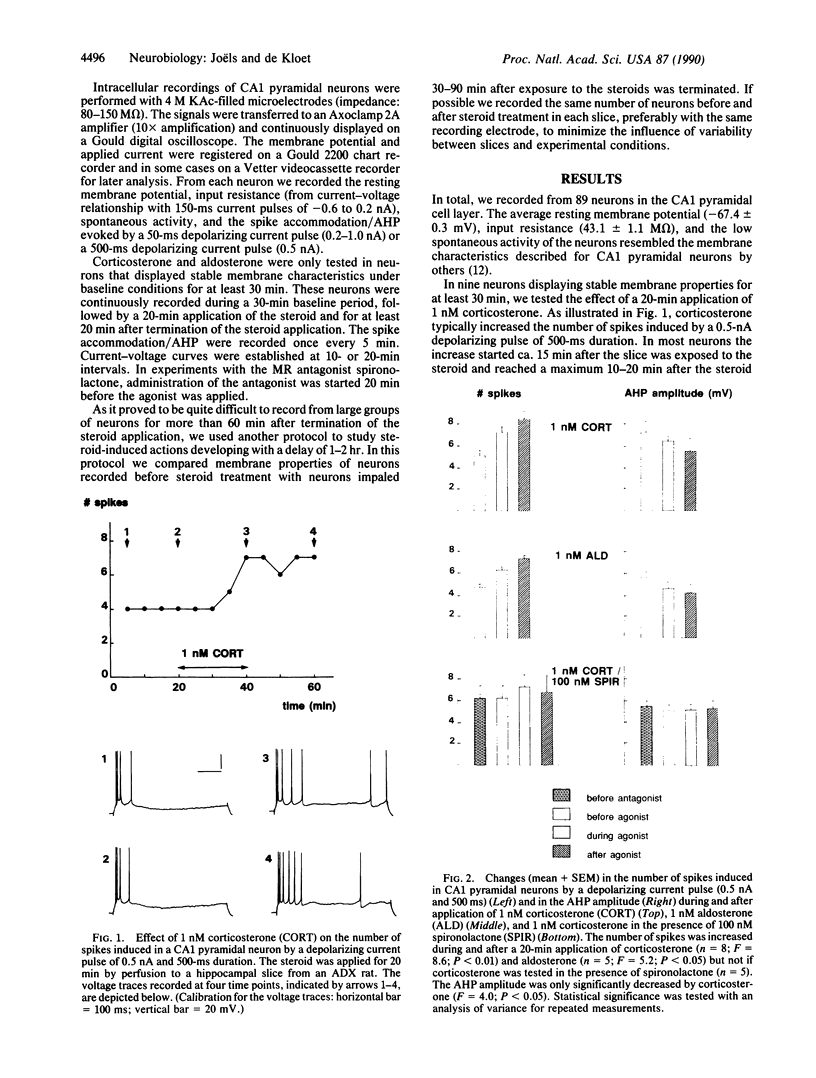
Abstract
Pyramidal neurons in the rat hippocampus contain mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs) to which the adrenal steroid corticosterone binds with differential affinity. We have used intracellular recording techniques to examine MR-mediated effects on membrane properties of CA1 pyramidal neurons in hippocampal slices from adrenalectomized rats. Low doses of corticosterone (1 nM) applied by perfusion for 20 min decreased the spike accommodation observed during a depolarizing current pulse (0.5 nA for 500 ms) and the amplitude of the subsequent afterhyperpolarization without affecting other membrane properties tested. The decrease became apparent ca. 15 min after steroid perfusion was started and reached its peak value 10-20 min after the steroid perfusion was terminated. The steroid effect was blocked by the MR antagonist spironolactone and mimicked by the natural MR ligand aldosterone (1 nM). Neurons recorded 30-90 min after termination of aldosterone application still displayed a decreased spike accommodation. However, 30-90 min after corticosterone application, the decrease in spike accommodation/afterhyperpolarization appeared to be reversed. Higher doses of corticosterone (greater than or equal to 30 nM) induced a significant increase in accommodation and amplitude of the afterhyperpolarization, as was previously observed for selective GR ligands. The data indicate that MR and GR activations induce opposite actions on the spike accommodation/afterhyperpolarization of CA1 pyramidal neurons, an important intrinsic mechanism of these neurons to regulate their response to excitatory input. We suggest that occupation of both MR and GR by the endogenous ligand corticosterone will result in an initial MR-mediated enhanced cellular excitability, which is gradually reversed and overridden by a GR-mediated suppression of cellular activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronsson M., Fuxe K., Dong Y., Agnati L. F., Okret S., Gustafsson J. A. Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9331–9335. doi: 10.1073/pnas.85.23.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza J. L., Simerly R. B., Swanson L. W., Evans R. M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988 Nov;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Barak Y. B., Gutnick M. J., Feldman S. Iontophoretically applied corticosteroids do not affect the firing of hippocampal neurons. Neuroendocrinology. 1977;23(4):248–256. doi: 10.1159/000122672. [DOI] [PubMed] [Google Scholar]
- Bohus B., de Kloet E. R. Adrenal steroids and extinction behavior: antagonism by progesterone, deoxycorticosterone and dexamethasone of a specific effect of corticosterone. Life Sci. 1981 Jan 26;28(4):433–440. doi: 10.1016/0024-3205(81)90090-4. [DOI] [PubMed] [Google Scholar]
- Dafny N., Phillips M. I., Taylor A. N., Gilman S. Dose effects of cortisol on single unit activity in hypothalamus, reticular formation and hippocampus of freely behaving rats correlated with plasma steroid levels. Brain Res. 1973 Sep 14;59:257–272. doi: 10.1016/0006-8993(73)90265-5. [DOI] [PubMed] [Google Scholar]
- De Kloet E. R., Reul J. M. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12(2):83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Arriza J. L. A molecular framework for the actions of glucocorticoid hormones in the nervous system. Neuron. 1989 Feb;2(2):1105–1112. doi: 10.1016/0896-6273(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Funder J. W. Adrenal steroids: new answers, new questions. Science. 1987 Jul 17;237(4812):236–237. doi: 10.1126/science.3603018. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Evidence for two types of afterhyperpolarization in CA1 pyramidal cells in the hippocampus. Brain Res. 1981 Feb 16;206(2):462–468. doi: 10.1016/0006-8993(81)90548-5. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Joëls M., de Kloet E. R. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science. 1989 Sep 29;245(4925):1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- Kerr D. S., Campbell L. W., Hao S. Y., Landfield P. W. Corticosteroid modulation of hippocampal potentials: increased effect with aging. Science. 1989 Sep 29;245(4925):1505–1509. doi: 10.1126/science.2781293. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984 Sep;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., De Kloet E. R., Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986 Oct;66(4):1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Weiss J. M., Schwartz L. S. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968 Nov 30;220(5170):911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Mosher K. M., Young D. A., Munck A. Evidence for irreversible, actinomycin D-sensitive, and temperature-sensitive steps following binding of cortisol to glucocorticoid receptors and preceding effects on glucose metabolism in rat thymus cells. J Biol Chem. 1971 Feb 10;246(3):654–659. [PubMed] [Google Scholar]
- Nichols N. R., Lerner S. P., Masters J. N., May P. C., Millar S. L., Finch C. E. Rapid corticosterone-induced changes in gene expression in rat hippocampus display type II glucocorticoid receptor specificity. Mol Endocrinol. 1988 Mar;2(3):284–290. doi: 10.1210/mend-2-3-284. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The coupling of neurotransmitter receptors to ion channels in the brain. Science. 1988 Jul 29;241(4865):545–551. doi: 10.1126/science.2456612. [DOI] [PubMed] [Google Scholar]
- Ratka A., Sutanto W., De Kloet E. R. Long-lasting glucocorticoid suppression of opioid-induced antinociception. Neuroendocrinology. 1988 Oct;48(4):439–444. doi: 10.1159/000125046. [DOI] [PubMed] [Google Scholar]
- Reiheld C. T., Teyler T. J., Vardaris R. M. Effects of corticosterone on the electrophysiology of hippocampal CA1 pyramidal cells in vitro. Brain Res Bull. 1984 Apr;12(4):349–353. doi: 10.1016/0361-9230(84)90102-3. [DOI] [PubMed] [Google Scholar]
- Reul J. M., de Kloet E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985 Dec;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rey M., Carlier E., Soumireu-Mourat B. Effects of RU 486 on hippocampal slice electrophysiology in normal and adrenalectomized BALB/c mice. Neuroendocrinology. 1989 Feb;49(2):120–124. doi: 10.1159/000125102. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975 Mar 7;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eekelen J. A., Jiang W., De Kloet E. R., Bohn M. C. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res. 1988 Sep;21(1):88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- Vidal C., Jordan W., Zieglgänsberger W. Corticosterone reduces the excitability of hippocampal pyramidal cells in vitro. Brain Res. 1986 Sep 24;383(1-2):54–59. doi: 10.1016/0006-8993(86)90007-7. [DOI] [PubMed] [Google Scholar]




