Abstract
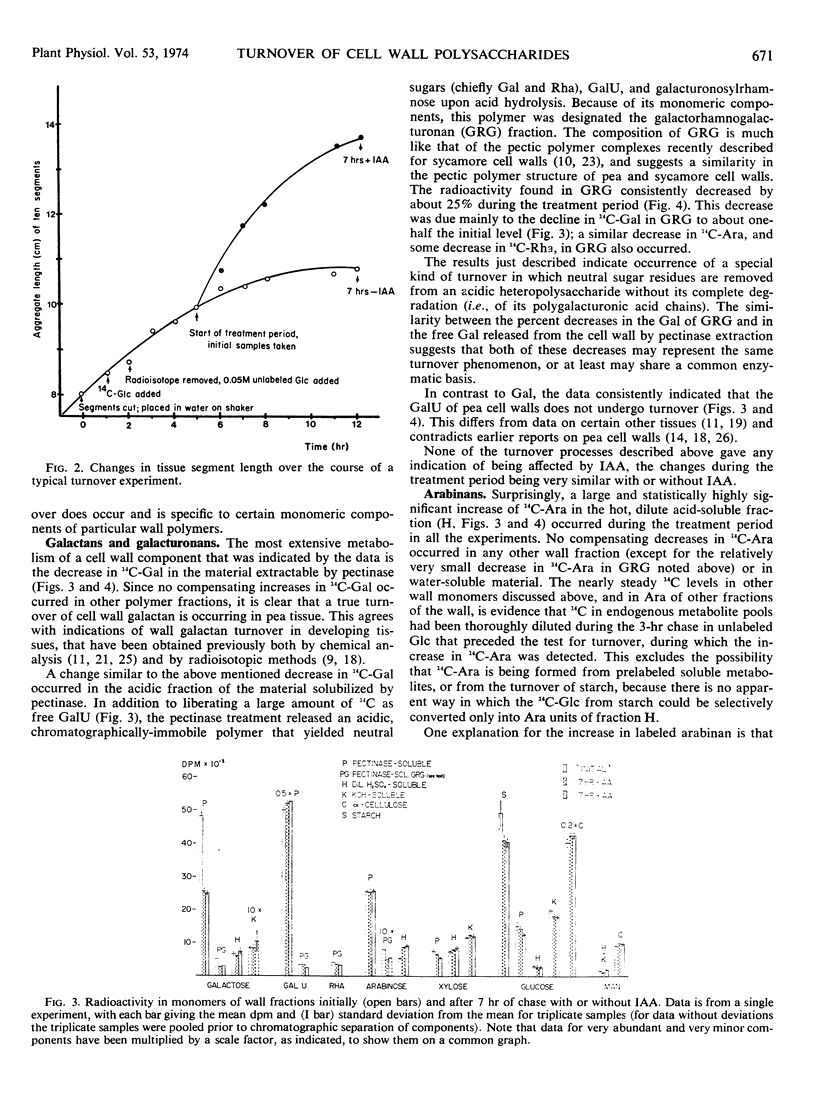
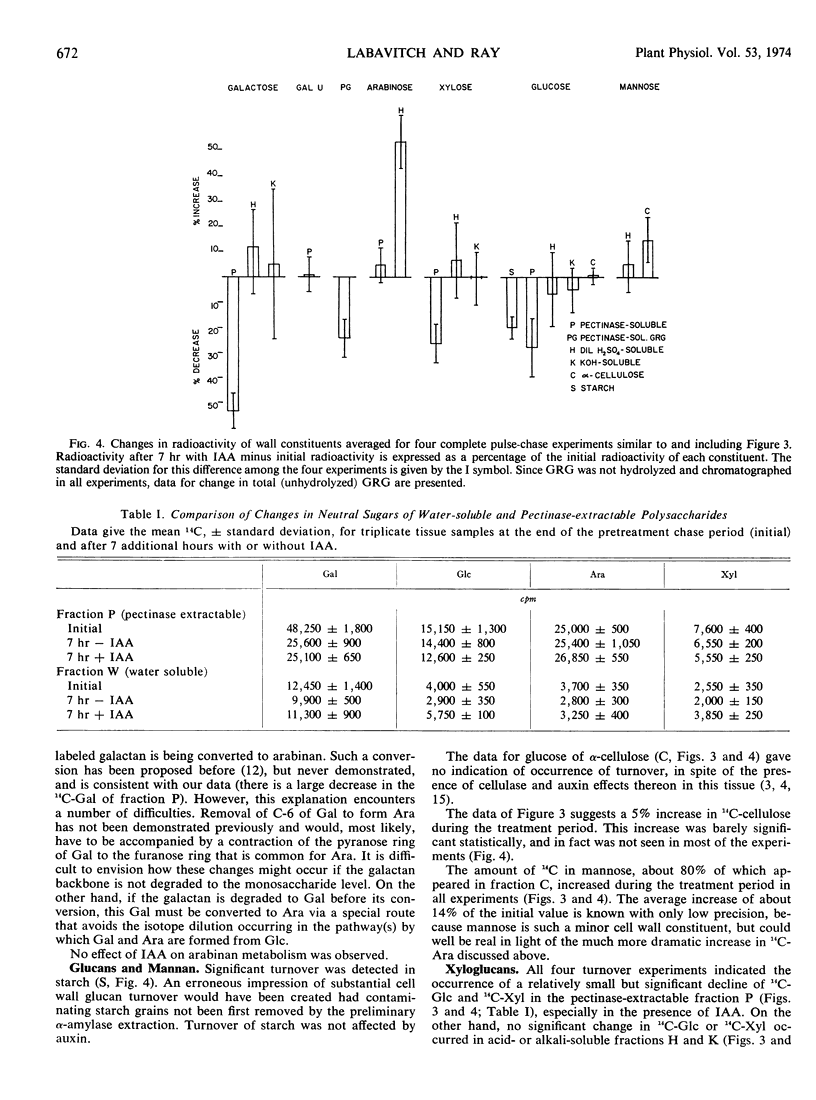
Turnover of cell wall polysaccharides and effects of auxin thereon were examined after prelabeling polysaccharides by feeding pea (Pisum sativum var. Alaska) stem segments 14C-glucose, then keeping the tissue 7 hours in unlabeled glucose with or without indoleacetic acid. There followed an extraction, hydrolysis, and chromatography procedure by which labeled monosaccharides and uronic acids were released and separated with consistently high recovery. Most wall polymers, including galacturonan and cellulose, did not undergo appreciable turnover. About 20% turnover of starch, which normally contaminates cell wall preparations but which was removed by a preliminary step in this procedure, occurred in 7 hours. Quantitatively, the principal wall polymer turnover process observed was a 50% decrease in galactose in the pectinase-extractable fraction, including galactose attached to a pectinase-resistant rhamnogalacturonan. Other pectinase-resistant galactan(s) did not undergo turnover. No turnover was observed in arabinans, but a doubling of radioactivity in arabinose of the pectinase-resistant, hot-acid-degradable fraction occurred in 7 hours, possibly indicating conversion of galactan into arabinan. None of the above changes was affected by indoleacetic acid, but a quantitatively minor turnover of a pectinase-degradable xyloglucan was found to be consistently promoted by indole-acetic acid. This was accompanied by a reciprocal increase in water-soluble xyloglucan, suggesting that indoleacetic acid induces conversion of wall xyloglucan from insoluble to water-soluble form. The results indicate a highly selective pattern of wall turnover processes with an even more specific influence of auxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A., Ray P. M. Regulation by auxin of carbohydrate metabolism involved in cell wall synthesis by pea stem tissue. Plant Physiol. 1971 Apr;47(4):537–544. doi: 10.1104/pp.47.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. E., Rees D. A., Wight N. J. Polysaccharides in germination. Xyloglucans ( amyloids') from the cotyledons of white mustard. Biochem J. 1971 Aug;124(1):47–53. doi: 10.1042/bj1240047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Ordin L. Metabolic turnover in cell wall constituents of Avena sativa L. coleoptile sections. Biochim Biophys Acta. 1967 Jun 13;141(1):118–125. doi: 10.1016/0304-4165(67)90250-4. [DOI] [PubMed] [Google Scholar]
- Keegstra K., Talmadge K. W., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: III. A Model of the Walls of Suspension-cultured Sycamore Cells Based on the Interconnections of the Macromolecular Components. Plant Physiol. 1973 Jan;51(1):188–197. doi: 10.1104/pp.51.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y., Yamamoto R. Effect of auxin on beta-1, 3-glucanase activity in Avena coleoptile. Dev Growth Differ. 1970 Mar;11(4):287–296. doi: 10.1111/j.1440-169x.1970.00287.x. [DOI] [PubMed] [Google Scholar]
- Nevins D. J., English P. D., Albersheim P. Changes in cell wall polysaccharides associated with growth. Plant Physiol. 1968 Jun;43(6):914–922. doi: 10.1104/pp.43.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Rottenberg D. A. Uronic acid constituents of oat-coleoptile cell walls. Biochem J. 1964 Mar;90(3):646–655. doi: 10.1042/bj0900646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. II. Carbohydrate constituents of each main component. Biochem J. 1961 Dec;81:455–464. doi: 10.1042/bj0810455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YACHNIN S. Non-antigenicity of synthetic polyribonucleotides and apurinic acid. Nature. 1962 Sep 29;195:1319–1319. doi: 10.1038/1951319a0. [DOI] [PubMed] [Google Scholar]


