Abstract
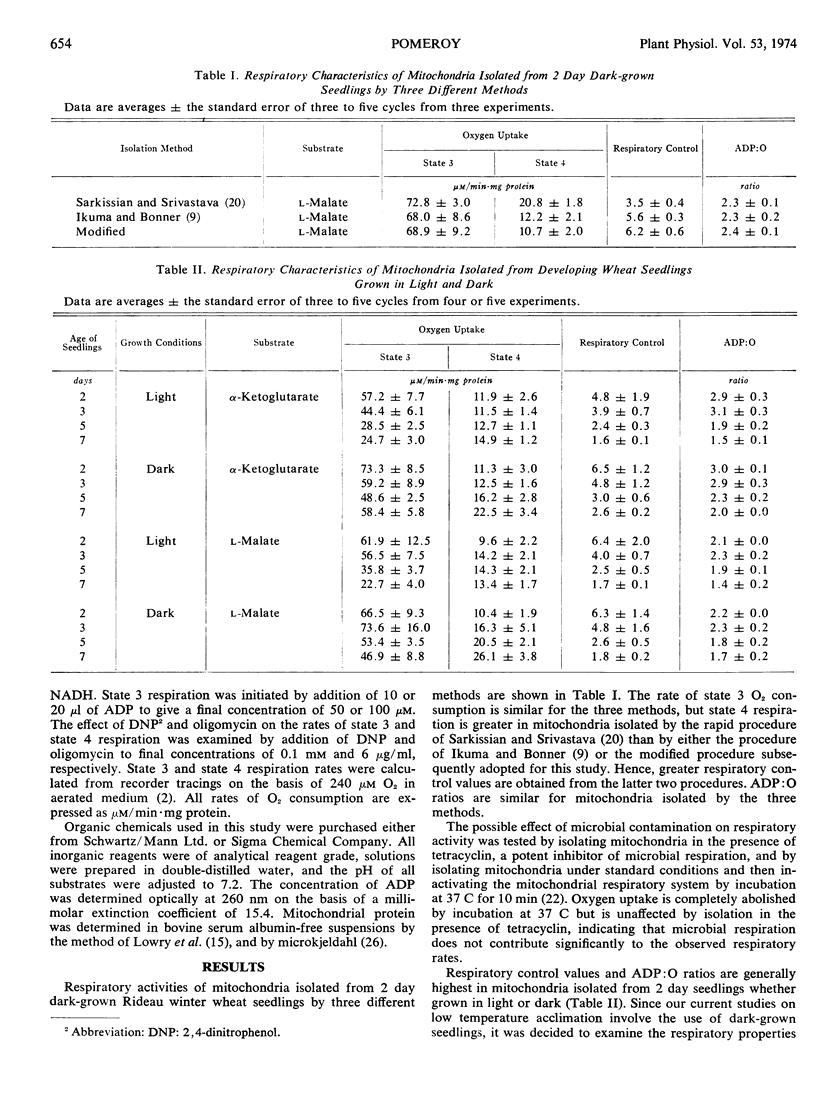
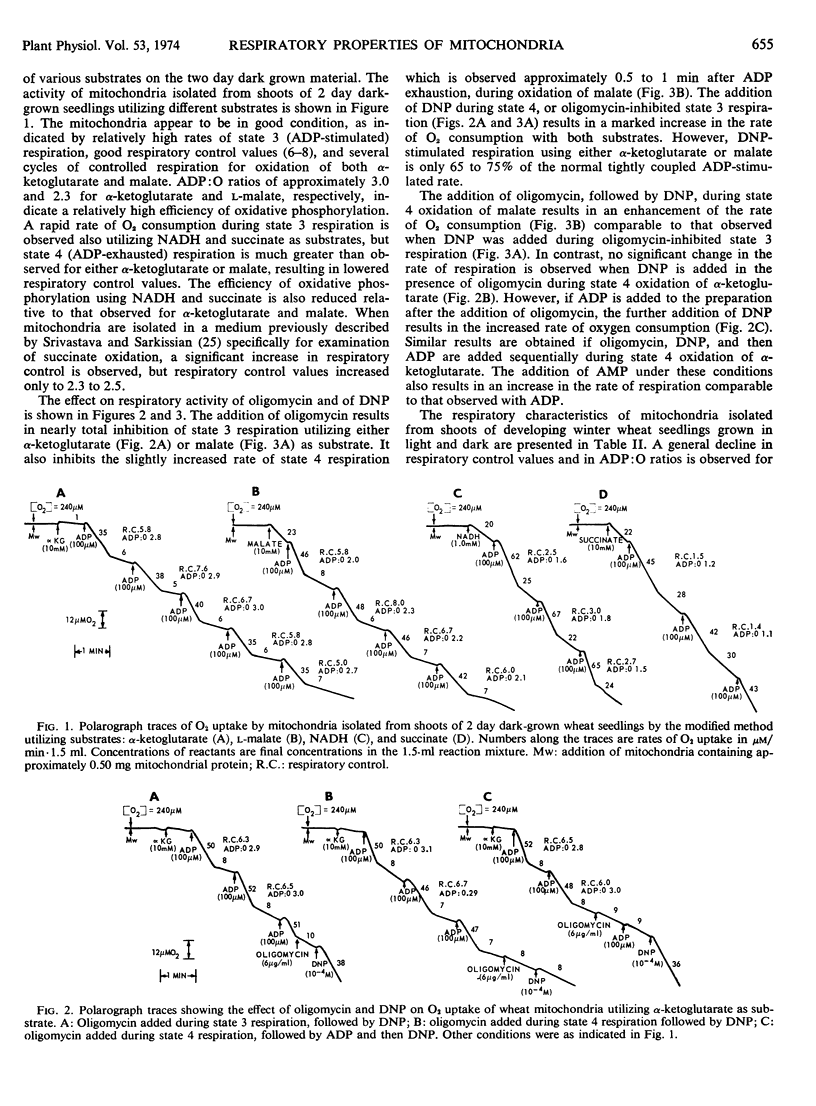
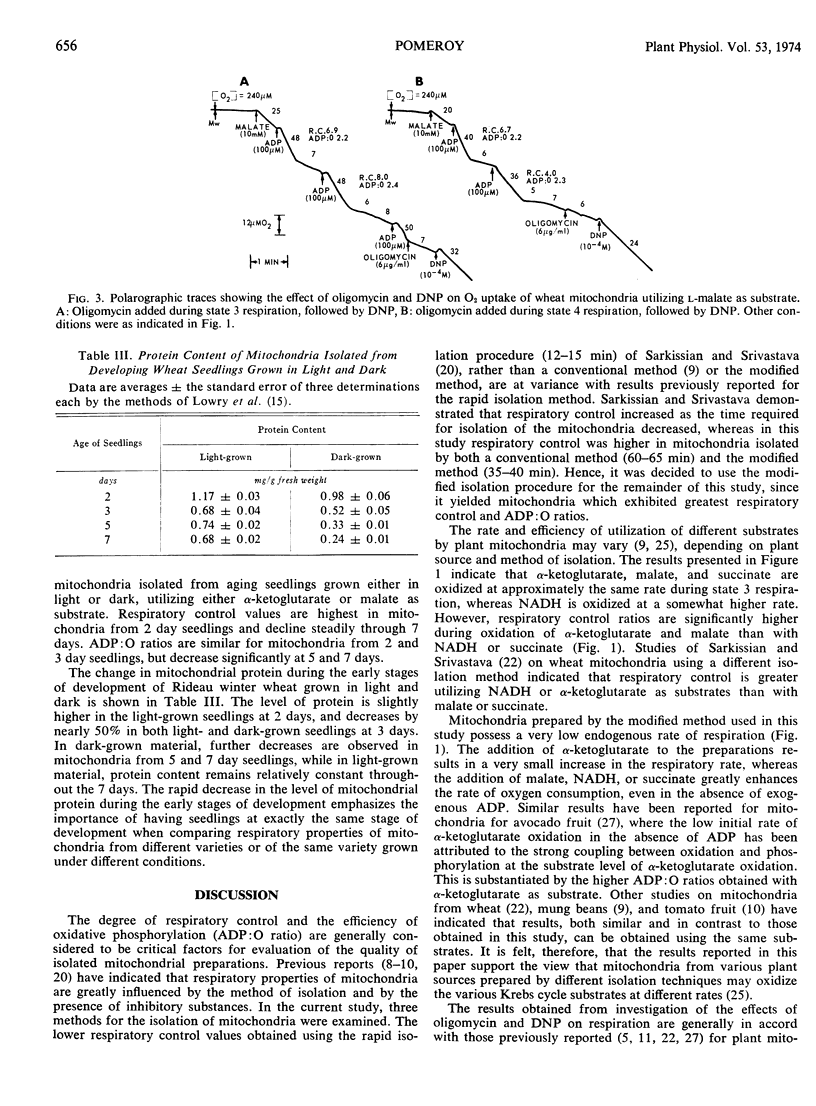
Mitochondria isolated from shoots of 2 days, light- and dark-grown winter wheat (Triticum aestivum L. cv. Rideau) seedlings oxidize α-ketoglutarate and l-malate with good respiratory control and ADP: O ratios. The efficiency of oxidative phosphorylation, and respiratory control are both reduced significantly when succinate or NADH is employed as substrate. Respiratory control values and ADP: O ratios show a general decline in mitochondria from seedlings of increasing age, whether grown in light or dark. In light-grown seedlings, the decrease in respiratory control with aging is due principally to a decrease in the rate of state 3 respiration, while in dark-grown material, the decrease appears to be due mainly to an increased rate of state 4 respiration. In both light- and dark-grown seedlings, oxygen consumption during state 3 respiration is severely inhibited by oligomycin. During state 4 respiration, 2,4-dinitrophenol stimulates oxygen uptake to a level approximately two-thirds the normal ADP-stimulated rate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS H., WALKER D. A. The oxidative activity of particulate fractions from germinating castor beans. Biochem J. 1956 Jan;62(1):114–120. doi: 10.1042/bj0620114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku H. S., Pratt H. K., Spurr A. R., Harris W. M. Isolation of active mitochondria from tomato fruit. Plant Physiol. 1968 Jun;43(6):883–887. doi: 10.1104/pp.43.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- LOOMIS W. F., LIPMANN F. Reversible inhibition of the coupling between phosphorylation and oxidation. J Biol Chem. 1948 Apr;173(2):807–807. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lance C., Hobson G. E., Young R. E., Biale J. B. Metabolic processes in cytoplasmic particles of the avocado fruit. VII. Oxidative and phosphorylative activities throughout the climacteric cycle. Plant Physiol. 1965 Nov;40(6):1116–1123. doi: 10.1104/pp.40.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. W., de la Roche I., Pomeroy M. K. Structural and functional responses of wheat mitochondrial membranes to growth at low temperatures. Plant Physiol. 1974 Mar;53(3):426–433. doi: 10.1104/pp.53.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M. The influence of mitochondrial concentration and storage on the respiratory control of isolated plant mitochondria. Plant Physiol. 1970 Apr;45(4):382–385. doi: 10.1104/pp.45.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkissian I. V., Srivastava H. K. A very simple trick separates plant mitochondria from starch. Life Sci. 1969 Nov 15;8(22):1201–1205. doi: 10.1016/0024-3205(69)90175-1. [DOI] [PubMed] [Google Scholar]
- Sarkissian I. V., Srivastava H. K. High efficiency of oxidative phosphorylation in mitochondria of wheat. Can J Biochem. 1970 Jun;48(6):692–698. doi: 10.1139/o70-109. [DOI] [PubMed] [Google Scholar]
- Sarkissian I. V., Srivastava H. K. On methods of isolation of active, tightly coupled mitochondria of wheat seedlings. Plant Physiol. 1968 Sep;43(9):1406–1410. doi: 10.1104/pp.43.9.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D., Rheaume B., Pomeroy K., Lepage M. Phospholipid, protein, and nucleic acid increases in protoplasm and membrane structures associated with development of extreme freezing resistance in black locust tree cells. Cryobiology. 1968 Nov-Dec;5(3):202–225. doi: 10.1016/s0011-2240(68)80164-6. [DOI] [PubMed] [Google Scholar]
- Wiskich J. T., Young R. E., Biale J. B. Metabolic Processes in Cytoplasmic Particles of the Avocado Fruit. VI. Controlled Oxidations and Coupled Phosphorylations. Plant Physiol. 1964 May;39(3):312–322. doi: 10.1104/pp.39.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche I. A., Andrews C. J. Changes in Phospholipid Composition of a Winter Wheat Cultivar during Germination at 2 C and 24 C. Plant Physiol. 1973 Mar;51(3):468–473. doi: 10.1104/pp.51.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]


