Abstract
Background
Specimens collected after antibiotic exposure may reduce culture-based bacterial detections. The impact on culture-independent diagnostic tests is unclear. We assessed the effect of antibiotic exposure on both of these test results among patients hospitalized with community-acquired pneumonia (CAP).
Methods
Culture-based bacterial testing included blood cultures and high-quality sputum or endotracheal tube (ET) aspirates; culture-independent testing included urinary antigen testing (adults) for Streptococcus pneumoniae and Legionella pneumophila and polymerase chain reaction (PCR) on nasopharyngeal and oropharyngeal (NP/OP) swabs for Mycoplasma pneumoniae and Chlamydia pneumoniae. The proportion of bacterial detections was compared between specimens collected before and after either any antibiotic exposure (prehospital and/or inpatient) or only prehospital antibiotics and increasing time after initiation of inpatient antibiotics.
Results
Of 4678 CAP patients, 4383 (94%) received antibiotics: 3712 (85%) only inpatient, 642 (15%) both inpatient and prehospital, and 29 (<1%) only prehospital. There were more bacterial detections in specimens collected before antibiotics for blood cultures (5.2% vs 2.6%; P < .01) and sputum/ET cultures (50.0% vs 26.8%; P < .01) but not urine antigen (7.0% vs 5.7%; P = .53) or NP/OP PCR (6.7% vs 5.4%; P = .31). For all diagnostic testing, bacterial detections declined with increasing time between inpatient antibiotic administration and specimen collection.
Conclusions
Bacteria were less frequently detected in culture-based tests collected after antibiotics and in culture-independent tests that had longer intervals between antibiotic exposure and specimen collection. Bacterial yield could improve if specimens were collected promptly, preferably before antibiotics, providing data for improved antibiotic selection.
Keywords: antibiotic stewardship, antibiotic use, bacterial disease, Pneumonia, pneumonia diagnostics
Community-acquired pneumonia (CAP) is an important infectious cause of morbidity and mortality in the United States [1–3]. Historically, Streptococcus pneumoniae has been reported as the leading bacterial cause of CAP. However, since the widespread implementation of pediatric pneumococcal conjugate vaccination in the United States, hospitalizations due to pneumococcal pneumonia have declined in both children and adults [1]. With advances in respiratory virus molecular diagnostics, viruses are more commonly detected than bacteria among patients hospitalized with CAP [4, 5].
The 2007 Infectious Diseases Society of America/American Thoracic Society adult CAP guidelines recommended the routine use of urinary antigen testing for Legionella pneumophila serogroup 1 and S pneumoniae in addition to blood and sputum cultures in adults hospitalized with severe CAP [2]. The guidelines also recommended starting empiric antibiotic therapy for patients hospitalized with CAP [2]. Empiric antibiotic therapy and a prior Centers for Medicare and Medicaid Services CAP performance standard that mandated the initiation of antibiotic therapy within 6 hours of registration in emergency departments may have de-emphasized diagnostic testing for hospitalized CAP patients [6].
It is generally recommended to start empiric antibiotic therapy for CAP as soon as possible after diagnosis and collect samples for etiology determinations before initiation of therapy. However, data evaluating the impact of prior antibiotic use on bacterial detections in both culture-based and culture-independent diagnostic tests among CAP patients are limited. Some studies indicate that prior antibiotic exposure leads to reduced bacterial detections in patients for culture-based diagnostic tests [7, 8]. The low sensitivity of culture-based diagnostic tests compounds this problem [9]. Other studies suggest that the sensitivity of culture-independent diagnostic tests (eg, pneumococcal and L pneumophila serogroup 1 urine antigen testing or molecular detection) is not influenced by antibiotic exposure [10, 11].
The Etiology of Pneumonia in the Community (EPIC) study, a large, multicenter, active, population-based surveillance study of hospitalized patients with CAP, systematically captured results of diagnostic testing, antibiotic prescribing, and specimen collection for each patient [4, 5]. We evaluated the influence of antibiotics on both culture-based and culture-independent bacterial diagnostic test results among hospitalized patients with CAP enrolled in the EPIC study.
PATIENTS AND METHODS
The Etiology of Pneumonia in the Community Study
From January 1, 2010 to June 30, 2012, patients hospitalized with clinical and radiographically confirmed CAP were enrolled into the EPIC study [4, 5]. Children <18 years of age were enrolled at 3 hospitals, 1 each in Memphis, Nashville, and Salt Lake City. Adults ≥18 years of age were enrolled at 5 hospitals, 3 in Chicago and 2 in Nashville. Patients were interviewed, medical charts were abstracted, and blood, urine, and respiratory specimens were obtained. Only specimens obtained within 72 hours after admission were included in the analysis. The study protocol was approved by the institutional review board at each institution and the Centers for Disease Control and Prevention.
Culture-Based Diagnostic Tests
Blood for culture was collected from children and adults. Contaminants were previously defined [4, 5]. Endotracheal (ET) aspirates were collected in children and adults only per clinical care. Expectorated sputum specimens were collected for bacterial culture only in adults with productive cough. The pediatric site in Memphis also cultured induced sputum specimens via inhalation of albuterol followed by 7% saline to induce deep cough [12]. Suctioning through the nose or mouth was used in children who were too young to expectorate. For sputum specimens (both expectorated and induced) and ET aspirates, only high-quality specimens were included in the analysis, defined as follows: ≤10 epithelial cells and ≥25 white blood cells/low-power field. Because there were few ET aspirate cultures performed, we combined high-quality sputum and ET aspirate culture results in the analysis. There were only 3 pleural fluid and 4 bronchoalveolar-lavage specimens obtained before antibiotic administration so they were excluded from the analysis.
Culture-Independent Diagnostic Tests
Trained staff obtained nasopharyngeal and oropharyngeal (NP/OP) swabs for real-time polymerase chain reaction (PCR) assays to detect Chlamydia pneumoniae and Mycoplasma pneumoniae. Urine specimens from adult CAP patients were tested for S pneumoniae and L pneumophila serogroup 1 antigens using BinaxNOW. For each specimen obtained, date and time of collection were recorded.
Antibiotic Exposure Assessment
Any patient who self-reported antibiotic use during the 5 days before hospitalization was considered to have prehospital antibiotic exposure. Inpatient antibiotic exposure, including date and time of administration of the first inpatient antibiotic, was abstracted from the medical charts. For analyses involving C pneumoniae and M pneumoniae, antibiotics were grouped into classes with (macrolide or fluoroquinolone) and without (β-lactams, clindamycin, other) activity against these atypical bacteria. Specimens with no date or time documented for antibiotic administration or specimen collection were excluded.
Analytic Approach
First, we determined the number of patients who received antibiotics in inpatient, prehospital, or both settings, and we compared the proportion of bacterial detections among specimens collected before and after either inpatient, prehospital, or any (inpatient and/or prehospital) antibiotic exposure for each diagnostic test. Second, to assess the influence of prehospital antibiotic exposure, we excluded specimens that were collected after inpatient antibiotic exposure and compared the proportion of detections among specimens collected with and without prehospital antibiotic exposure. Third, to examine the effect of time after first antibiotic exposure on detections, we evaluated diagnostic yield at different time points. We excluded specimens with prehospital antibiotic exposure and divided exposure time into quartiles and compared the proportion of bacterial detections in each quartile to the proportion in specimens with no antibiotic exposure. The χ2 or Fisher’s exact tests were used for comparisons as appropriate. The Cochran-Armitage trend test was used to compare the proportion of bacterial detections over time. All comparisons were 2-sided, and a P value of <.05 was considered significant. Data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
There were 4676 hospitalized patients with radiographically confirmed CAP (2320 adults and 2356 children) enrolled. Table 1 shows patients receiving antibiotics by inpatient and/or prehospital setting.
Table 1.
Proportion of Adults and Children Hospitalized With Community-Acquired Pneumonia Who Received Antibiotics
| Antibiotics receipt by settingb | Adult, n (%) | Child, n (%) | Total, N (%) |
|---|---|---|---|
| n (row %) | 2320 (49.6) | 2356 (50.4) | 4676 (100) |
| n (column %) | |||
| Received inpatient antibiotics only | 2043 (88.1) | 1667 (70.7) | 3710 (79.3) |
| Received prehospitala antibiotics only | 2 (0.1) | 27 (1.1) | 29 (0.6) |
| Received both inpatient and prehospitala antibiotics | 244 (10.5) | 398 (16.9) | 642 (13.7) |
| No antibiotics received | 31 (1.3) | 264 (11.2) | 295 (6.3) |
Prehospital antibiotic exposure was defined as receiving an antibiotic during the 5 days before admission.
For 61 patients, it was unknown whether they received inpatient and/or prehospital antibiotic.
There were 275 (6.5%) of 4246 blood cultures, 17 (3.8%) of 445 sputum/ET cultures, 26 (1.3%) of 1941 urine antigen tests, and 320 (7.0%) of 4550 NP/OP samples excluded because of unknown date and time of antibiotic administration. The number and proportion of specimens collected before prehospital or inpatient antibiotics included the following: 2679 (67.5%) of 3971 blood cultures, 36 (8.4%) of 428 sputum/ET cultures, 158 (8.3%) of 1915 urine antigen tests, and 405 (9.6 %) of 4230 NP/OP samples.
Culture-Dependent Tests
There were 139 (5.2%) bacterial detections in 2679 blood cultures collected before any antibiotics (prehospital and/or inpatient) compared with 33 (2.6%) bacterial detections in 1292 blood cultures collected after any antibiotics (P < .01) (Table 2). Detection of S pneumoniae was lower among blood cultures collected after any antibiotics (Table 2) compared with those collected before antibiotics. For children, 37 of 1164 blood cultures collected before antibiotics were positive compared with 14 of 725 blood cultures collected after antibiotics (3.2% vs 1.9%; P = .10). For adults, 102 of 1515 blood cultures collected before antibiotics were positive compared with 19 of 567 blood cultures collected after antibiotics (6.7% vs 3.4%; P < .01). Analyzing the effect of prehospital antibiotics, there were 147 positive blood cultures from 3073 specimens collected from patients without prehospital antibiotic exposure compared with 16 positive blood cultures from 600 specimens from patients who reported taking prehospital antibiotics (4.8% vs 2.7%; P = .02).
Table 2.
Proportion of Bacterial Detections Based on Specimens Collected Before and After Inpatient and/or Prehospital Antibiotics for Each Diagnostic Test
| Diagnostic Tests | Specimens Obtained Before Antibiotics | |||||
|---|---|---|---|---|---|---|
| Yes (%) | No (%) | |||||
| Adult | Child | Total | Adult | Child | Total | |
| Blood cultures, n | 1515 | 1164 | 2679 | 567 | 725 | 1292 |
| Total bacterial detections | 102 (6.7)a | 37 (3.2) | 139 (5.2)d | 19 (3.4) | 14 (1.9) | 33 (2.6) |
| Streptococcus pneumoniae | 37 (2.4) | 18 (1.5)c | 55 (2.1)c | 7 (1.2) | 2 (0.3) | 9 (0.7) |
| Streptococcus pyogenes | 4 (0.3) | 3 (0.3) | 7 (0.3) | 2 (0.4) | 1 (0.1) | 3 (0.2) |
| Streptococcus aureus | 18 (1.2) | 3 (0.3) | 21 (0.8) | 3 (0.5) | 3 (0.4) | 6 (0.5) |
| Haemophilus influenzae | 7 (0.5) | 1 (0.1) | 8 (0.3) | 2 (0.4) | 2 (0.3) | 4 (0.3) |
| Other pathogensa | 36 (2.4)c | 13 (1.1) | 49 (1.8)c | 5 (0.9) | 7 (1.0) | 12 (0.9) |
| High-quality sputum or ET cultures, n | 13 | 23 | 36 | 260 | 132 | 392 |
| Total bacterial detections | 2 (15.4) | 16 (69.6) | 18 (50.0)c | 29 (11.2) | 76 (57.6) | 105 (26.8) |
| S pneumoniae | 2 (15.4) | 4 (17.4) | 6 (16.7) | 11 (4.2) | 18 (13.6) | 29 (7.4) |
| S pyogenes | 0 (0.0) | 1 (4.3) | 1 (2.8) | 3 (1.2) | 3 (2.3) | 6 (1.5) |
| S aureus | 0 (0.0) | 6 (26.1) | 6 (16.7) | 11 (4.2) | 29 (22.0) | 40 (10.2) |
| H influenzae | 0 (0.0) | 6 (26.1) | 6 (16.7)c | 0 (0.0) | 23 (17.4) | 23 (5.9) |
| Other pathogensb | 0 (0.0) | 10 (43.5)c | 10 (27.8)d | 4 (1.5) | 32 (24.2) | 36 (9.2) |
| Urine antigen test, n (only performed in adults) | 158 | 158 | 1757 | 1757 | ||
| Total detections | 11 (7.0) | NA | 11 (7.0) | 101 (5.7) | NA | 101 (5.7) |
| Pneumococcal antigen detection | 7 (4.4) | NA | 7 (4.4) | 74 (4.2) | NA | 74 (4.2) |
| Legionella antigen detection | 4 (2.5) | NA | 4 (2.5) | 26 (1.5) | NA | 26 (1.5) |
| Combined pneumococcal and Legionella antigen detections | 0 (0.0) | NA | 0 (0.0) | 1 (0.1) | NA | 1 (0.1) |
| NP/OP swab PCR assay, n | 126 | 279 | 405 | 2118 | 1707 | 3825 |
| Total Mycoplasma pneumoniae or Chlamydia pneumoniae detections | 7 (5.6)c | 20 (7.2) | 27 (6.7) | 45 (2.1) | 163 (9.5) | 208 (5.4) |
Abbreviations: ET, endotracheal tube; NA, nonapplicable; NP/OP, nasopharyngeal and oropharyngeal; PCR, polymearase chain reaction.
Columns may not add to 100% because some specimens may have had more than 1 pathogen detected. The following bacteria were considered contaminants and were excluded from the analyses: Aeroccocus, Alcaligenes, Bacillus, Citrobacter, coagulase-negative Staphylococcus, Corynebacterium, Enterococcus, Micrococcus, Neisseria subflava, Propionibacterium, Stomatococcus, Streptococcus bovis, and Veillonella.
Other pathogens (specimens before antibiotics: yes/no): Escherichia coli (13/0); viridans streptococci species (9/4); Klebsiella (6/0); streptococcal groups B, C, or G (7/3); Fusobacterium (3/1); Pseudomonas (2/1); Moraxella (1/1); Acinobacter (2/0); Enterobacter (1/0); Pasteurella (1/0); Proteus (1/0); or codetections (3/2).
Other pathogens (specimens before antibiotics: yes/no): Moraxella (10/30), Pseudomonas (0/4), E coli (0/1), Enterobacter (0/1).
P value <.05 compared with specimens obtained after antibiotics.
P value <.001 compared with specimens obtained after antibiotics.
With increasing time between inpatient antibiotic administration and specimen collection, the proportion of blood cultures with a bacterial detection decreased with increased time; the proportion positive was 5.2% for specimens collected before inpatient antibiotic administration compared with 1.7% for specimens collected >15 hours after initial inpatient antibiotic exposure (P for trend <.01) (Table 3).
Table 3.
Proportion of Bacterial Detections Comparing Specimens Collected Before and After Inpatient Antibiotic Administration According to Time Elapsed Between Antibiotic Administration and Specimen Collection Stratified Into Quartilesa
| Blood cultures (n = 3369) | n | Bacterial Detections (%) | P Value for Trendb |
|---|---|---|---|
| Before antibiotics | 2679 | 139 (5.2) | <.01 |
| >0–1 hours after antibiotics | 163 | 8 (4.9) | |
| >1–4 hours after antibiotics | 176 | 5 (2.8) | |
| >4–15 hours after antibiotics | 176 | 1 (0.6) | |
| >15 hours after antibiotics | 175 | 3 (1.7) | |
| ET/Sputum Cultures (n = 378) | |||
| Before antibiotics | 36 | 18 (50.0) | <.01 |
| >0–5 hours after antibiotics | 98 | 46 (46.9) | |
| >5–10 hours after antibiotics | 76 | 23 (30.3) | |
| >10–20 hours after antibiotics | 86 | 17 (19.8) | |
| >20 hours after antibiotics | 82 | 10 (12.2) | |
| Urine Pneumococcal or Legionella test (n = 1693) | |||
| Before antibiotics | 157 | 11 (7.0) | .01 |
| >0–6 hours after antibiotics | 374 | 33 (8.8) | |
| >6–15 hours after antibiotics | 395 | 22 (5.6) | |
| >15–23 hours after antibiotics | 363 | 19 (5.2) | |
| >23 hours after antibiotics | 404 | 14 (3.5) | |
| NP/OP PCR for Mycoplasma pneumoniae or Chlamydia pneumoniae (n = 3557) | |||
| Before antibiotics | 401 | 27 (6.7) | .01 |
| >0–5 hours after antibiotics | 752 | 39 (5.2) | |
| >5–14 hours after antibiotics | 845 | 29 (3.4) | |
| >14–21 hours after antibiotics | 739 | 27 (3.7) | |
| >21 hours after antibiotics | 820 | 28 (3.4) | |
Abbreviations: ET, endotracheal tube; NP/OP, nasopharyngeal and oropharyngeal; PCR, polymearase chain reaction.
Patients with prehospital antibiotic exposure, and/or unknown timing of inpatient antibiotic exposure, were excluded from analysis.
Cochran-Armitage trend test.
There were 18 (50.0%) bacterial detections in 36 high-quality sputum/ET specimens collected before antibiotics compared with 105 (26.8%) bacterial detections in 392 sputum/ET specimens collected after antibiotics (prehospital and/or inpatient) (P < .01) (Table 2). For children at the Memphis site, 16 of 23 sputum/ET specimens collected before antibiotics yielded pathogens compared with 76 of 132 sputum/ET specimens collected after antibiotics (69.6% vs 57.6%; P = .28). For adults, 2 of 13 sputum/ET specimens collected before antibiotics yielded pathogens compared with 29 of 260 sputum/ET specimens collected after antibiotics (15.4% vs 4.2%; P = .24).
Analyzing the effect of prehospital antibiotics, 47 of 90 sputum/ET cultures from specimens collected without prehospital antibiotic exposure yielded pathogens compared with 8 of 49 sputum/ET cultures from patients who reported taking prehospital antibiotics (52.2% vs 16.3%; P < .01).
With increasing time between inpatient antibiotic administration and specimen collection, the proportion of sputum specimens with a bacterial detection decreased from 50.0% for specimens collected before antibiotic exposure compared with 12.2% for specimens collected >20 hours after initial inpatient antibiotic exposure (P for trend <.01) (Table 3).
Culture-Independent Tests
Of 158 urine specimens collected before antibiotics, there were 11 (7.0%) urinary pneumococcal or L pneumophila serogroup 1 detections, not significantly different from specimens collected after antibiotics (inpatient and/or prehospital) (101 of 1757 [5.7%]; P = .53) (Table 2). We found that urinary antigen detection rates were similar when comparing specimens with and without prehospital antibiotic exposure (6.8% vs 6.0%; P = .74). With increasing time between inpatient antibiotic administration and specimen collection, the proportion of pneumococcal or L pneumophila serogroup 1 antigen detections decreased from 7.0% for specimens collected before antibiotic exposure to 3.5% for specimens collected >23 hours after initial inpatient antibiotic exposure (P for trend <.01) (Table 3).
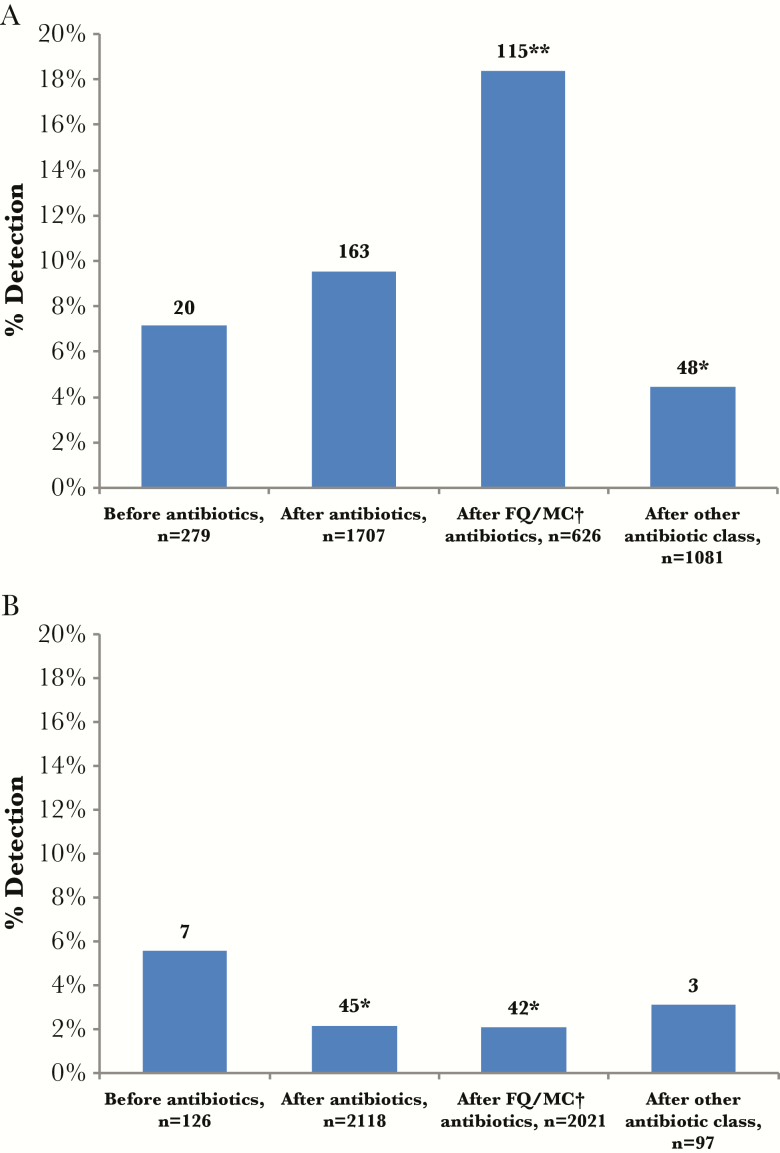
Mycoplasma pneumoniae and C pneumoniae were detected by PCR from 27 (6.7%) of 405 NP/OP specimens collected before antibiotics compared with 208 (5.4%) detected in 3825 NP/OP specimens collected after antibiotics (prehospital and/or inpatient) (P = .31) (Table 2). Among adults, M pneumoniae or C pneumoniae was detected in 7 of 126 NP/OP specimens collected before antibiotics and 45 of 2118 NP/OP specimens collected after antibiotics (5.6% vs 2.1%; P < .05). For children, there were 20 bacterial detections in 279 NP/OP specimens collected before antibiotics and 163 bacterial detections in 1707 NP/OP specimens collected after antibiotics (7.2% vs 9.5%; P = .20). The proportion of M pneumoniae or C pneumoniae detection was significantly higher in children receiving either a fluoroquinolone or macrolide antibiotic class and was lower for children receiving other antibiotic classes (Figure 1A). Subgrouping children further, we found a similar yield before and after antibiotics for young children under 5 years (4.5% vs 3.9%; P = .65); however, for children 5 to 17 years, we found 11 detections in 81 specimens collected before antibiotics compared with 96 detections in 338 specimens collected after antibiotics (13.6% vs 21.8%; P = .08). This finding became statistically significant when we compared the yield before antibiotics to yield after exposure to a fluoroquinolone or macrolide antibiotic (13.6% vs 28.4%; P < .01) for children aged 5 to 17 years. For adults, the proportion of M pneumoniae or C pneumoniae detections was lower in adults who received either a fluoroquinolone or macrolide antibiotic before NP/OP specimen collection compared with specimens collected after antibiotics (Figure 1B).
Figure 1.
(A) Proportion of Chlamydia pneumoniae or Mycoplasma pneumoniae detections among nasopharyngeal and oropharyngeal (NP/OP) specimens collected before and after inpatient and/or prehospital antibiotics overall and by antibiotic class in children. Numbers above bars represent the number of bacterial detections. (B) Proportion of C pneumoniae and M pneumoniae detections among NP/OP specimens collected before and after inpatient and/or prehospital antibiotics overall and by antibiotic class in adults. Numbers above bars represent the number of bacterial detections. ‡NP/OP swabs for real-time polymerase chain reaction assays to detect C pneumoniae and M pneumoniae. †FQ/MC, fluoroquinolone or macrolide. *P value <.05 compared with specimens obtained before antibiotics. **P value <.001 compared with specimens obtained before antibiotics.
Analyzing the effect of prehospital antibiotics, we found that PCR detected M pneumoniae or C pneumoniae in 51 of 913 NP/OP specimens collected from patients without prehospital antibiotic exposure compared with 81 of 647 specimens from patients who reported taking prehospital antibiotics (5.6% vs 12.5%; P < .01). With increasing time between inpatient antibiotic administration and specimen collection, the proportion of M pneumoniae and C pneumoniae detections decreased from 6.7% for specimens collected before antibiotic exposure compared with 3.4% for specimens collected >21 hours after initial antibiotic exposure (P for trend <.01) (Table 3).
DISCUSSION
Our analysis showed that bacteria were less frequently detected in culture-based tests collected after antibiotic exposure and for culture-independent tests that had longer intervals between antibiotic exposure and specimen collection. Antibiotic exposure was associated with reduced bacterial yield from blood cultures, sputum, and tracheal aspirates by approximately 50%. The incremental increase in time after inpatient antibiotic exposure was also associated with decreased bacterial yield, emphasizing the need to obtain cultures as close to the time of antibiotic administration as possible. Antibiotic administration did not appear to be associated with yield from urinary antigen detection assays for S pneumoniae or L pneumophila serogroup 1. Among adults but not children, antibiotic exposure, particularly exposure to fluoroquinolones or macrolides, was associated with decreased yield from PCR assays for M pneumoniae and C pneumoniae.
In our study, most blood cultures were collected before antibiotics, and we found a lower proportion of bacterial detections in specimens collected after antibiotics. Historically, studies have shown that 6%–16% of hospitalized patients with CAP had documented bacteremia [13–16]. In the EPIC study, 2.5% of children and 5.7% of adults had documented bacteremia [4, 5]; the decreased proportion of bacterial detections may be a result of widespread implementation of pediatric pneumococcal conjugate vaccination. In our analysis, blood cultures collected before antibiotics were positive in 6.7% of adults and 3.2% of children. This dropped substantially after prehospital and/or inpatient antibiotics, similar to a surveillance study of 35 639 patient blood cultures from 29 883 hospitalized patients with pneumonia in Thailand that reported a 4.1% drop in pathogen detections [7]. We found a decreased proportion of bacterial detections as the time between antibiotic exposure and specimen collection increased, and this became significant >4 hours between antibiotic exposure and specimen collection.
Studies have shown that the diagnostic yield for sputum cultures varies from 10% to 86% depending upon specimen quality and previous antibiotic use [17]. A small study of 85 hospitalized adult patients in the United States with bacteremic pneumococcal pneumonia found that the frequency of pneumococcal growth in sputum specimens decreased with increasing time from antibiotic initiation [18]. A study among elderly hospitalized patients with CAP also showed a decrease in S pneumoniae detection after antibiotic exposure when using culture-based diagnostic tests [19]. In our study, the proportion of S pneumoniae was lower in high-quality sputum/ET specimens collected after antibiotics, although this was not statistically significant. To maximize diagnostic yield for culture-based diagnostic testing, specimens should be collected before or as soon as possible after antibiotic administration.
For the culture-independent urine antigen tests for S pneumoniae and L pneumophila serogroup 1 in adults, there were fewer detections in specimens collected after antibiotics compared with before, although this was not statistically significant. Urinary antigen tests for pneumococcus were responsible for the majority (67%) of pneumococcal detections in the EPIC study [4]. The urine antigen diagnostic tests offer both high sensitivity and specificity for identifying pneumococcus or L pneumophila serogroup 1 in adult patients with CAP [17]. Previous studies have reported continued antigen detection despite antibiotic exposure [11, 19]. Although we found a lower proportion of urine antigen detections after antibiotic exposure, this was not statistically significant. Other studies have reported fewer urinary pneumococcal antigen detections after prehospital antibiotic exposure [8, 20]. When we examined urine antigen detections based on self-reported prehospital antibiotic exposure within 5 days before admission, we found no significant difference in detections before or after antibiotic exposure. Streptococcus pneumoniae remains an important cause of bacterial CAP [4, 5]; thus, early and increased use of rapid pneumococcal urine antigen tests for adults with CAP could improve antibiotic selection and allow for subsequent antibiotic de-escalation [21, 22]. However, a recent national survey among practicing US infectious disease clinicians suggested that the urine pneumococcal antigen test is only used by 65% of providers [23]. Urinary antigen testing for S pneumoniae or L pneumophila serogroup 1 in an adults hospitalized with CAP should be pursued in accordance with current clinical guidelines [2], although we observed false-positive results in some patients who received 23-valent pneumococcal polysaccharide vaccine before urine collection in the EPIC study [24].
Atypical bacterial pathogens were the most common bacteria detected among pediatric patients hospitalized with CAP in the EPIC study [5], and these were detected in 8% of children and 2% of adults (4, 5). Although multiplex PCR is often used for respiratory virus detection, atypical bacteria have not routinely been included in these panels for clinical use [17]. However, a multiplex PCR for detection of M pneumoniae and C pneumoniae was approved by the US Food and Drug Administration in 2013 [25], which could help inform antibiotic use and choice. In our study, only 10.6% of these research NP/OP specimens were collected before antibiotic exposure. Although more atypical bacteria were detected by PCR in specimens exposed to prehospital antibiotics compared with specimens not exposed, when we excluded specimens with prehospital antibiotic exposure, we found the proportion of M pneumoniae and C pneumoniae detections by PCR in NP/OP specimens significantly decreased with increasing time after antibiotic exposure compared with specimens collected before antibiotics. When examined by age, adults also had significantly fewer PCR detections when exposed to antibiotics. Although children more frequently had atypical bacterial detection by PCR after antibiotic exposure, higher detection was primarily in older children aged 5–17 years who received a fluoroquinolone or a macrolide. Potential explanations include clinicians choosing a macrolide or fluoroquinolone in children with a heightened risk of atypical bacterial infection, or that fluoroquinolones or macrolides may result in bacterial killing that releases the molecular material in the specimen increasing the likelihood of a PCR-based detection.
Our study was subject to several limitations. First, the EPIC study was not specifically designed to answer the question of whether pretest antibiotic exposure effects bacterial detection, and thus serial samples on the same patient collected before and after antibiotic exposure were not obtained. Second, the analysis is subject to uncontrolled confounding for patient characteristics associated with either earlier or later timing of specimen collection and/or antibiotic administration. In addition, although specimens, particularly blood, were collected as soon as possible from patients per clinical care, NP/OP, sputum, and urine specimens were often collected at the time of enrollment and after informed consent per the study protocol. Third, certain specimen types included in this study are not routinely collected in clinical practice such as expectorated sputum, ET aspirates, urine, and NP/OP swab specimens, which may limit the generalizability of our findings. Fourth, a recall bias might exist in patients self-reporting prehospital antibiotic use.
CONCLUSIONS
In conclusion, the frequency of bacterial detections decreased with increasing time between inpatient antibiotic exposure and specimen collection for culture-based diagnostic tests, as well as for M pneumoniae and C pneumoniae PCR detection in adult NP/OP specimens. Urinary antigen testing for L pneumophila and S pneumoniae was less effected by antibiotic exposure, and these tests should be used in accordance with clinical guidelines. Our results suggest that bacteria could be detected more commonly if specimens were collected before or early after administering antibiotic therapy, leading to improved pathogen detection and targeted antibiotic therapy. Newer rapid and accurate bacterial diagnostics, especially culture-independent tests, are urgently needed for hospitalized CAP to improve pathogen detection and better inform and facilitate antibiotic de-escalation [26].
Acknowledgments
We thank the children and families who graciously consented to participate in the Etiology of Pneumonia in the Community (EPIC) study. We also thank Dr. Chris Stockmann (University of Utah Health Services Center) for his contributions to this study.
Author contributions. A. M. H. designed the analytic plan, analyzed and interpreted the data, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted; A. M. B. created and managed the database, helped design the study, assisted with data analysis and interpretation, critically reviewed the manuscript, and approved the final manuscript as submitted. S. J. obtained funding to conduct the study, supervised the study, helped design the study, helped analyze and interpret the data, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. L. A. H. contributed to study design, supervised A. M. H. in the analysis and interpretation of the data, critically reviewed the manuscript as a subject matter expert, and approved the final manuscript as submitted. S. R. A., K. A., W. H. S., D. J. W., E. J. A., C. G. G., J. A. M., A. T. P., R. G. W., and K. M. E. obtained funding to conduct the study, enrolled patients and collected data at the study sites, contributed to study design, helped interpret the data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support. The EPIC study was supported by the Influenza Division in the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. W. H. S. was supported in part by K23GM110469 from the National Institute of General Medical Sciences. C. G. G. was supported in part by R01AG043471 from the National Institute on Aging. D. J. W. was supported in part by K23AI104779 from the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. W. H. S. serves on scientific advisory boards for BioFire Diagnostics and Venaxis, Inc., consults for Abbott Point of Care, and received research funding from Pfizer, ThermoFisher, and BioMerieux. E. J. A. received research funding from Novavax and research funding and editorial assistance from MedImmune, and he consults for AbbVie. R. G. W. consults with Accelerate Diagnostics and GenMark, and his institution received grants from bioMerieux and Pfizer. A. T. P. received research funding from BioFire Diagnostics. C. G. G. has served as a consultant for Pfizer Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med 2013; 369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44Suppl 2:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med 2014; 371:1619–28. [DOI] [PubMed] [Google Scholar]
- 4. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lindenauer PK, Remus D, Roman S, et al. Public reporting and pay for performance in hospital quality improvement. N Engl J Med 2007; 356:486–96. [DOI] [PubMed] [Google Scholar]
- 7. Rhodes J, Hyder JA, Peruski LF, et al. Antibiotic use in Thailand: quantifying impact on blood culture yield and estimates of pneumococcal bacteremia incidence. Am J Trop Med Hyg 2010; 83:301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Value of intensive diagnostic microbiological investigation in low- and high-risk patients with community-acquired pneumonia. Eur J Clin Microbiol Infect Dis 2005; 24:241–9. [DOI] [PubMed] [Google Scholar]
- 9. Campbell SG, Marrie TJ, Anstey R, et al. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest 2003; 123:1142–50. [DOI] [PubMed] [Google Scholar]
- 10. Dorigo-Zetsma JW, Verkooyen RP, van Helden HP, et al. Molecular detection of Mycoplasma pneumoniae in adults with community-acquired pneumonia requiring hospitalization. J Clin Microbiol 2001; 39:1184–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murdoch DR, Laing RT, Mills GD, et al. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol 2001; 39:3495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lahti E, Peltola V, Waris M, et al. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax 2009; 64:252–7. [DOI] [PubMed] [Google Scholar]
- 13. Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest 1995; 108:932–6. [DOI] [PubMed] [Google Scholar]
- 14. Falguera M, Trujillano J, Caro S, et al. A prediction rule for estimating the risk of bacteremia in patients with community-acquired pneumonia. Clin Infect Dis 2009; 49:409–16. [DOI] [PubMed] [Google Scholar]
- 15. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J 2013; 32:736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shariatzadeh MR, Huang JQ, Tyrrell GJ, et al. Bacteremic pneumococcal pneumonia: a prospective study in Edmonton and neighboring municipalities. Medicine (Baltimore) 2005; 84:147–61. [DOI] [PubMed] [Google Scholar]
- 17. Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clin Infect Dis 2011; 52Suppl 4:S296–304. [DOI] [PubMed] [Google Scholar]
- 18. Abers MS, Musher DM. The yield of sputum culture in bacteremic pneumococcal pneumonia after initiation of antibiotics. Clin Infect Dis 2014; 58:1782–3. [DOI] [PubMed] [Google Scholar]
- 19. Saukkoriipi A, Palmu AA, Jokinen J, et al. Effect of antimicrobial use on pneumococcal diagnostic tests in elderly patients with community-acquired pneumonia. Eur J Clin Microbiol Infect Dis 2015; 34:697–704. [DOI] [PubMed] [Google Scholar]
- 20. Gutiérrez F, Masiá M, Rodríguez JC, et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis 2003; 36:286–92. [DOI] [PubMed] [Google Scholar]
- 21. Guchev IA, Yu VL, Sinopalnikov A, et al. Management of nonsevere pneumonia in military trainees with the urinary antigen test for Streptococcus pneumoniae: an innovative approach to targeted therapy. Clin Infect Dis 2005; 40:1608–16. [DOI] [PubMed] [Google Scholar]
- 22. Strålin K, Holmberg H. Usefulness of the Streptococcus pneumoniae urinary antigen test in the treatment of community-acquired pneumonia. Clin Infect Dis 2005; 41:1209–10. [DOI] [PubMed] [Google Scholar]
- 23. Harris AM, Beekmann SE, Polgreen PM, Moore MR. Rapid urine antigen testing for Streptococcus pneumoniae in adults with community-acquired pneumonia: clinical use and barriers. Diagn Microbiol Infect Dis 2014; 79:454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grijalva CG, Wunderink RG, Zhu Y, et al. In-hospital pneumococcal polysaccharide vaccination is associated with detection of pneumococcal vaccine serotypes in adults hospitalized for community-acquired pneumonia. Open Forum Infect Dis 2015; 2:ofv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baron EJ, Miller JM, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis 2013; 57:e22–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caliendo AM. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin Infect Dis 2011; 52Suppl 4:S326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]