Abstract
Procalcitonin levels rise in response to systemic inflammation, especially of bacterial origin. Multiple randomized controlled trials have demonstrated that procalcitonin-based algorithms can safely reduce antibiotic use in 2 clinical scenarios. First, in stable, low-risk patients with respiratory infections, procalcitonin levels of <0.25 µg/L can guide the decision to withhold antibiotics or stop therapy early. Second, in critically ill patients with suspected sepsis, clinicians should not initially withhold antibiotics, but procalcitonin levels of <0.5 µg/L or levels that decrease by ≥80% from peak can guide discontinuation once patients stabilize. The recent stop antibiotics on procalcitonin guidance study (SAPS), the largest procalcitonin trial to date, demonstrated reduction in both antibiotic exposure and mortality in critically ill patients. Although procalcitonin is ready for routine use, future research should examine optimal strategies for implementation in hospitals, its real-world impact on clinical outcomes and costs, its applicability to immunocompromised patients, and the generalizability of trials to the US population.
Keywords: antibiotic stewardship, biomarkers, procalcitonin, respiratory infections, sepsis
Up to 30%–50% of antibiotics given to hospitalized patients may be unnecessary [1]. Treatment courses commonly exceed recommended durations or are targeted towards colonizing or contaminating microorganisms [2]. Antibiotic overprescription has fueled the emergence of antibiotic-resistant bacteria as well as unnecessary drug adverse events and propagation of Clostridium difficile infection [3]. This global public health threat has stimulated national calls for hospitals to improve antibiotic prescribing practices and implement stewardship programs. However, in the face of imperfect diagnostic tools, clinicians are reluctant to withhold antibiotics when infection is suspected. This pressure is compounded by national quality measures compelling clinicians to immediately start antibiotics in potentially septic patients, even when the diagnosis is uncertain [4]. Subsequent antibiotic de-escalation can be challenging because 40% or more of patients with sepsis never have a pathogen identified [5].
Additional tools to help guide antibiotic prescribing are thus sorely needed. Biomarkers of bacterial infection are attractive because they can provide objective data to augment clinicians’ intuition in starting, withholding, or stopping antibiotics. Of the various biomarkers, procalcitonin has been the most extensively studied. This review will focus on the literature supporting procalcitonin’s utility as a biomarker of bacterial infection and its role in guiding antibiotic therapy in adult patients.
PROCALCITONIN AS A BIOMARKER FOR BACTERIAL INFECTION AND SEPSIS
Procalcitonin is a precursor hormone of calcitonin that is undetectable in healthy states, but it is upregulated by cytokines released in response to bacterial infections [6, 7]. Conversely, procalcitonin production is blocked by interferon-gamma, a cytokine released in response to viral infections [8]. Procalcitonin has been used in Europe for many years and was also approved for use in the United States by the US Food and Drug Administration (FDA) as a diagnostic aid for sepsis in 2005. It also gained an FDA indication in 2016 for serial use to assess sepsis progression and 28-day mortality risk. Several different procalcitonin assays exist, but all demonstrate good concordance at clinically relevant cutoffs [9].
Serum procalcitonin levels rise rapidly in response to systemic inflammatory insults, with peak levels that correlate with the intensity of the stimulus. Procalcitonin has a short half-life (25–30 hours), and levels decline rapidly with resolution of inflammation [6, 10]. These properties make it potentially useful in helping decide whether to start antibiotics and when to stop antibiotics in a clinically improving patient. The kinetics also explain the prognostic value of both the initial procalcitonin level and subsequent trends [11]. The nonbinary nature of the test also means that different procalcitonin thresholds differ in their sensitivity and specificity, while allowing for potential variation in algorithms used to guide antibiotic therapy.
Although procalcitonin is more specific for bacterial infections than other inflammatory markers such as C-reactive protein [12], false positives can occur. Major stressors that cause systemic inflammation, such as severe trauma, circulatory shock, surgery, burns, inhalation injury, and pancreatitis, can also elevate procalcitonin levels, possibly through gut translocation of lipopolysaccharide or other bacterial products [13]. False negatives can also occur, notably in contained localized infections such as mediastinitis, empyema, or abscesses, or if procalcitonin is drawn too early in the course of infection [14].
The performance of procalcitonin as a diagnostic biomarker for sepsis in intensive care unit (ICU) patients has been examined in several studies and summarized in a recent meta-analysis by Wacker et al [15]. This meta-analysis pooled 30 studies, with a total of 3244 patients, and found moderate accuracy (overall sensitivity of 77% and specificity of 79%), with substantial heterogeneity between studies. However, procalcitonin levels are significantly higher in culture-positive sepsis versus culture negative-sepsis, and thus diagnostic performance may be better in the former group of patients [5, 16]. Furthermore, in critically ill patients with microbiologically documented infection, procalcitonin levels differ by site of infection, with the highest levels in those with positive blood cultures and lowest with pulmonary cultures [17]. Unfortunately, it is in the septic patients where no organism is identified that accurate biomarkers of infection would be most clinically helpful. Attempts to characterize the accuracy of procalcitonin in these patients are hampered by the lack of a true gold standard for diagnosing sepsis, because even experienced clinicians often disagree on whether or not patients were septic in retrospect [18]. It is similarly impossible to characterize procalcitonin’s accuracy in pneumonia, because the absence of positive cultures does not rule out bacterial infection or coinfection when viral pathogens are identified.
Because of the lack of a reliable reference standard for identifying bacterial pneumonia and sepsis, particularly in culture-negative patients, the utility of procalcitonin cannot be determined through observational studies alone. However, numerous randomized intervention studies have examined whether procalcitonin-based algorithms can help clinicians decide whether to start or stop antibiotic therapy, without adversely impacting patients through delayed antibiotic therapy or inadequate treatment courses. These studies have primarily demonstrated efficacy in 2 clinical scenarios: adult patients with suspected respiratory infections, and critically ill adults with any type of suspected infection. Improving antibiotic use in these 2 clinical scenarios carries the potential for significant benefit given that respiratory infections are the most common indication for antibiotics in hospitalized patients and antibiotic use is most prevalent in ICUs [19].
PROCALCITONIN TO GUIDE ANTIBIOTIC THERAPY FOR RESPIRATORY INFECTIONS
Randomized controlled trials that have shown that procalcitonin guidance can help safely decrease antibiotic exposure in patients with suspected or proven respiratory infections are summarized in Table 1. Christ-Crain et al [20] first demonstrated this in a single hospital in Switzerland in patients who presented to the emergency department with suspected lower respiratory tract infections. Procalcitonin was used to help determine whether not to initiate antibiotics, with a cutoff threshold of 0.25 µg/L. The procalcitonin group had a 47% reduced rate of antibiotic exposure, with no difference in laboratory or clinical outcomes. Follow-up trials in the same hospital, using similar procalcitonin algorithms, demonstrated efficacy in safely reducing antibiotic exposure in patients hospitalized for suspected community-acquired pneumonia and exacerbations of chronic obstructive pulmonary disease [21, 22].
Table 1.
Procalcitonin Randomized Controlled Trials for Respiratory Tract Infections in Adult Patients
| First Author (Year) [Reference] Trial Name |
Setting (Country) |
Number and Type of Infection | PCT Algorithm | Exclusion Criteria | Antibiotic Reduction Outcomes | Clinical Outcomes |
|---|---|---|---|---|---|---|
| Christ-Crain (2004) [20] | 1 hospital (Switzerland) | 243 patients with LRTI | Initiation only: antibiotics strongly discouraged (<0.1 µg/L), discouraged (0.1–0.25), encouraged (0.25–0.5), strongly encouraged (≥0.5). Repeat PCT after 6–24 hours if antibiotics withheld | Severely immunocompromised, cystic fibrosis, active tuberculosis, hospital-acquired pneumonia | 47% reduction in antibiotic use (P < .0001) | No difference (including hospital mortality or long- term mortality at mean 5.3 months, hospital or ICU LOS, laboratory outcomes) |
| Christ-Crain (2006) [21] ProCAP |
1 hospital (Switzerland) | 302 patients with CAP | Initiation and Discontinuation: antibiotics strongly discouraged (<0.1 µg/L), discouraged (0.1–0.25), encouraged (0.25–0.5), strongly encouraged (≥0.5). Repeat PCT after 6–24 hours if antibiotics withheld, and at days 4, 6, and 8 | Severely immunocompromised, cystic fibrosis, active tuberculosis, hospital-acquired pneumonia | 52% relative risk of antibiotic exposure and median 5 vs 12 days of antibiotic treatment (P < .001 for both) | No difference (including mortality, ICU admission, treatment success, laboratory outcomes) |
| Stolz (2007) [22] |
1 hospital (Switzerland) | 208 patients with COPD exacerbation | Initiation only: antibiotics discouraged (<0.1 µg/L), discouraged if clinically stable (0.1–0.25), encouraged (>0.25). Repeat PCT after 6–24 hours if antibiotics withheld | Immunosuppression, asthma, cystic fibrosis, infiltrates on chest radiograph, psychiatric illness | 56% relative risk of antibiotic exposure; 40% vs 72% overall antibiotic use (P < .0001 for both) | No difference (including death, treatment success, hospital LOS, ICU admission, improvement in FEV1 at 14 days and 6 months, 6 month rehospitalization rate, mean time to next exacerbation) |
| Briel (2008) [25] | 53 outpatient physicians in (Switzerland) | 458 outpatients with acute respiratory tract infections | Initiation and Discontinuation: antibiotics strongly discouraged (≤0.1 µg/L), discouraged (0.1–0.25), encouraged (>0.25). Repeat PCT after 6–24 hours if antibiotics withheld | Antibiotics within prior 28 days, psychiatric disorders, severe immunosuppression, cystic fibrosis, active tuberculosis, need for immediate hospitalization | 72% decrease in antibiotic use (95% CI, 66%–78%) | No difference in activity restriction at 14 days, or ongoing symptoms or relapsing infection at 28 days |
| Kristoffersen (2009) [45] | 3 hospitals (Denmark) | 223 patients with LRTI | Initiation only: antibiotics discouraged (<0.25 µg/L), encouraged (0.25–0.5), and strongly encouraged (≥0.5). Single PCT measurement | None described | Mean 5.1 vs 6.8 days of antibiotic therapy (P = .007) | No difference (including hospital LOS, ICU admission, hospital mortality) |
| Schuetz (2009) [23] ProHOSP |
6 hospitals (Switzerland) | 1359 patients with LRTI | Initiation and Discontinuation: antibiotics strongly discouraged (≤0.1 µg/L), discouraged (0.1–0.25), encouraged (0.25–0.5), strongly encouraged (≥0.5). Repeat PCT after 6–24 hours if antibiotics withheld, and at days 3, 5, 7, and discharge | Active intravenous drug use, severe immunosuppression, life-threatening comorbidities, hospital-acquired pneumonia, chronic infection requiring antibiotics | Mean 5.7 vs 8.7 days of antibiotic therapy (relative change, −34.8%; 95% CI, −40.3% to −28.7%) | No difference (composite of 30-day adverse outcomes including death, ICU admission, and disease-specific complications, and recurrent LRTI); less frequent antibiotic-associated adverse events (19.8% vs 28.1%) |
| Burkhardt (2010) [24] | 15 primary care practices (Germany) | 550 patients with mild respiratory tract infection | Initiation only: no antibiotics (<0.25 µg/L) or yes antibiotics (≥0.25) | Recent antibiotics, chronic liver disease, recent major surgery, autoimmune or systemic inflammatory disorders, dialysis, medullary C-cell carcinoma | 21.5% vs 36.7% of patients received antibiotics | No difference in significant health impairment after 14 days |
| Long (2011) [46] |
1 hospital (China) | 172 patients with low- risk CAP (discharged from ED) | Initiation only: antibiotics strongly discouraged (≤0.1 µg/L), discouraged (0.1–0.25), encouraged (>0.25). Repeat PCT after 6–12 hours if antibiotics withheld | Pregnancy, antibiotics started ≥48 hours before enrollment, immunocompromised, withholding of life-support, active tuberculosis | 55% relative risk of antibiotic exposure (P = .003); median 5 vs 7 days of antibiotic treatment (P < .001) | No difference (all survived at 4 weeks, with similar clinical and laboratory outcomes) |
| Tang (2013) [47] |
1 hospital (China) |
225 patients with asthma exacerbation | Initiation only: antibiotics strongly discouraged (≤0.1 µg/L), discouraged (0.1–0.25), encouraged (>0.25). Repeat PCT after 6–8 hours if initial PCT <0.25 | Recent antibiotics, other bacterial infections, pneumonia on chest radiograph, other chronic respiratory disease, severe organ dysfunction | 46.1% vs 74.8% of patients received antibiotics (P < .001) | No difference (including asthma control, secondary ED visits, hospital readmission, additional steroids or antibiotics, FEV1) |
| Branche (2015) [48] | 1 hospital (United States) | 300 patients with nonpneumonic LRTI | Initiation only: antibiotics strongly discouraged (≤0.1 µg/L), discouraged (0.1–0.25), encouraged (0.25–0.5), strongly encouraged (≥0.5). PCT intervention arm coupled with viral PCR testing. | Definitive infiltrates on chest radiograph, immunosuppression, hypotension, ICU requirement, ≥15% peripheral bands, conditions known to increase PCT, antibiotics before admission | No difference overall, but less antibiotics in algorithm-adherent patients (2.0 vs 4.0 days; P = .004) | No difference in adverse events (no deaths in either arm; same median hospital LOS and number of posthospitalization healthcare visits) |
Abbreviations: CAP, community-acquired pneumonia; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; FEV1, forced expiratory volume at 1 second; ICU, intensive care unit; LOS, length of stay; LRTI, lower respiratory tract illness; PCR, polymerase chain reaction; PCT, procalcitonin.
The largest study to date examining procalcitonin for respiratory infections, the Procalcitonin Guided Antibiotic Therapy and Hospitalisation in Patients With Lower Respiratory Tract Infections (ProHOSP) study, was a multicenter randomized controlled trial involving 1359 adults at 6 hospitals in Switzerland who presented to the emergency department with any type of suspected respiratory tract infection [23]. Investigators in this study tested the utility of procalcitonin to inform both treatment initiation as well as discontinuation. As in prior studies, clinicians were encouraged to withhold antibiotics for patients with procalcitonin values of ≤0.25 µg/L. The study then required repeat procalcitonin testing on days 3, 5, 7, and at discharge. The investigators also recommended stopping antibiotics if procalcitonin levels decreased by ≥80% when the initial procalcitonin was >5–10 µg/L. Clinicians were allowed to overrule the algorithm and start antibiotics in patients with respiratory or hemodynamic instability, pneumonia due to Legionella pneumophila, or other risk factors for poor outcomes regardless of procalcitonin level. The procalcitonin group had a significantly lower mean duration of antibiotics (5.7 vs 8.7 days), with no difference in adverse events (including death, ICU admission, and recurrent infection). The procalcitonin algorithm also safely facilitated earlier antibiotic discontinuation in the subgroup of patients with confirmed community-acquired pneumonia, including those with documented bacteremia. Overall, 75% of patients in the procalcitonin group received any antibiotics, vs 88% in the control group. The algorithm was overruled by the physician based on their clinical judgment in only 9.2% of cases.
Although the trials studying procalcitonin for respiratory infections have generally focused on patients presenting to the emergency room (or in some cases the outpatient setting [24, 25]), one study demonstrated efficacy of procalcitonin in safely guiding antibiotic discontinuation in ICU patients with ventilator-associated pneumonia [26]. A subsequent Cochrane review concluded that procalcitonin guidance for respiratory patients in a variety of settings (including the emergency department, ICU, and primary care settings) resulted in a significant reduction in total antibiotic exposure (median 4 days vs 8 days), with no difference in mortality or rates of treatment failure [27].
PROCALCITONIN TO GUIDE ANTIBIOTIC THERAPY IN CRITICALLY ILL PATIENTS
Compared with stable patients with respiratory infections, it is less realistic that clinicians will withhold antibiotics in critically ill patients with suspected infection, regardless of procalcitonin results. This is reasonable in light of the imperfect sensitivity of procalcitonin for bacterial sepsis, and the potential to miss severe infections if only looking at a single measurement taken early in the course of disease. Thus, in randomized controlled trials examining the ICU patient population (summarized in Table 2), procalcitonin has generally been used as an aid to discontinue therapy after clinical stabilization. Procalcitonin cutoffs to stop antibiotics in ICU patients have been higher (0.5 µg/L, or even greater in some studies) than cutoffs used for stable patients with respiratory tract infections (0.25 µg/L). Small, single-center studies in medical-surgical or surgical ICUs in Switzerland and Germany first demonstrated that procalcitonin used in this manner could safely reduce antibiotic therapy in patients with suspected sepsis [17, 28, 29].
Table 2.
Procalcitonin Randomized Controlled Trials for Infections in Critically Ill Adult Patients
| First Author (Year) [Reference] Trial Name |
Setting (Country) |
Number and Type of Infection | PCT Algorithm | Exclusion Criteria | Antibiotic Reduction Outcomes | Clinical Outcomes |
|---|---|---|---|---|---|---|
| Nobre (2008) [17] | 1 Medical-Surgical ICU (Switzerland) | 79 patients with severe sepsis/septic shock | Discontinuation only: stop antibiotics if PCT decreased 90% from initial value, but not before day 3 (if baseline <1 µg/L) or day 5 (if baseline ≥1) | Organisms or conditions requiring prolonged duration of therapy, severe viral or parasitic infections, antibiotics started ≥48 hours before enrollment, severely immunocompromised, withholding of life support | 4 day reduction in median duration of antibiotic therapy (P = .003) | No different in mortality and recurrent infection; reduced ICU LOS by 2 days (P = .03) |
| Hochreiter (2009) [28] | 1 Surgical ICU (Germany) | 110 patients with suspected or confirmed sepsis | Discontinuation only: stop antibiotics if PCT <1 µg/L or decrease to 25%–35% of initial value over 3 days | Antibiotics started before ICU admission, therapy limitation due to goals of care | Mean 5.9 vs 7.9 days (P < .001) | No difference (treatment success, ICU LOS, SOFA score, hospital mortality) |
| Schroeder (2009) [29] | 1 Surgical ICU (Germany) | 27 patients with severe sepsis | Discontinuation only: stop antibiotics if PCT <1 µg/L or decrease to <35% of initial value over 3 days | Antibiotics started before ICU admission | Mean 6.6 vs 8.3 days (P < .001) | No difference (SAPS II or SOFA score, ICU stay, hospital mortality) |
| Stolz (2009) [26] ProVAP |
7 ICUs in 3 hospitals (Switzerland, United States) | 101 patients with ventilator-associated pneumonia | Discontinuation only: after 72 hours, antibiotic cessation strongly encouraged (<0.25 µg/L), encouraged (0.25–0.5 or decrease by ≥80%), discouraged (≥0.5 or decrease by <80%), strongly discouraged (>1) | Pregnant, enrolled in another trial, immunosuppressed, coexisting extrapulmonary infection requiring antibiotics for >3 days | 13 vs 9.5 antibiotic-free days alive 28 days after ventilator-associated pneumonia onset (overall 27% reduction in antibiotic therapy, P = .038) | No difference (mechanical ventilation-free days, ICU-free days alive, hospital LOS, 28-day mortality) |
| Boudama (2010) [30] PRORATA |
5 medical ICUs and 2 surgical ICUs (France) | 621 patients with suspected infection | Initiation and Discontinuation: antibiotics strongly discouraged (<0.25 µg/L), discouraged (0.25–0.5), encouraged (0.5–1), strongly encouraged (≥1) (daily PCT measurements). Discontinuation also if PCT decreased ≥80% from peak | Pregnancy, bone marrow transplant, or neutropenic, infections requiring long-term antibiotics, poor chance of survival, and do-not-resuscitate orders | Mean 11.6 vs 14.3 days of therapy (P < .0001) | No difference in noninferiority analysis (28-day and 60-day mortality), but trend towards increased 60-day mortality (+3.8%). No difference in infection relapse or superinfection, mechanical ventilation, ICU and hospital LOS |
| Annane (2013) [49] | 8 ICUs (France) | 58 patients with culture-negative severe sepsis | Initiation and Discontinuation: withhold or stop antibiotics (<0.25 µg/L); antibiotics strongly discouraged (0.25–0.5), recommended (0.5–5), strongly recommended (≥0.5). Higher cutoffs used for postsurgical patients |
Pregnancy, severe burns, trauma, cardiac arrest, postorthopedic surgery, neutropenic, withholding of life-supportive therapies, indisputable clinical infection, antibiotic exposure ≥48 hours before ICU admission | Nonsignificant trend: 67% vs 81% of patients on antibiotics at day 5 (P = .24) | No difference in day 5 mortality, ICU or hospital LOS or mortality, SOFA score at day 3 or 5 (but study terminated early due to low incidence of eligible patients) |
| Shehabi (2014) [31] ProGUARD |
11 ICUs (Australia) | 394 patients with suspected sepsis | Discontinuation only: stop antibiotics if PCT <0.1 µg/L, or 0.1–0.25 and infection is highly unlikely, or subsequent PCT declines >90% from baseline (daily PCT measurements) |
Antibiotics for surgical prophylaxis or proven infection requiring >3 weeks of therapy, fungal or viral infections, immunosuppressed, cardiac surgery or trauma or heat stroke within 48 hours, medullary thyroid or small cell lung cancer, not expected to survive, pregnancy | Nonsignificant trend: median 9 vs 11 days of antibiotic therapy (P = .58) | No difference (ventilation time, ICU and hospital LOS, hospital and 90-day mortality) |
| de Jong (2016) [32] SAPS |
ICUs at 15 hospitals (Netherlands) | 1546 patients with suspected or proven infection | Discontinuation only: stop antibiotics if PCT decreased to ≥80% of peak value, or ≤0.5 µg/L (daily PCT measurements) | Antibiotics for prophylaxis only or gut decontamination, expected ICU stay <24 hours, severe immunosuppression, severe viral or parasitic or tuberculosis infections, moribund, chronic infection (eg, endocarditis) | Median antibiotic consumption of 7.5 vs 9.3 daily defined doses (P < .0001), median treatment duration 5 vs 7 days (P < .0001) | Decreased 28-day mortality (20% vs 25%, P = .0122) and 1-year mortality (36% vs 43%, P = .0188). No difference in hospital and ICU LOS or requirement for additional antibiotics within 28 days. But 5% vs 3% rate of reinfection by same pathogen (P = .0492) |
| Bloos (2016) [50] SISPCT |
33 ICUs (Germany) | 1089 patients with severe sepsis or septic shock | Discontinuation or “Alert”: PCT measured on days 0, 1, 4, 7, 10, and 14. On day 4: change antibiotics or intensify source control efforts if PCT not decreased by ≥50% from baseline value. Other days: stop antibiotics if PCT <1 or decreased by ≥50% from previous value. (2 × 2 factorial study design with sodium selenite administration and PCT arms) | Pregnancy or lactation, selenium intoxication, infections with long recommended treatment durations, immunocompromised, imminent death or treatment limitations | 4.5% reduction in antibiotic exposure per 1000 ICU days (823 days vs 862 days) (P = .02) | No difference in 28-day mortality (25.6% vs 28.2%, P = .34), no differences in procedures for infection source control or diagnosis |
Abbreviations: ICU, intensive care unit; PCT, procalcitonin; LOS, length of stay; SAPS II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment score.
The first large multicenter study, the PROcalcitonin to Reduce Antibiotic Treatments in Acutely ill patients (PRORATA) trial, was conducted in 5 academic hospitals in France and included 621 adult patients with suspected bacterial infection at admission or during their stay in the ICU [30]. This study was unique in the ICU-based trials in that the algorithm included an initial procalcitonin to help assess whether to start antibiotics in addition to subsequent daily procalcitonin levels to help decide when to stop antibiotics. The cutoff procalcitonin value for discontinuation was <0.5 µg/L or a decrease from peak value by ≥80%. Overall, more than 80% of patients were retrospectively adjudicated to have microbiologically or clinically documented infection. The procalcitonin group had significantly more days at 28 days without antibiotics (14.3 days vs 11.6 days) and an overall 23% relative reduction in days of antibiotic exposure (mean 10.3 vs 13.3 days). The algorithm was overruled in 53% of cases (a much higher rate than in ProHOSP). The most common reason for nonadherence was physician reluctance in stopping antibiotic therapy in patients with low procalcitonin levels but ongoing clinical instability. The study showed noninferiority for 28-day and 60-day mortality, with no difference in other clinical outcomes. However, many critics were concerned about the trend towards higher 60-day mortality seen in the procalcitonin group (30.0% vs 26.1%), even though it did not meet the 10% noninferiority boundary set by the trial. Although none of the 60-day deaths in the procalcitonin group appeared to be attributable to relapsed or recurrent infection, this finding has made some critical care clinicians reticent to use procalcitonin in this way.
The next multicenter randomized controlled trial, the ProGUARD study, was conducted in 11 Australian ICUs and enrolled 400 adult patients with suspected sepsis [31]. The algorithm was much more conservative than PRORATA, because it advised stopping antibiotics if procalcitonin was <0.1 µg/L or levels decreased by >90% from baseline (vs 0.5 µg/L or ≥80% decline in PRORATA). The control arm explicitly included antimicrobial stewardship guidance. There was a nonsignificant trend towards decreased antibiotic use in the procalcitonin group (median treatment duration of 9 vs 11 days, P = .58), with no difference in clinical outcomes, despite high (>97%) protocol compliance. The more conservative procalcitonin threshold used may explain the lack of benefit compared with PRORATA. In addition, the study was likely underpowered because it was designed to detect an ambitious reduction of antibiotic therapy by 3.75 days.
The largest trial to date was the Stop Antibiotics on Procalcitonin guidance Study (SAPS), a multicenter randomized trial in patients with suspected infection admitted to the ICU in 3 university medical centers and 12 teaching hospitals [32]. Importantly, this trial was conducted in the Netherlands, a country known to have judicious antibiotic prescribing practices. Baseline procalcitonin measurements were obtained around time of initiation of antibiotics and each day until ICU discharge or 3 days after systemic antibiotics were stopped. The algorithm in SAPS was similar to PRORATA, as clinicians were advised to stop antibiotics if procalcitonin was ≤0.5 µg/L or if it decreased by ≥80% of peak value. Unlike PRORATA, the algorithm was not designed to encourage or discourage antibiotic initiation at the time of suspected infection.
Overall, the procalcitonin group had significantly lower median antibiotic consumption: 7.5 vs 9.3 defined daily doses and 5 vs 7 median days of treatment. As expected in this country with low antibiotic prescribing rates, treatment durations were lower in both arms compared with ProGUARD and PRORATA. Although this was designed as a noninferiority study, the procalcitonin group had statistically significant lower mortality at 28 days (20% vs 25% in control group, P = .0122) and at 1 year (36% vs 43%, P = .0188). There was 53% physician adherence to the algorithm within 48 hours. As in PRORATA, the main reason for nonadherence was concern about stopping antibiotics when the patient was not yet clinically stable. Although clinical outcomes were generally superior (mortality) or no different (hospital and ICU length of stay and requirement for additional courses of antibiotics within 28 days), 5% of the procalcitonin group required a second course of antibiotics for proven reinfection by the same pathogen, vs 3% in the standard care group (P = .0492).
Despite the mild increase in reinfection seen in the intervention arm, the SAPS trial provides the strongest evidence to date that procalcitonin algorithms can be used to help safely decrease antibiotic exposure in critically ill patients, even when baseline antibiotic treatment courses are fairly short. More importantly, SAPS alleviated the concerning trend towards higher mortality seen in the PRORATA trial. Although the reasons for the lower mortality were not explicitly studied, the study authors speculated that high procalcitonin levels may lead physicians to feel reassured about their initial diagnosis of sepsis, whereas low procalcitonin levels may lead physicians to seek noninfectious diagnoses at an earlier stage than they would otherwise and thus treat patients’ true causes of illness sooner [32]. In addition, antibiotic reduction may decrease adverse effects, selection of resistant pathogens, and C difficile.
LIMITATIONS AND AREAS FOR FUTURE RESEARCH
Despite the strength of evidence described above, there are several limitations to these studies that clinicians should be aware of before using procalcitonin in their own practice. First, in all procalcitonin-based trials, treatment group blinding is impossible, so the possibility of treatment bias cannot be ruled out. Randomized controlled trials are also accompanied by education and close guidance to physicians on how to apply the procalcitonin algorithms. Despite this, protocol adherence in the ICU-based trials has typically been approximately 50%. How closely physicians outside of clinical trials adhere to evidence-based algorithms is unclear. Large, ideally prospective studies are thus needed to examine the way procalcitonin is being used as well as its impact in real-world situations. Future studies quantifying procalcitonin impact should also examine its effects on antibiotic-associated adverse reactions, C difficile infections, and antibiotic resistance. Cost-effectiveness should also be carefully evaluated; so far, however, several small studies do suggest a net positive benefit in terms of reduction of overall costs of care [33–35]. One retrospective propensity-matched observational study using a large US database also suggested that procalcitonin testing on ICU admission was associated with lower hospital and ICU length of stay and costs [36].
Second, the optimal strategy for implementing procalcitonin algorithms in hospitals to maximize compliance and its impact on antibiotic use, outcomes, and cost remains unknown. Procalcitonin algorithms may not be well understood or trusted by front-line clinicians, and lessons can be learned from the literature showing that rapid diagnostics for bloodstream infections have greatest impact on patients and resource utilization when integrated with an antibiotic stewardship program that receives results in real-time and can intervene [37]. Furthermore, although the SAPS trial shows that procalcitonin can reduce antibiotic exposure when baseline treatment courses are already short, it remains to be seen whether similar results can be achieved simply by shortening global duration of treatment recommendations and implementing rigorous antibiotic stewardship programs. This is particularly relevant in light of recent randomized controlled trials and guidelines supporting shorter treatment courses for various infections [38–40].
Third, immunocompromised patients have generally been excluded from randomized controlled trials. However, evidence exists that procalcitonin is still a sensitive marker of sepsis in these patients [41]. Given that immunocompromised patients account for a substantial amount of antibiotic use, future research is required to determine whether procalcitonin algorithms can help safely guide therapy in this patient population. Furthermore, optimal diagnostic cutoff points for sepsis may be higher in surgical versus medical patients with sepsis [42]. Surgical patients were a minority of cases in the large ICU-based trials, and it is unclear whether different algorithms should be applied in these patients. Other patient populations that have generally been excluded, but in whom guidance regarding antibiotic therapy duration would be valuable, include pregnant patients, patients with cystic fibrosis, and those with infections generally treated with prolonged antibiotic courses (such as osteomyelitis or endocarditis).
Fourth, although the procalcitonin cutoffs used for respiratory tract infections and ICU patients has been generally consistent (0.25 µg/L and 0.5 µg/L, respectively), there have been slight variations in the algorithms in different studies. As demonstrated by the ProGUARD study, where the procalcitonin cutoff level for discontinuing antibiotics was a conservative 0.1 µg/L, the benefit of procalcitonin may depend on what algorithm is used. At this point, however, it seems reasonable for hospitals to adopt the algorithms used in the largest studies for respiratory infections (ProHOSP) and ICU patients (PRORATA and SAPS). Future studies should aim (1) to explore the comparative effectiveness of different algorithms to optimize the use of procalcitonin and (2) to demonstrate whether procalcitonin guidance is safe and effective for all types of suspected or proven infections in non-ICU patients, not just those with respiratory infections.
Finally, most of the trials have been conducted in Europe. Thus, the generalizability of these findings to the US population has not yet been clearly demonstrated. However, the Procalcitonin Antibiotic Consensus (ProACT) study, a US-based multicenter trial, is underway and hopefully will help close this particular knowledge gap [43]. This trial is similar to the ProHOSP study and aims to study the effect of a procalcitonin algorithm in noncritically ill adult patients presenting to the emergency department with lower respiratory tract infections. A positive result from ProACT, and a study replicating SAPS in the United States, would undoubtedly go a long way in reassuring US-based practitioners about the safety and efficacy of procalcitonin for antibiotic guidance in the ICU.
PROCALCITONIN AS AN ALERT TOOL TO GUIDE ANTIBIOTIC ESCALATION
Although most of the ICU-based trials to date have focused on using procalcitonin as a tool to guide antibiotic discontinuation, there has also been interest in using procalcitonin-based screening to alert physicians to worsening sepsis. The Procalcitonin and Survival Study (PASS) was a randomized controlled trial at 9 university hospitals in Denmark that enrolled 1200 adults within 24 hours of ICU admission, regardless of whether or not infection was initially suspected, and measured daily procalcitonin levels [44]. If procalcitonin levels were ≥1.0 µg/L and not decreasing at least 10% from prior day, clinicians were required to increase the spectrum of antibiotic coverage and/or intensify infection diagnostic efforts. The study showed no benefit in 28-day mortality. Although the intervention increased the number of cultures taken and broad-spectrum antibiotic use, it did not improve time to appropriate antibiotics, except in patients with bloodstream infections. There was evidence of harm with increased ICU length of stay and increased rates of mechanical ventilation, vasopressor use, and renal injury.
SUMMARY AND RECOMMENDATIONS
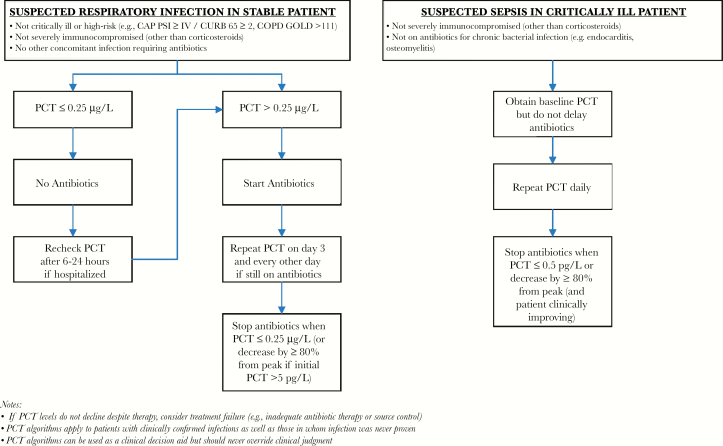
Procalcitonin has been extensively studied as a biomarker of bacterial infection, and most studies suggest good but imperfect diagnostic performance. However, based on over a dozen randomized controlled trials, it can be a useful aid to guide antibiotic therapy in the following 2 scenarios (summarized in Figure 1):
Figure 1.
Suggested algorithms for using procalcitonin to guide antibiotic therapy in stable patients with respiratory infections and critically ill patients with sepsis. CAP, community-acquired pneumonia; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PCT, procalcitonin; PSI, Pneumonia Severity Index.
-
1.
Non-critically ill patients with suspected or proven respiratory infection. Procalcitonin can be safely used at a cutoff of <0.25 µg/L to withhold antibiotics in stable, low-risk patients with suspected respiratory infections. If antibiotics are given, procalcitonin can help inform early discontinuation of antibiotics based on serial measurements, even in those with documented bacterial infections.
-
2.
Critically ill patients with suspected infection/sepsis. Clinicians are understandably reluctant to withhold antibiotics when sepsis is first suspected in unstable patients, regardless of procalcitonin levels, but serial procalcitonin measurements can be used at a cutoff of <0.5 µg/L or ≥80% decrease in peak level to help guide early antibiotic discontinuation once patients stabilize. Using procalcitonin in this fashion is safe, even in those with documented infections, and the largest trial to date (SAPS) suggests that it may even reduce mortality.
CONCLUSIONS
Clinicians must remember that false positives and negatives can occur with procalcitonin testing and that sepsis is a complex and heterogeneous syndrome. In most of the procalcitonin trials, overruling of the algorithm was allowed and in fact common in the ICU-based studies, underscoring the fact that procalcitonin should not replace clinical judgment. Clinicians should also be aware of the patients typically excluded from trials, particularly severely immunocompromised patients. However, when used properly as a clinical decision aid, the evidence is robust that procalcitonin can be a powerful antibiotic stewardship tool.
Acknowledgments
I thank Drs. Richard Platt and Michael Klompas for reviewing the manuscript.
Potential conflicts of interest. The author: No reported conflicts.The author has submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 2. Hecker MT, Aron DC, Patel NP, et al. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003; 163:972–8. [DOI] [PubMed] [Google Scholar]
- 3. Huttner A, Harbarth S, Carlet J, et al. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2013; 2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care–reasons for caution. N Engl J Med 2014; 370:1673–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care 2013; 17:R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harbarth S, Holeckova K, Froidevaux C, et al. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med 2001; 164:396–402. [DOI] [PubMed] [Google Scholar]
- 8. Müller B, Becker KL, Schächinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 2000; 28:977–83. [DOI] [PubMed] [Google Scholar]
- 9. Dipalo M GL, Micca G, Pittalis S, et al. Multicenter comparison of automated procalcitonin immunoassays. Practical Laboratory Medicine 2015; 2: 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dandona P, Nix D, Wilson MF, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994; 79:1605–8. [DOI] [PubMed] [Google Scholar]
- 11. Schuetz P, Maurer P, Punjabi V, et al. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care 2013; 17:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. BalcI C, Sungurtekin H, Gürses E, et al. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit Care 2003; 7:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 2008; 36:941–52. [DOI] [PubMed] [Google Scholar]
- 14. Christ-Crain M, Müller B. Procalcitonin in bacterial infections—hype, hope, more or less? Swiss Med Wkly 2005; 135:451–60. [DOI] [PubMed] [Google Scholar]
- 15. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:426–35. [DOI] [PubMed] [Google Scholar]
- 16. Anand D, Das S, Bhargava S, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. J Crit Care 2015; 30:218.e7–12. [DOI] [PubMed] [Google Scholar]
- 17. Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008; 177:498–505. [DOI] [PubMed] [Google Scholar]
- 18. Rhee C, Kadri SS, Danner RL, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care 2016; 20:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA 2014; 312: 1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet 2004; 363:600–7. [DOI] [PubMed] [Google Scholar]
- 21. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med 2006; 174:84–93. [DOI] [PubMed] [Google Scholar]
- 22. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007; 131:9–19. [DOI] [PubMed] [Google Scholar]
- 23. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302:1059–66. [DOI] [PubMed] [Google Scholar]
- 24. Burkhardt O, Ewig S, Haagen U, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J 2010; 36:601–7. [DOI] [PubMed] [Google Scholar]
- 25. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med 2008; 168: 2000–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 26. Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J 2009; 34:1364–75. [DOI] [PubMed] [Google Scholar]
- 27. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2012; CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hochreiter M, Köhler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 2009; 13:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schroeder S, Hochreiter M, Koehler T, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 2009; 394:221–6. [DOI] [PubMed] [Google Scholar]
- 30. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375:463–74. [DOI] [PubMed] [Google Scholar]
- 31. Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med 2014; 190:1102–10. [DOI] [PubMed] [Google Scholar]
- 32. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis 2016; 16:819–27. [DOI] [PubMed] [Google Scholar]
- 33. Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Crit Care Med 2011; 39:1792–9. [DOI] [PubMed] [Google Scholar]
- 34. Michaelidis CI, Zimmerman RK, Nowalk MP, et al. Cost-effectiveness of procalcitonin-guided antibiotic therapy for outpatient management of acute respiratory tract infections in adults. J Gen Intern Med 2014; 29:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrison M, Collins CD. Is procalcitonin-guided antimicrobial use cost-effective in adult patients with suspected bacterial infection and sepsis? Infect Control Hosp Epidemiol 2015; 36:265–72. [DOI] [PubMed] [Google Scholar]
- 36. Balk RA, Bozzette SA, Cao Z, et al. Effect of procalcitonin testing on healthcare utilization and costs in critically Ill patients in the United States. Chest 2016. doi:10.1016/j.chest.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rubach MP, Hanson KE. ID learning unit-diagnostics update: current laboratory methods for rapid pathogen identification in patients with bloodstream infections. Open Forum Infect Dis 2015; 2:ofv174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016; 176:1257–65. [DOI] [PubMed] [Google Scholar]
- 39. Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med 2015; 372:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalil AC, Metersky ML, Klompas M, et al. Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bele N, Darmon M, Coquet I, et al. Diagnostic accuracy of procalcitonin in critically ill immunocompromised patients. BMC Infect Dis 2011; 11:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clec’h C, Fosse JP, Karoubi P, et al. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med 2006; 34:102–7. [DOI] [PubMed] [Google Scholar]
- 43. ClinicalTrials.gov. Procalcitonin Antibiotic Consensus Trial (ProACT). Available at: https://clinicaltrials.gov/ct2/show/NCT02130986 Accessed 11 September 2016. [Google Scholar]
- 44. Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011; 39:2048–58. [DOI] [PubMed] [Google Scholar]
- 45. Kristoffersen KB, Søgaard OS, Wejse C, et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission–a randomized trial. Clin Microbiol Infect 2009; 15:481–7. [DOI] [PubMed] [Google Scholar]
- 46. Long W, Deng X, Zhang Y, et al. Procalcitonin guidance for reduction of antibiotic use in low-risk outpatients with community-acquired pneumonia. Respirology 2011; 16:819–24. [DOI] [PubMed] [Google Scholar]
- 47. Tang J, Long W, Yan L, et al. Procalcitonin guided antibiotic therapy of acute exacerbations of asthma: a randomized controlled trial. BMC Infect Dis 2013; 13:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Branche AR, Walsh EE, Vargas R, et al. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis 2015; 212:1692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Annane D, Maxime V, Faller JP, et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open 2013; 3: e002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bloos F, Trips E, Nierhaus A, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med 2016; 176:1266–76. [DOI] [PubMed] [Google Scholar]