without HCV)d | $140–$1050 | $70–$1575 | [33] |
| HCV-related medical costs, $ per month | |||
| No cirrhosis (SD) | $245 ($60) | $185 ($45)–$305 ($75) | [34] |
| Mild to moderate cirrhosis (SD) | $440 ($125) | $315 ($90)–$550 ($150) | [34] |
| Decompensated cirrhosis (SD) | $830 ($215) | $620 ($160)–$1050 ($260) | [34] |
| Costs multiplier after achieving SVR | 0.50 | 0–0.70 | [34] |
| HCV Therapy Costs, $ per 4 Weeks | |||
| Provider visit costse | $120 | $60–180 | [52, 53] |
| Pegylated-interferonf | $720 | $370–$1200 | [1] |
| Ribaviring | $1200 | $560–$1700 | [1] |
| Sofosbuvir | $26500 | $13000–$40000 | [1] |
| Sofosbuvir/ledipasvir | $29000 | $15000–$43000 | [1] |
| Simeprevir | $21000 | $11000–$32000 | [1] |
| Paritaprevir-ritonavir/ombitasvir/dasabuvir | $26000 | $13000–$39000 | [1] |
| Daclatasvir | $19300 | $9500–$28500 | [1] |
| Filgrastimh | $2800 | $1500–$5100 | [1] |
| Complete HCV therapy costs, $ | [1, 52, 53] | ||
| Pegylated-interferon/ribavirin 48 weeks | $52600 | $26000–$79500 | |
| Pegylated-interferon/ribavirin/simeprevir 24 weeks | $75000 | $37500–$113000 | |
| Pegylated-interferon/ribavirin/sofosbuvir 12 weeks | $91000 | $45500–$137000 | |
| Sofosbuvir/ledipasvir 8 weeks | $58200 | $29000–$88200 | |
| Sofosbuvir/ledipasvir 12 weeks | $87300 | $47300–$107300 | |
| Sofosbuvir/ledipasvir 24 weeks | $175000 | $115000–$215000 | |
| Sofosbuvir/ledipasvir/ribavirin 12 weeks | $90600 | $31000–$112000 | |
| Paritaprevir-ritonavir/ombitasvir/dasabuvir 12 weeks | $77000 | $23000–$97000 | |
| Paritaprevir-ritonavir/ombitasvir/dasabuvir/ribavirin 12 weeks | $80300 | $30000–$100300 | |
| Paritaprevir-ritonavir/ombitasvir/dasabuvir/ribavirin 24 weeks | $161000 | $101000–$201000 | |
| Sofosbuvir/simeprevir 12 weeks | $139000 | $69500–$209000 | |
| Daclatasvir/sofosbuvir 12 weeks | $137000 | $68500–$206000 | |
| Daclatasvir/sofosbuvir 24 weeks | $274000 | $137000–$411000 | |
| Daclatasvir/sofosbuvir/ribavirin 24 weeks | $280000 | $140000–$420000 | |
| One-time costs, $ | |||
| Managing treatment-ending toxicity on interferon-containing therapy | $465–$877 | $360–$1200 | [1, 19, 20, 32, 52–55] |
| Managing treatment-ending toxicity on interferon-free therapy | $241 | $100–$610 | [29, 52–55] |
| Quality of lifei | |||
| After achieving SVR | 0.74–0.92 | 0.60–1 | [37] |
| With HCV Infection | |||
| No-to-moderate fibrosis | 0.89 | 0.75–1 | [14–16] |
| Cirrhosis | 0.62 | 0.55–0.75 | [14, 15] |
| Decompensated cirrhosis | 0.48 | 0.40–0.60 | [14, 15] |
| Receiving interferon-containing therapyj | 0.88 | 0.50–0.96 | [37, 38] |
| Receiving interferon-free therapyj | 0.99 | 0.95–1 | [38] |
| Major toxicity decrementk | 0.16 | 0.09–0.25 | [39] |
Abbreviations: F2, fibrosis stage 2; HCV, hepatitis C virus; PYs, person-years; SD, standard deviation; SVR, sustained virologic response.
(NOTE: All costs are in 2014 US dollars and discounted at an annual rate of 3%.).
Sensitivity analyses on HCV therapy efficacy were probabilistic as opposed to deterministic (see Methods). The table provides the approximate mean and SD of the beta distribution developed to reflect uncertainty in efficacy estimates.
Because HCV-attributable mortality is only applied in the model once individuals are cirrhotic, this probability is applied only to individuals who had cirrhosis before initiating treatment and subsequently attained SVR.
Efficacy estimates for patient subgroups (eg, genotype 1b treatment-naive with cirrhosis) are informed by clinical trials.
Costs varied as a function of age and sex.
Cost in first month is higher ($750).
15% of patients on pegylated-interferon/ribavirin and sofosbuvir/pegylated-interferon/ribavirin therapy receive a reduced weekly dose of 135 mcg due to nontreatment-ending neutropenia (absolute neutrophil count <750/mL but ≥500/mL) in addition to twice weekly filgrastim 300 mcg [32].
26% of patients on pegylated-interferon/ribavirin, 20% of patients pegylated-interferon/ribavirin/ sofosbuvir therapy, 23% of patients on pegylated-interferon/ribavirin/simeprevir, and 6% of patients on paritaprevir-ritonavir/ombitasvir/dasabuvir/ribavirin therapy were treated with a reduced dose of daily ribavirin in response to nontreatment-ending anemia (grade 3–4 adverse event of hemoglobin <10 g/dL) [19–21, 29].
The cost of a nurse visit ($20.40) is also included for anemia management [52].
Utility without HCV infection is a function of age. To estimate utility in a given month, the model uses a multiplicative assumption to combine HCV-related utility with age- and sex-stratified utility without HCV. For example, the utility estimate without HCV infection for a 55-year-old is 0.84. The estimated utility of living with compensated cirrhosis is 0.62. A 55-year-old with compensated cirrhosis would have a modeled utility of 0.84 × 0.62 = 0.52.
This utility weight was multiplied by an individual’s health state utility during the months that the individual received HCV therapy without major toxicity.
This utility loss was subtracted from a patient’s health state utility during the month of a major toxicity event.
Competing Causes of Death
In every month, individuals in the model are exposed to age- and sex-stratified risk of death from causes other than HCV [18]. Because this analysis focuses specifically on HCV-infected patients who are engaged in medical care and ready to initiate HCV therapy, we assume that competing risks are similar to those of the general population, and we explore that assumption in sensitivity analyses.
Hepatitis C Virus Treatment Simulation
The probability of attaining SVR to treatment is specific to regimen and patient type, and it is a function of the probabilities of withdrawing from treatment for nonadherence or toxicity and achieving SVR conditional on completing treatment (Table 2). Patients taking pegylated-interferon/ribavirin or pegylated-interferon/ribavirin/simeprevir who have inadequate virologic response at treatment week 12 discontinue treatment [19–21]. There are no early stopping criteria for any other regimen [22–25]. We did not consider patients with previous exposure to HCV polymerase inhibitors or NS5A inhibitors.
In F2+ only strategies, noncirrhotic patients are eligible to start therapy only when their fibrosis is clinically identified. We assume annual fibrosis staging with test characteristics representative of the mean sensitivity and specificity reported for the Fibroscan and Fibrosis-4 tests (Table 2) to identify moderate fibrosis [26]. Thus, some early stage patients receive therapy, whereas other advanced patients are incorrectly delayed. We conducted a sensitivity analysis in which we assumed perfect staging.
We developed efficacy parameters using data from multiple clinical trials [19–25, 27–32]. We used a Bayesian approach to develop probability density functions around each efficacy parameter value. The expected value of each distribution matched the data reported from clinical trials, whereas the variance reflected uncertainty surrounding the data based on sample size (Supplemental Tables 1 and 2).
Costs
We denominate costs in 2014 US dollars, and when necessary input costs were inflated to 2014 dollars using the consumer price index. In each simulation month, individuals accrue non-HCV-related costs based on age and sex (Table 2) [33]. Monthly costs related to HCV increase with advancing disease due to the increasing risk of liver disease sequelae such as hepatocellular carcinoma and liver transplantation as well as extrahepatic manifestations of HCV [34]. Because HCV-related costs are variable across patients, we estimate probability density functions around these costs using the gamma distribution. We then assign each simulated patient a disease stage-specific monthly HCV-related cost by random draw from those distributions (Table 2).
The costs of HCV therapy include costs of HCV medications, clinic visits, laboratory tests, and on-treatment toxicity management (Table 2, Supplemental Table 3). We assume that the cost of HCV therapies includes the medication discount available to public payers such as Medicaid plans, which we model as the average wholesale price (AWP) less 23% [1, 35]. Due to the rapid evolution in the HCV treatment market, competitive negotiation, and price discounts, we performed extensive sensitivity analyses on medication cost.
Quality-of-Life
Quality-of-life reflects the combination of 3 utility functions: (1) utility related to non-HCV comorbidities, which is a function of age [36]; 2) HCV-specific utility, which is a function of fibrosis stage [14–16]; and (3) treatment-related utility, reflecting lower QoL on interferon-containing regimens as well as major toxicity events on treatment in all regimens [37–39]. In addition, because the QoL with early stage HCV is uncertain and has a potentially large impact on the cost-effectiveness of early HCV therapy, we included a scenario in which early stage HCV is assigned a QoL weight of 0.95 [6, 14, 40]. We assumed that effects of each of these utility functions on total utility are independent and proportional.
Benefits of Sustained Virologic Response
When patients attain SVR, fibrosis progression halts, HCV-attributable costs are reduced to 50% of an individual’s disease stage-specific cost before initiating therapy [34], and QoL reverts to that of HCV-uninfected individuals of the same age and sex [37]. In individuals who were cirrhotic before attaining SVR, liver-related mortality decreases by 94% [41].
RESULTS
Cost Control by Deferring Treatment Until Fibrosis Stage 2 (F2± Only)
Treating all noncirrhotic patients with an interferon-free regimen regardless of disease stage resulted in a nondiscounted 5-year payer cost in the range of $1.02 billion to $2.14 billion per 10000 patients treated, quality adjusted life expectancy of 14.4 to 14.7 QALY per patient, and a discounted lifetime medical cost of $227000 to $329000 per patient. Strategies that limited access to patients with F2 or greater fibrosis had lower 5-year total cost, but strategies that treated all patients resulted in better quality-adjusted life expectancy and provided good value for money.
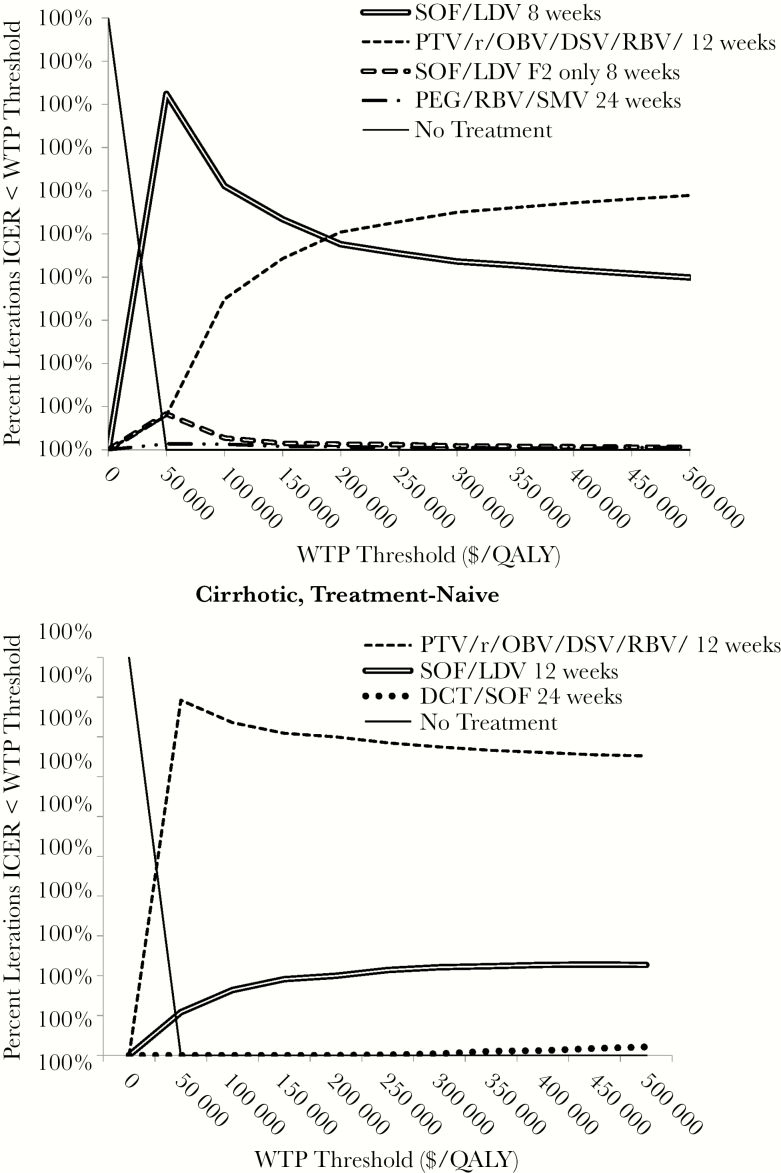
In all patient types, treat all strategies had economically attractive ICERs according to conventional benchmarks (ICER <$100000/QALY), and in many patient types treat-all provided better outcomes than F2+ only at a lower cost per QALY gained (Tables 3 and 4). These findings were robust in all age groups (mean age 42 and mean age 62) and with various assumptions about rates of fibrosis progression, mortality (including doubling the risk of death from non-HCV mortality), cost, and rates of HCV reinfection after SVR (Supplemental Tables 4–15) as well as QoL (Supplemental Figure 1). When we assumed high QoL with noncirrhotic HCV infection (0.95), the qualitative conclusions did not change (Supplemental Table 16). In probabilistic sensitivity analyses, F2+ only was almost never preferred for any patient type (Figure 1).
Table 3.
Cost Effectiveness of Treatment for Hepatitis C Virus Infection Among Genotype 1a and 1b Noncirrhotic Patients Assuming Both “Treat All” and “F2+ Only” Approaches
| Treatment Strategy | Cost, $ | Incremental Cost, $ | QALYs | Incremental QALYs | ICER, $/QALY | SVR, % | Nondiscounted Cost/10 000 Patients, Billion $ |
|---|---|---|---|---|---|---|---|
| Genotype 1a, Treatment-Naive, RNA <6 Million | |||||||
| No treatment | 165 000 | - | 11.5 | - | - | - | 0.37 |
| PEG/RBV 48 weeks | 206 000 | 40 800 | 12.8 | 1.3 | Dominateda | 43.4 | 0.84 |
| SOF/LDV 8 weeks treating only F2+ | 227 000 | 61 900 | 14.4 | 2.9 | 21 700 | 88.6 | 1.02 |
| PEG/RBV/SMV 24 weeks | 231 000 | 3800 | 13.9 | −0.4 | Dominateda | 77.3 | 1.13 |
| SOF/LDV 8 weeks | 233 000 | 5900 | 14.6 | 0.3 | 22 300 | 96.2 | 1.17 |
| PTV/r/OBV/DSV/RBV 12 weeks | 252 000 | 19 000 | 14.7 | 0.0 | 610 000 | 97.6 | 1.36 |
| PEG/RBV/SOF 12 weeks | 266 000 | 14 100 | 14.5 | −0.2 | Dominateda | 92.4 | 1.50 |
| DCV/SOF 12 weeks | 329 000 | 76 500 | 14.7 | 0.1 | 1 520 000 | 99.1 | 2.14 |
| Genotype 1a, Treatment-Naive, RNA ≥6 Million | |||||||
| No treatment | 165 000 | - | 11.5 | - | - | - | 0.37 |
| PEG/RBV 48 weeks | 206 000 | 41 000 | 12.8 | 1.3 | Dominateda | 43.6 | 0.84 |
| PEG/RBV/SMV 24 weeks | 231 000 | 24 700 | 13.9 | 1.1 | Dominateda | 77.2 | 1.13 |
| PTV/r/OBV/DSV/RBV 12 weeks treating only F2+ | 243 000 | 77 900 | 14.4 | 2.9 | 27 100 | 89.8 | 1.17 |
| PTV/r/OBV/DSV/RBV 12 weeks | 252 000 | 8900 | 14.7 | 0.3 | 32 600 | 97.5 | 1.36 |
| SOF/LDV 12 weeks | 261 000 | 8800 | 14.6 | 0.0 | Dominateda | 96.2 | 1.45 |
| PEG/RBV/SOF 12 weeks | 266 000 | 14 100 | 14.5 | −0.2 | Dominateda | 92.3 | 1.50 |
| DCV/SOF 12 weeks | 329 000 | 76 500 | 14.7 | 0.1 | 1 400 000 | 99.1 | 2.14 |
| Genotype 1a, Treatment-Experienced | |||||||
| No treatment | 156 000 | - | 11.5 | - | - | - | 0.39 |
| PTV/r/OBV/DSV/RBV 12 weeks treating only F2+ | 236 000 | 80 400 | 13.9 | 2.3 | Dominateda | 85.6 | 1.21 |
| PTV/r/OBV/DSV/RBV 12 weeks | 245 000 | 89 300 | 14.0 | 0.2 | 28 300 | 95.6 | 1.39 |
| SOF/LDV 12 weeks | 255 000 | 9500 | 14.0 | 0.0 | Dominateda | 94.7 | 1.48 |
| PEG/RBV/SOF 12 weeks | 258 000 | 13 000 | 13.9 | −0.1 | Dominateda | 92.3 | 1.52 |
| DCV/SOF 12 weeks | 320 000 | 74 600 | 14.1 | 0.1 | 700 000 | 98.9 | 2.15 |
| SMV/SOF 12 weeks | 321 000 | 1500 | 13.9 | −0.3 | Dominateda | 88.4 | 2.15 |
| Genotype 1b, Treatment-Naive, RNA <6 Million | |||||||
| No treatment | 165 000 | - | 11.5 | - | - | - | 0.37 |
| PEG/RBV 48 weeks | 206 000 | 40 700 | 12.8 | 1.3 | Dominateda | 43.5 | 0.84 |
| SOF/LDV 8 weeks treating only F2+ | 227 000 | 61 900 | 14.4 | 2.9 | 21 700 | 88.5 | 1.02 |
| PEG/RBV/SMV 24 weeks | 232 000 | 5400 | 14.3 | −0.1 | Dominateda | 87.9 | 1.16 |
| SOF/LDV 8 weeks | 233 000 | 5900 | 14.6 | 0.3 | 22 700 | 96.1 | 1.17 |
| PTV/r/OBV/DSV 12 weeks | 248 000 | 14 900 | 14.7 | 0.1 | 182 000 | 98.4 | 1.32 |
| PEG/RBV/SOF 12 weeks | 266 000 | 18 400 | 14.5 | −0.2 | Dominateda | 92.4 | 1.50 |
| DCV/SOF 12 weeks | 329 000 | 80 800 | 14.7 | 0.0 | 4 190 000 | 99.1 | 2.14 |
| Genotype 1b, Treatment-Naive, RNA ≥6 Million | |||||||
| No treatment | 165 000 | - | 11.5 | - | - | - | 0.37 |
| PEG/RBV 48 weeks | 206 000 | 41 100 | 12.8 | 1.3 | Dominateda | 43.5 | 0.84 |
| PEG/RBV/SMV 24 weeks | 232 000 | 67 200 | 14.3 | 2.8 | 24 400 | 87.8 | 1.16 |
| PTV/r/OBV/DSV 12 weeks treating only F2+ | 239 000 | 6800 | 14.4 | 0.2 | Dominateda | 90.7 | 1.13 |
| PTV/r/OBV/DSV/RBV 12 weeks | 248 000 | 15 200 | 14.7 | 0.4 | 35 500 | 98.4 | 1.32 |
| SOF/LDV 12 weeks | 261 000 | 13 200 | 14.6 | −0.1 | Dominateda | 96.3 | 1.45 |
| PEG/RBV/SOF 12 weeks | 266 000 | 18 400 | 14.5 | −0.2 | Dominateda | 92.4 | 1.50 |
| DCV/SOF 12 weeks | 329 000 | 80 800 | 14.7 | 0.0 | 4 470 000 | 99.1 | 2.14 |
| Genotype 1b, Treatment-Experienced | |||||||
| No treatment | 156 000 | - | 10.8 | - | - | - | 0.39 |
| PTV/r/OBV/DSV/RBV 12 weeks treating only F2+ | 236 000 | 80 200 | 13.7 | 2.8 | Dominateda | 86.1 | 1.21 |
| PTV/r/OBV/DSV/RBV 12 weeks | 245 000 | 89 100 | 14.0 | 3.2 | 28 000 | 96.2 | 1.39 |
| SOF/LDV 12 weeks | 255 000 | 9800 | 14.0 | 0.0 | Dominateda | 94.7 | 1.48 |
| PEG/RBV/SOF 12 weeks | 258 000 | 13 200 | 13.9 | −0.2 | Dominateda | 92.3 | 1.52 |
| DCV/SOF 12 weeks | 320 000 | 75 000 | 14.1 | 0.1 | 814 000 | 99.0 | 2.15 |
| SMV/SOF 12 weeks | 324 000 | 3700 | 14.1 | 0.0 | Dominateda | 98.7 | 2.19 |
Abbreviations: DCV, daclatasvir; DSV, dasabuvir; F2, fibrosis stage F2; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; LDV, ledipasvir; OBV, ombitasvir; PEG, pegylated-interferon; PTV, paritaprevir; QALY, quality-adjusted life year; r, ritonavir; RBV, ribavirin; RNA, ribonucleic acid; SMV, simeprevir; SOF, sofosbuvir; SVR, sustained virologic response.
(NOTE: All values are mean per-person values based on Monte-Carlo simulations of 1 000 000 individuals. Nondiscounted cost per 10000 patients is calculated over 5 years. Cost-effectiveness ratios may not match previous columns due to rounding.)
Dominated = strategies more costly and less effective than a competing strategy or strategies with an ICER greater than that of a more effective strategy.
Table 4.
Cost-Effectiveness of Treatment for Hepatitis C Virus Infection Among Genotype 1a and 1b Cirrhotic Patients
| Treatment Strategy | Cost, $ | Incremental Cost, $ | QALYs | Incremental QALYs | ICER, $/QALY | SVR, % | Nondiscounted Cost/10 000 Patients, Billion $ |
|---|---|---|---|---|---|---|---|
| Genotype 1a, Treatment-Naive | |||||||
| No treatment | 99 000 | - | 4.9 | - | - | - | 0.44 |
| PEG/RBV 48 weeks | 158 000 | 59 600 | 7.0 | 2.1 | Dominateda | 23.5 | 0.91 |
| PEG/RBV/SMV 24 weeks | 197 000 | 38 200 | 9.9 | 2.9 | Dominateda | 53.6 | 1.12 |
| PTV/r/OBV/DSV/RBV 12 weeks | 245 000 | 146 000 | 13.7 | 8.9 | 16 500 | 92.7 | 1.36 |
| PEG/RBV/SOF 12 weeks | 252 000 | 6900 | 12.3 | −1.4 | Dominateda | 78.6 | 1.52 |
| SOF/LDV 12 weeks | 255 000 | 10 000 | 14.0 | 0.2 | 43 000 | 95.1 | 1.45 |
| DCV/SOF 24 weeks | 453 000 | 198 000 | 14.1 | 0.1 | 2 370 000 | 96.9 | 3.46 |
| Genotype 1a, Treatment-Experienced | |||||||
| No treatment | 99 000 | - | 4.7 | - | - | - | 0.47 |
| PEG/RBV/SOF 12 weeks | 246 000 | 147 000 | 11.5 | 6.8 | Dominateda | 78.3 | 1.54 |
| SOF/LDV/RBV 12 weeks | 253 000 | 154 000 | 12.9 | 8.2 | 18 700 | 94.2 | 1.53 |
| PTV/r/OBV/DSV/RBV 24 weeks | 315 000 | 62 100 | 12.8 | −0.1 | Dominateda | 94.0 | 2.17 |
| SMV/SOF 12 weeks | 317 000 | 64 500 | 12.5 | −0.4 | Dominateda | 90.0 | 2.21 |
| SOF/LDV 24 weeks | 334 000 | 81 000 | 13.0 | 0.2 | 520 000 | 96.8 | 2.34 |
| DCV/SOF/RBV 24 weeks | 453 000 | 119 000 | 13.0 | 0.0 | Dominateda | 96.7 | 3.56 |
| Genotype 1b, Treatment-Naive | |||||||
| No treatment | 99 000 | - | 4.9 | - | - | - | 0.44 |
| PEG/RBV 48 weeks | 158 000 | 59 400 | 7.0 | 2.10 | Dominateda | 23.1 | 0.91 |
| PEG/RBV/SMV 24 weeks | 205 000 | 46 800 | 10.8 | 3.80 | Dominateda | 62.4 | 1.15 |
| PTV/r/OBV/DSV/RBV 12 weeks | 248 000 | 149 000 | 14.2 | 9.27 | 16 100 | 97.0 | 1.37 |
| PEG/RBV/SOF 12 weeks | 252 000 | 4000 | 12.4 | −1.79 | Dominateda | 78.5 | 1.52 |
| SOF/LDV 12 weeks | 255 000 | 7100 | 14.0 | −0.17 | Dominateda | 95.3 | 1.45 |
| DCV/SOF 24 weeks | 453 000 | 205 000 | 14.1 | −0.10 | Dominateda | 96.9 | 3.46 |
| Genotype 1b, Treatment-Experienced | |||||||
| No treatment | 98 000 | - | 4.6 | - | - | - | 0.46 |
| PTV/r/OBV/DSV/RBV 12 weeks | 239 000 | 141 000 | 13.0 | 8.40 | 16 800 | 96.3 | 1.38 |
| PEG/RBV/SOF 12 weeks | 245 000 | 5800 | 11.4 | −1.59 | Dominateda | 78.3 | 1.54 |
| SOF/LDV/RBV 12 weeks | 252 000 | 12 600 | 12.8 | −0.21 | Dominateda | 94.0 | 1.52 |
| SMV/SOF 12 weeks | 319 000 | 79 700 | 13.1 | 0.10 | 828 000 | 97.4 | 2.19 |
| SOF/LDV 24 weeks | 332 000 | 13 500 | 13.0 | −0.16 | Dominateda | 96.4 | 2.34 |
| DCV/SOF/RBV 24 weeks | 451 000 | 132 000 | 12.9 | −0.18 | Dominateda | 96.2 | 3.56 |
Abbreviations: DCV, daclatasvir; DSV, dasabuvir; F2, fibrosis stage F2; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; LDV, ledipasvir; OBV, ombitasvir; PEG, pegylated-interferon; PTV, paritaprevir; QALY, quality-adjusted life year; r, ritonavir; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir; SVR, sustained virologic response.
(NOTE: All values are mean per-person values based on Monte-Carlo simulations of 1000000 individuals. Nondiscounted cost per 10000 patients is calculated over 5 years. Cost-effectiveness ratios may not match previous columns due to rounding.)
Dominated = strategy is more costly and less effective than a competing strategy or strategies with an ICER greater than that of a more effective strategy.
Figure 1.
Cost-effectiveness acceptability curves for the treatment of hepatitis C virus (HCV) genotype 1b patients with and without cirrhosis. Each panel presents the results of probabilistic sensitivity analyses in which we performed multiple iterations of the cost-effectiveness simulation, each time drawing treatment efficacy parameters from defined probability density functions. The horizontal axis represents increasing societal willingness to pay thresholds. Each line represents a treatment strategy. For clarity, we excluded those strategies where the incremental cost-effectiveness ratio (ICER) was above a willingness-to-pay (WTP) threshold of $500 000 in >99% of iterations. The vertical axis depicts the percentage of the simulation iterations in which a given strategy was “preferred” from a cost-effectiveness perspective at a given societal willingness to pay. All costs are in 2014 US dollars and discounted at an annual rate of 3%. DCV, daclatasvir; DSV, dasabuvir; LDV, ledipasvir; OBV, ombitasvir; PTV, paritaprevir; QALY, quality-adjusted life year; r, ritonavir; RBV, ribavirin; SMV, simeprevir; SOF, sofosbuvir.
The F2+ only approach was not preferred for several reasons. First, patients had lower QoL as liver fibrosis advances, and therefore they lost QALYs while waiting for therapy. In sensitivity analyses, treat-all strategies were preferred unless the utility weight of early stage HCV infection was >0.97 (base case 0.89) (Supplemental Figure 1). Second, most patients eventually reached F2 and were ultimately treated for HCV. For example, among GT1a, treatment-naive, noncirrhotic patients, 92% were ultimately treated for HCV. In sensitivity analysis with slower fibrosis progression rates (median time to cirrhosis = 40 years), treat all remained preferred. Only when the discount rate was greater than 10% (base case 3% in accordance with current guidelines for economic analysis) did F2+ only strategies begin to have ICERs <$100000/QALY. Third, noninvasive fibrosis staging modalities are imperfect [26], and some patients with advanced fibrosis were inappropriately deferred. Eliminating uncertainty in fibrosis staging improved outcomes and decreased the ICER of F2+ only, but the ICER of treat all remained attractive (<$100000/QALY) (Supplemental Table 17).
Cost Control by Negotiating Price Discounts and Requiring Use of Preferred Drugs
Noncirrhotic Patients
Among noncirrhotic patients, genotypes 1a and 1b, the choice of which interferon-free regimen to use depended on regimen cost. For example, among GT 1a and 1b treatment-naive, noncirrhotic patients with HCV RNA <6 million copies/mL, for whom sofosbuvir/ledipasvir is an 8-week regimen, sofosbuvir/ledipasvir dominated interferon-containing regimens, and the ICER of sofosbuvir/ledipasvir compared with “no treatment” was $21700/QALY. In such patients, paritaprevir/ritonavir/ombitasvir/dasabuvir ± ribavirin was estimated to be slightly more effective than sofosbuvir/ledipasvir (97%–98% SVR vs 96% SVR), but because the 12-week paritaprevir/ritonavir/ombitasvir/dasabuvir ± ribavirin treatment course greatly increased cost relative to 8 weeks of sofosbuvir/ledipasvir, the ICER of paritaprevir/ritonavir/ombitasvir/dasabuvir ± ribavirin among treatment-naive patients with RNA <6 million copies/mL was more than $600 000/QALY gained. Likewise, in GT1a and GT1b noncirrhotic patients, sofosbuvir/daclatasvir had the highest modeled treatment efficacy, but because of its high cost, it was never preferred for noncirrhotic patients.
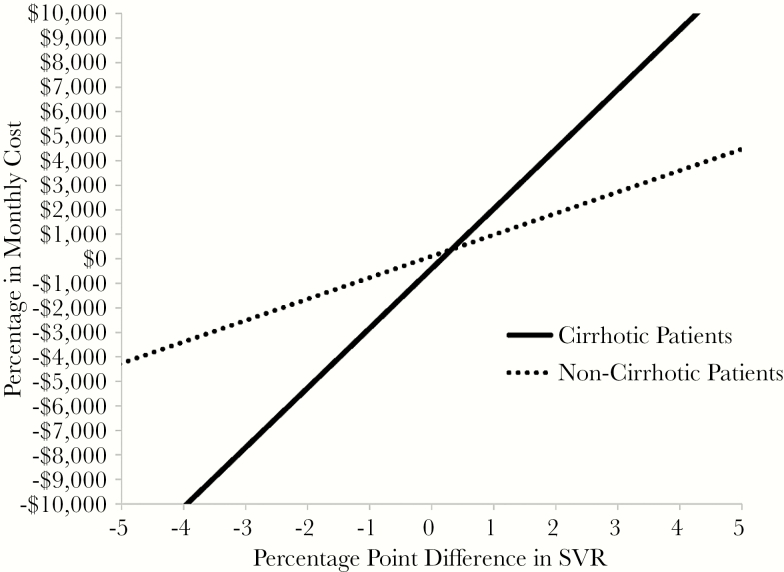
In two-way sensitivity analyses among noncirrhotic patients, each percentage point improvement in interferon-free regimen efficacy could support an additional cost of only $875 per month of treatment ($2625 for a 12-week course). Larger cost increases resulted in the more effective regimen having an ICER >$100000/QALY gained compared with the less effective regimen (Figure 2).
Figure 2.
Two-way sensitivity analysis on interferon-free regimen efficacy and cost. The analysis holds the efficacy and cost of 1 interferon-free treatment (“regimen A”) constant, while varying the efficacy and cost of a competing interferon-free regimen (“regimen B”). To improve generalizability such that the analysis applies to future interferon-free treatment options, we defined the ranges of drug cost and efficacy based on those of current competing drugs, but the analysis is not based on a single regimen. The horizontal axis depicts the relative efficacy of regimen B compared with regimen A. The vertical axis depicts the relative cost. Each line depicts the threshold cost that results in regimen B having an incremental cost-effectiveness ratio (ICER) <$100 000/quality-adjusted life year compared with regimen A at the given relative efficacy. The slope of the line thus represents the economic value of an additional percentage point increase in treatment efficacy. The solid line represents cirrhotic patients, and the dotted line represents noncirrhotic patients. All costs are in 2014 US dollars and discounted at an annual rate of 3%. SVR, sustained virologic response.
In probabilistic sensitivity analyses, interferon-free therapy had an ICER <$100000/QALY in >99% of simulations, with the least costly interferon-free regimen always preferred when the threshold for willingness to pay for each year of healthy life was $100000/QALY gained (Supplemental Figure 2).
Cirrhotic Patients
Among cirrhotic patients, genotype 1a and 1b, regimen efficacy played a larger role in determining the choice of which interferon-free regimen was cost effective. In two-way sensitivity analyses, each additional percentage point of regimen efficacy could be associated with up to a $2400 increase in monthly regimen cost ($7200 for a 12-week treatment course) for the more effective regimen to remain cost effective with an ICER <$100000/QALY (Figure 1). Even among cirrhotic patients, however, very small differences in treatment efficacy did not provide adequate improvement in outcomes to justify the very high cost of some regimens. For example, sofosbuvir/daclatasvir, which was the most effective regimen for many cirrhotic patients, had a very high ICER due to its high cost. In probabilistic sensitivity analyses, interferon-free therapy had an ICER <$100000/QALY in >99% of simulations, and the more effective interferon-free regimen was preferred in >75% of simulations (Supplemental Figure 3).
DISCUSSION
Our analysis demonstrates that interferon-free therapies to treat HCV GT1 provide good value for the resources required to use them broadly in the United States, but they are costly for payers. Cost-control strategies in which noncirrhotic patients are eligible for treatment only when they reach F2 or greater fibrosis do limit cost; however, treating all patients regardless of fibrosis stage results in longer quality-adjusted life-expectancy (between 0.2 and 0.3 QALYs) and is cost effective. If seeking rational cost control, payers should focus on price negotiations rather than treatment restrictions. At this time, with multiple interferon-free treatment options available, there are HCV treatment regimens that remain appealing to providers, but they are not cost effective. In treatment-naive, noncirrhotic patients with low viral load, for example, who are eligible for an 8-week course with sofosbuvir/ledipasvir, choosing a 12-week regimen in hope of gaining treatment efficacy is not cost effective. It will be possible to attain better population-level outcomes by negotiating best prices and treating the greatest number possible, even if the preferred first-line regimen is somewhat less effective than another option.
Our work should be read in context of a growing body of literature. We independently confirm that (1) interferon-free options are cost effective [2–4] and that (2) treating early stage disease is preferred to F2+ only approaches [5, 6]. The analysis extends those findings by determining the value of small improvements in efficacy in treatment-naive patients. We find that among competing regimens with efficacy >90%, cost should be the primary driver of formulary decisions. Negotiating prices and limiting formulary for noncirrhotic patients is a better approach to cost control than current disease stage treatment restrictions. To our knowledge, this paper provides one of the first quantitative comparisons of cost-control options.
This analysis has several limitations. First, our finding that F2+ only strategies are not preferred depends in part on assumptions about QoL with early stage HCV. Utility values are difficult to estimate and are imprecise. In sensitivity analyses, however, we identified the threshold utility value that results in treat F2+ only becoming preferred. We note that the threshold value (0.97) is substantially higher than base case (0.89) and higher than most reports in the literature [14]. Readers can use these results to calibrate conclusions with any emerging data about QoL. Second, similar to other cost-effectiveness analyses [2–4], our efficacy inputs are based on clinical trials and not real-world effectiveness. For instance, if twice-daily regimens are less effective than indicated in efficacy trials, whereas single tablet, once daily regimens are similar, then costly once daily regimens could appear to be economically attractive. However, our sensitivity analyses demonstrate that among noncirrhotic patients, the gap in treatment effectiveness would need to be large to justify the substantially higher price: for example, a large gap of 5% better effectiveness would only support $4000 in increased price. In addition, we also use the clinical trials to inform model demographics, which may be different from the general HCV population, although our conclusions do not change in our sensitivity analyses varying age, mortality, and QoL (all of which may differ with demographics) (Supplemental Tables 4, 5, 13, 14). Third, because knowledge of the optimal combination of available medications to treat HCV is evolving, this analysis excludes treatment regimens recently approved. For instance, we did not include recommended regimes of elbasvir/grazoprevir or sofosbuvir/velpatasvir [42]; however, this approach is unlikely to be significantly different from other highly efficacious regiments. Likewise, our analysis was completed shortly before the US Food and Drug Administration issued a “black box warning” about the use of paritaprevir/ritonavir/ombitasvir/dasabuvir in patients with advanced liver disease [43]. Nevertheless, we demonstrate that tradeoffs between price and efficacy will be relevant to considering the cost effectiveness of these future regimens compared with the current standard of care. Fourth, because discounts negotiated with pharmaceutical companies typically include a nondisclosure agreement, it is not possible to know what each payer pays for HCV medications. In the base case, we assumed an average discount of 23% off of AWP, and we explored that cost in extensive sensitivity analysis (Supplemental Tables 11–12). Fifth, although we account for increased costs and mortality and decreased utility of advancing liver disease, we did not model specific manifestations of this such as hepatocellular carcinoma, liver transplantation, or other extrahepatic consequences of HCV. Modeling these effects separately would not change our overall results, but it may be beneficial for stakeholders who want to find another way to quantify the effect of treatment on health outcomes. Sixth, although we assumed that fibrosis progression halts after SVR, some studies suggest that progression may continue at a much slower rate or that fibrosis would regress instead. In sensitivity analysis where patients were subject to lower QoL after SVR (if progression continued, for example), our conclusions were unchanged, although the ICERs increased. The effect of fibrosis regression is not well understood in the literature, although if present we would expect treatment to become more attractive and for ICERs to fall given the additional benefit of regression. In addition, our model assumed a linear progression through fibrosis stages with a 25-year median time to cirrhosis among the cohort. More recent literature suggests that fibrosis progression is not linear, with faster progression through early stages of disease [44]. However, the main driver of cost-effectiveness conclusions in the model was the time to becoming cirrhotic. We experimented with a broad array of median times to cirrhosis, and we found that our conclusions are consistent. Seventh, our analysis assumed a healthcare system perspective that included the costs of medical care (HCV and non-HCV), but it did not include costs related to lost labor productivity and disability. Were we to incorporate those costs in the model, the net cost of HCV therapy would be less, although it is difficult to imagine a scenario in which preventing disability provides so much benefit as to entirely offset the high cost of treatment. Finally, we assumed that competing risks of death for HCV-infected patients initiating therapy are similar to those of the general US population. If history of substance use or other risk factors that led to HCV infection also have an impact on long-term mortality, then the cost-effectiveness ratios of treatment may be higher than those reported.
CONCLUSIONS
New therapies to treat HCV GT1 have changed the paradigm of HCV care from chronic, moderately effective disease management to short-term, curative therapy [45]. Our analysis demonstrates that expanding treatment access, including to patients with early stage disease, will improve clinical outcomes and is cost effective, but it will have a very high cost. Currently, many payers restrict access to HCV therapy based on fibrosis stage [9, 46], and although some have been forced to relax restrictions through litigation [47], these policy changes are occurring without clear evidence of the right cost-control measures and without knowledge of the long-term consequences of these policies on human health and local budgets. We show that such strategies are not the best approach to control spending on HCV treatment from the perspective of the health system. We also find that lower drug costs for interferon-free regimens to treat noncirrhotic patients in a competitive drug market will improve cost effectiveness and reduce short-term payer costs, suggesting that payers should focus on price negotiations as a cost-control strategy. Now that there are multiple effective HCV regimens and there is greater competition in this market, it is important to expand efforts to identify and treat patients with chronic HCV infection in the United States.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Financial support. This work was funded by the National Institute on Drug Abuse and National Institute of Allergy and Infectious Diseases (Grants P30A1042853, R01DA031059, and P30DA040500; to B. P. L. and J. M.). K. A. F. received a National Institutes of Health grant (to Massachusetts General Hospital).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Micromedex 2.0. Drug Topics Red Book Online. Avalable at: http://www.micromedexsolutions.com Accessed 22 December 2015
- 2. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med 2015; 162:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med 2015; 162:407–19. [DOI] [PubMed] [Google Scholar]
- 4. Rein DB, Wittenborn JS, Smith BD, et al. The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis 2015; 61:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leidner AJ, Chesson HW, Xu F, et al. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology 2015; 61:1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chahal HS, Marseille EA, Tice JA, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med 2016; 176:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014; 160:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. New York State Department of Health. New York State Medicaid Drug Utilization Review Meeting February 26, 2015. Available at: http://www.health.ny.gov/health_care/medicaid/program/dur/meetings/2015/02/sum_0226_15_durbs.pdf Accessed 22 December 2015
- 9. Barua S, Greenwald R, Grebely J, et al. Restrictions for medicaid reimbursement of Sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 10. Linas BP, Barter DM, Leff JA, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One 2014; 9:e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linas BP, Barter DM, Morgan JR, et al. The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C genotypes 2 and 3. Ann Intern Med 2015; 162:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 13. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014; 17:5–14. [DOI] [PubMed] [Google Scholar]
- 14. Chong CA, Gulamhussein A, Heathcote EJ, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003; 98:630–8. [DOI] [PubMed] [Google Scholar]
- 15. Grieve R, Roberts J, Wright M, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 2006; 55:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stein K, Dalziel K, Walker A, et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess 2002; 6:1–122. [PubMed] [Google Scholar]
- 17. Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol 2009; 104:1147–58. [DOI] [PubMed] [Google Scholar]
- 18. Arias E. United States life tables, 2008. Natl Vital Stat Rep 2012; 61:1–63. [PubMed] [Google Scholar]
- 19. McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361:580–93. [DOI] [PubMed] [Google Scholar]
- 20. Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2014; 384:403–13. [DOI] [PubMed] [Google Scholar]
- 21. Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2014; 384:414–26. [DOI] [PubMed] [Google Scholar]
- 22. Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–93. [DOI] [PubMed] [Google Scholar]
- 23. Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 24. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014; 384:1756–65. [DOI] [PubMed] [Google Scholar]
- 25. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370:211–21. [DOI] [PubMed] [Google Scholar]
- 26. Poynard T, de Ledinghen V, Zarski JP, et al. Relative performances of FibroTest, Fibroscan, and biopsy for the assessment of the stage of liver fibrosis in patients with chronic hepatitis C: a step toward the truth in the absence of a gold standard. J Hepatol 2012; 56:541–8. [DOI] [PubMed] [Google Scholar]
- 27. Welzel TM, Herzer K, Ferenci P, et al. Daclatasvir plus sofosbuvir with or without ribavirin for the treatment of HCV in patients with severe liver disease: interim results of a multicenter compassionate use program. European Association for the Study of the Liver; Vienna, Austria: April 22–26, 2015. [Google Scholar]
- 28. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–88. [DOI] [PubMed] [Google Scholar]
- 29. Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014; 370:1983–92. [DOI] [PubMed] [Google Scholar]
- 30. Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370:1604–14. [DOI] [PubMed] [Google Scholar]
- 31. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370:1973–82. [DOI] [PubMed] [Google Scholar]
- 32. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–87. [DOI] [PubMed] [Google Scholar]
- 33. Agency for Healthcare Research and Quality. Total health services-mean and median expenses per person with expense and distribution of expenses by source of payment: medical expenditure panel survey household component data. Generated interactively. Available at: http://meps.ahrq.gov/mepsweb/ Accessed 15 July 2014
- 34. Davis KL, Mitra D, Medjedovic J, et al. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol 2011; 45:e17–24. [DOI] [PubMed] [Google Scholar]
- 35. Levinson D. Medicaid drug price comparisons: average manufacturer price to published prices 2005. Available at: http://oig.hhs.gov/oei/reports/oei-05-05-00240.pdf Accessed 22 December 2015
- 36. Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006; 26:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sherman K, Muir A, Aggarwal J, et al. 904 health-related quality-of-life among Genotype 1 treatment-experienced chronic hepatitis C patients: post-hoc analyses from the Realize Study. J Hepatol 2013; 58:S372. [Google Scholar]
- 38. Stepanova M, Nader F, Cure S, et al. Patients’ preferences and health utility assessment with SF-6D and EQ-5D in patients with chronic hepatitis C treated with sofosbuvir regimens. Aliment Pharmacol Ther 2014; 40:676–85. [DOI] [PubMed] [Google Scholar]
- 39. Schackman BR, Teixeira PA, Weitzman G, et al. Quality-of-life tradeoffs for hepatitis C treatment: do patients and providers agree? Med Decis Making 2008; 28:233–42. [DOI] [PubMed] [Google Scholar]
- 40. Townsend R, McEwan P, Kim R, Yuan Y. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health 2011; 14:1068–77. [DOI] [PubMed] [Google Scholar]
- 41. van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584–93. [DOI] [PubMed] [Google Scholar]
- 42. AASLD/IDSA/IAS–USA. Recommendations for testing, managing, and treating hepatitis C. Available at: www.hcvguidelines.org Accessed 22 October 2016
- 43. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm468634.htm Accessed 16 December 2015
- 44. Zeremski M, Dimova RB, Pillardy J, et al. Fibrosis progression in patients with chronic hepatitis C virus infection. J Infect Dis 2016; 214:1164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C–the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther 2015; 41:497–520. [DOI] [PubMed] [Google Scholar]
- 46. National Viral Hepatitis Roundtable, Center for Health Law & Policy Innovation. Hepatitis C: The State of Medicaid Access. Available at: http://www.chlpi.org/wp-content/uploads/2013/12/HCV-Report-Card-National-Summary_FINAL.pdf Accessed 29 November 2016
- 47. Ampel C. United Health expands hepatitis C drug coverage to settle national class action. Available at: http://www.dailybusinessreview.com/id=1202767146286/Insurer-Expands-Hepatitis-Cure-Coverage-to-Settle-Nationwide-Class-Action?slreturn=20160821164916 Accessed 5 September 2016
- 48. Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418–31. [DOI] [PubMed] [Google Scholar]
- 49. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–32. [DOI] [PubMed] [Google Scholar]
- 50. Gilead Sciences. Havoni Package Insert. Availble at: http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf Accessed 11 March 2015
- 51. Reddy KR, Bourlière M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 2015; 62:79–86. [DOI] [PubMed] [Google Scholar]
- 52. United States Department of Health and Human Services Center for Medicare Services. Physician Fee Schedule. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSched Accessed 22 December 2015
- 53. United States Department of Health and Human Services Center for Medicare Services. Clinical Diagnostic Laboratory Fee Schedule. Available at: http://www.cms.gov/ClinicalLabFeesched/ Accessed 22 December 2015
- 54. Chaiwat O, Lang JD, Vavilala MS, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology 2009; 110:351–60. [DOI] [PubMed] [Google Scholar]
- 55. Gao X, Stephens JM, Carter JA, et al. Impact of adverse events on costs and quality of life in protease inhibitor-based combination therapy for hepatitis C. Expert Rev Pharmacoecon Outcomes Res 2012; 12:335–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.