Abstract
Background
The aim of this study was to explore whether interactions between FTO rs9939609 and ABCA1 rs9282541 affect BMI and waist circumference (WC), and could explain previously reported population differences in FTO-obesity and FTO-BMI associations in the Mexican and European populations.
Methods
A total of 3938 adults and 636 school-aged children from Central Mexico were genotyped for both polymorphisms. Subcutaneous and visceral adipose tissue biopsies from 22 class III obesity patients were analyzed for FTO and ABCA1 mRNA expression. Generalized linear models were used to test for associations and gene-gene interactions affecting BMI, WC and FTO expression.
Results
FTO and ABCA1 risk alleles were not individually associated with higher BMI or WC. However, in the absence of the ABCA1 risk allele, the FTO risk variant was significantly associated with higher BMI (P = 0.043) and marginally associated with higher WC (P = 0.067), as reported in Europeans. The gene-gene interaction affecting BMI and WC was statistically significant only in adults. FTO mRNA expression in subcutaneous abdominal adipose tissue according to ABCA1 genotype was consistent with these findings.
Conclusions
This is the first report showing evidence of FTO and ABCA1 gene variant interactions affecting BMI, which may explain previously reported population differences. Further studies are needed to confirm this interaction.
Electronic supplementary material
The online version of this article (doi:10.1186/s12881-017-0410-y) contains supplementary material, which is available to authorized users.
Keywords: Body mass index, FTO and ABCA1 variants, Interaction
Background
The FTO rs9939609 gene variant has been consistently associated with BMI and obesity, however clear population differences have been identified [1]. Despite the high prevalence of obesity in Mexico, the FTO risk allele is considerably less frequent, both in admixed and Native populations as compared to Europeans (0.21, 0.06 and 0.46, respectively). Interestingly, rs9939609 has been associated only with class III obesity, but not with overall obesity or BMI in admixed Mexican adults [2, 3], and rs1421085, in high linkage disequilibrium with rs9939609, was not associated with obesity or BMI in admixed Mexican children [4]. It has been stated that loci that are specific to a single ancestry might contribute to genetic susceptibility across populations [5]. The ABCA1-R230C variant (rs9282541) is an ancestry-specific polymorphism private to the Americas and has been strongly associated with low HDL-cholesterol (HDL-C), although its association with BMI and obesity is inconsistent [6–8]. This allele is of particular interest, because it is relatively frequent in the Mexican mestizo population (0.11), is functional and was found to interact with BMI affecting abdominal fat distribution particularly in Mexican premenopausal women [8]. The aim of this study was to analyze possible FTO rs9939609 - ABCA1 rs9282541 interactions affecting BMI, waist circumference (WC) and HDL-C levels in individuals from Central Mexico, which could help explain the differences observed between this and European populations.
Methods
Study population description
The studied population included 3938 DNA samples of unrelated Mexican mestizo adults from 4 different cohorts and 636 DNA samples of unrelated school-aged children. All cohorts include samples from Central Mexico and have been previously described (Table 1). Protocols for each cohort were approved by their respective Institutional Ethics Committee. Fully informed written consent for participation was attained from all participants or legal guardians.
Table 1.
Description of the study cohorts
| Study (Reference) | Sample size (% ancestrya) | Region (State) | Males (%) | Mean age (years ± SD) | Mean BMI (Kg/m2 ± SD) | Mean WC (cm ± SD) | Mean HDL-C (mg/dL ± SD) |
|---|---|---|---|---|---|---|---|
| Romero-Hidalgo et al. [16] | 525 (67.5) | Central Mexico (Mexico City, Hidalgo, Edo. De México, Morelos, Querétaro) | 31.2% | 46.2 ± 13.6 | 27.7 ± 4.5 | 92.0 ± 14.1 | 44.8 ± 12.7 |
| Velázquez-Cruz et al. [17] | 1207 (50.6) | Central Mexico (Morelos) | 30.3% | 50.9 ± 15.3 | 27.0 ± 4.5 | 93.7 ± 10.8 | 44.3 ± 11.4 |
| Villarreal-Molina et al. [8] | 1511 (72.5) | Central Mexico (Mexico City) | 50.9% | 53.1 ± 9.3 | 28.4 ± 4.3 | 94.8 ± 11.6 | 45.9 ± 13.3 |
| Villalobos-Comparán et al. [2] | 695 (67.5) | Central Mexico (Mexico City) | 36.0% | 40.0 ± 13.6 | 27.2 ± 5.2 | 89.1 ± 13.4 | 46.3 ± 12.5 |
| León-Mimila et al. [3] | 636 | Central Mexico (Mexico City) | 48.23% | 9.4 ± 1.85 | 20.0 ± 3.84 | 70.2 ± 11.3 | 46.8 ± 10.9 |
aProportion of individuals with an ancestry estimation
Genotyping
The FTO rs9939609 and ABCA1 rs9282541 variants were genotyped in 3938 DNA samples using TaqMan assays (ABI Prism 7900HT Sequence Detection System, Applied Biosystems). In addition, because the Mexican-Mestizo population is admixed, individual ancestry estimates were analyzed for 2354 individuals to test whether the results could be confounded by population stratification. Different panels of ancestry informative markers were used for each cohort (Additional file 1: Table S1).
Expression analysis
FTO and ABCA1 mRNA expression was measured in subcutaneous (SAT) and visceral (VAT) adipose tissue biopsies from 22 admixed Mexican patients (16 female and 6 male), aged 25 to 55 years with BMI > =40 kg/m2, who underwent bariatric surgery at the Hospital General Rubén Leñero in Mexico City. Total RNA was extracted with RNeasy Lipid Tissue Mini Kit (Qiagen), cDNA was reverse transcribed with TaqMan Reverse Transcription Reagents Kit (Applied Biosystems). Expression was analyzed using GeneChip Human Genome 2.0 ST Array (Affymetrix). FTO and ABCA1 expression were validated by Real-Time PCR (LightCycler 480 II, Roche), using the following primers and probes: ctcggagaattagtttaggatatttca (forward) tctgacccccaaagatgatg (reverse) and probe #59 for FTO, and tgctgcatagtcttgggactc (forward), acctcctgtcgcatgtcact (reverse) and probe #17 for ABCA1. Hypoxanthine phosphoribosyl transferase (HPRT) expression was measured as reference [2].
Statistical methods
HDL-C measurements were log-transformed for the analysis. Generalized linear regression (GLM) models were used to evaluate the individual effect of each single nucleotide variant and the interaction between FTO and ABCA1 risk variants, adjusting for age, gender, ancestry and BMI as appropriate. GLM models were also used to compare mean values of subcutaneous and visceral FTO gene expression, adjusted for age and gender. Thus a model with main effects for risk variants and the adjusted variables plus the interactions between risk variants was fitted. All statistical analyses were performed using SPSS v.15.
Results
FTO and ABCA1 risk allele frequencies were 20.3 and 10.0% in the overall population, respectively. In order to avoid potential population stratification, all individuals included in the analysis were from Central Mexico.
In the overall analysis and under additive inheritance models, the FTO “A” risk allele was not significantly associated with higher BMI (β = 0.187, P = 0.143) or higher WC (β = 0.409, P = 0.208), nor was the ABCA1 “T” risk allele associated with BMI or WC (β = 0.247, P = 0.154 and β = 0.369, P = 0.403, respectively). Furthermore, although HDL-C levels were higher in FTO “A” homozygous individuals, the association did not reach statistical significance (β = 0.005, P = 0.097). As expected, the ABCA1 “T” allele was strongly associated with lower HDL-C levels (β = −0.03, P = 2.37 × 10−12) (Fig. 1).
Fig. 1.
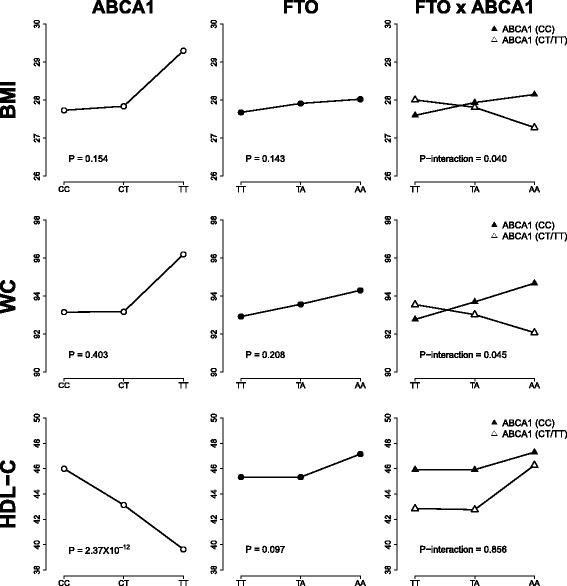
Association of FTO rs9939609 and ABCA1 rs9282541 risk variants, independently and stratified, with BMI, waist circumference (WC) and HDL-cholesterol (HDL-C) levels, adjusting for age, gender and BMI as appropriate
In order to assess a possible FTO-ABCA1 gene interaction, we sought associations between the FTO risk variant and BMI, WC and HDL-C, stratified by the absence or presence of the ABCA1 risk allele (“CC” and “CT/TT” genotypes, respectively). Interestingly, in the absence of the ABCA1 risk “T” allele, the FTO risk variant was significantly associated with higher BMI (β = 0.284, P = 0.042, n = 3191) and marginally associated with higher WC (β = 0.650, P = 0.063, n = 3191). In contrast, in the presence of the ABCA1 risk allele, the FTO risk variant was not associated with BMI (P = 0.421, n = 747) or WC (P = 0.376, n = 747). The interaction analyses between FTO rs9939609 and ABCA1 rs9282541 affecting BMI and WC were statistically significant (P = 0.040 and P = 0.045, respectively). ABCA1 and FTO gene variants showed no significant interaction affecting HDL-C levels (P = 0.856) (Fig. 1).
Individual ancestry estimates were available in 60% of the samples. After adjusting for Native American ancestry, the statistical significance of interactions between FTO rs9939609 and ABCA1 rs9282541 affecting BMI and WC was borderline significant (P = 0.054 and P = 0.063, respectively). This drop of significance is probably due to the lower sample size. Figure 2 shows the mean proportion of Native American component according to FTO genotype, stratified by the absence or presence of the ABCA1 risk allele (“CC” and “CT/TT” genotypes). As expected, the Native American component was lower in individuals with 1 and 2 FTO risk alleles, regardless of the presence of the ABCA1 risk allele. This suggests that the interactions are not confounded by ancestry.
Fig. 2.

Mean Native American ancestry proportion according to FTO rs99399609 genotype, stratified by ABCA1 rs9282541 genotype in 2354 individuals
We sought to evaluate this finding in an independent cohort of 636 children. As observed in adults, in the overall analysis using an additive inheritance model, the association of FTO “A” and the ABCA1 “T” risk alleles with BMI percentile did not reach statistical significance (β = 3.116, P = 0.084 and β = 4.002, P = 0.058, respectively). However, in the absence of the ABCA1 “T” risk variant, the effect of FTO risk allele over the BMI was higher and significant (β = 4.20, P = 0.043, n = 505), although the interaction did not reach significance (P = 0.356).
We further explored whether FTO mRNA expression is affected by ABCA1 genotypes. Figure 3 shows differences in relative FTO mRNA expression levels in human adipose tissue biopsies according to FTO rs9939609 genotypes under a dominant model. In the overall analysis of SAT biopsies, while FTO mRNA expression was higher for “TA/AA” than those with “TT” genotypes, the difference did not reach statistical significance (9.084 vs 8.961 AU, respectively; P = 0.068). However, considering only biopsies of individuals not bearing the ABCA1 risk allele (wild-type), FTO “TA/AA” SAT biopsies showed significantly higher FTO mRNA expression than those with “TT” genotypes (9.112 vs 8.943 AU, respectively; P = 0.003). Comparisons of FTO mRNA expression according to genotype in individuals bearing the ABCA1 risk variant were limited because only one individual carried the FTO “TT” genotype. However, the FTO “TA/AA” SAT biopsies showed significantly lower FTO mRNA expression levels in the presence of the ABCA1 risk allele (9.043 vs 9.112, P = 0.045). In VAT biopsies, FTO mRNA expression did not differ significantly according to genotype in the overall population (TT: 8.879 vs TA/AA: 8.975, P = 0.857), or in absence of the R230C risk allele (TT: 8.942; TA/AA: 8.965, P = 0.371). ABCA1 expression was not significantly affected by FTO rs9939609 genotypes (data not shown).
Fig. 3.

FTO mRNA expression levels in subcutaneous (SAT) and visceral adipose tissue (VAT) biopsies, in all tested biopsies (n = 22), and in individuals not bearing the ABCA1 rs9282541 risk variant (wild-type, n = 15). P-values were obtained adjusting by age and gender
Discussion
According to WHO, Mexico has one of the highest rates of adulthood and childhood obesity. This higher prevalence of obesity as compared to European populations could be explained by the Native American component as the result of adaptive processes related to energy saving, or could be the result ancestry-specific allele combinations derived from the admixture process. We thus analyzed whether the functional private ABCA1-R230C risk allele might interact with the most replicated obesity risk allele FTO rs9939609.
FTO and ABCA1 risk allele frequencies were 20.3% y 10%, respectively, similar to previous reports [2, 3]. Individually, FTO and ABCA1 risk alleles showed no significant association with BMI or WC. However, in the absence of the ABCA1 risk variant, the effect of FTO on BMI and WC became stronger, statistically significant and similar to what has been reported in European populations [9]. Observations from the cohort of children showed a similar trend, although the gene-gene interaction reached statistical significance only in the adult cohort. The lack of significance in children was likely due to the small sample size. Replication studies in independent adult and childhood cohorts are necessary to confirm this interaction. This type of interaction may explain differences in FTO associations with obesity between Mexican and European populations, but they do not explain the higher prevalence of obesity in Mexico.
FTO mRNA expression was significantly higher in SAT than in VAT, in accordance with previous studies in other populations [10]. Interestingly, allele-specific FTO expression in SAT differed significantly only in the absence of the ABCA1 risk allele, which is consistent with the interactions described above. It is noteworthy that no significant differences in allele-specific FTO expression have been observed in SAT biopsies from European individuals [11, 12]. However, a previous study in Mexican patients with morbid obesity, the rs9939609 “TA” genotype was significantly associated with higher FTO expression [2]. Although the latter biopsies were not genotyped for ABCA1-R230C, it is noteworthy that the only independent studies reporting allele-specific differences were performed in Mexican patients.
Although FTO and ABCA1 are both known to play a role in adipose tissue function, there is no previous experimental evidence directly linking the function of both genes [13, 14]. However, previous evidence supports the role of ABCA1 in body fat distribution both in the Mexican population [8], and in a recent multi-ethnic meta-analysis that identified ABCA1 rs10991437 as a variant associated with higher waist-hip ratio adjusted for BMI [15].
Conclusions
To our knowledge this is the first report showing evidence of FTO and ABCA1 gene variant interactions affecting BMI, which may explain previously reported population differences. Further studies are needed to understand the possible biological mechanisms underlying this interaction.
Acknowledgments
Funding
This research was partially supported by Fundación Miguel Alemán A.C. and Consejo Nacional de Ciencia y Tecnología grant numbers: SALUD-2009-01-111663, SALUD-2012-01-182801, SALUD-2009-01-112547, SALUD-2007-01-69473 and SALUD-2010-1-139795.
Availability of data and materials
All relevant data are available within the manuscript and its supporting information documents.
Authors’ contributions
MVC, BAP, SRH, MTVM responsible for the study design, and writing the manuscript. MVC, SRH, DJ participate in data analysis. MTVM, SCQ, RVC, SRH responsible for the cohort studies conception and design. PVLM, HVR, OESH, JAGB, MQ, JLMG, MRTB, MERA, CPR, GBA, FCP, JSC responsible of acquisition of samples, data and carried out the experiments. All authors reviewed and approved the submitted version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Protocols and informed consent forms for each cohort were approved by their respective Institutional Ethics Committee as follows: Ethics Committee of the National Institute of Public Health [16]; IMSS Research Ethics Committee [17]; Biomedical Research in Humans of the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” (INCMNSZ) [2, 3]; Instituto Nacional de Cardiología “Ignacio Chávez” (INCICH) and the Ethics Committee of the Instituto Nacional de Medicina Genoómica (INMEGEN) [8]. Fully informed written consent for participation was attained from all participants or legal guardians.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ABCA1
ATP Binding cassette Transporter A-1
- BMI
Body mass index
- FTO
Fat mass and obesity associated gene
- HDL-C
High Density Lipoprotein Cholesterol
- WC
Waist circumference
- WHO
World Health Organization
Additional file
Panels of ancestry informative markers used for each cohort [18]. (DOC 30 kb)
Contributor Information
Marisela Villalobos-Comparán, Email: marvico82@hotmail.com.
Bárbara Antuna-Puente, Email: bantuna@inmegen.gob.mx.
María Teresa Villarreal-Molina, Email: tvillareal@inmegen.gob.mx.
Samuel Canizales-Quinteros, Email: scanizales@inmegen.gob.mx.
Rafael Velázquez-Cruz, Email: rvelazquez@inmegen.gob.mx.
Paola León-Mimila, Email: paov_lemi@yahoo.com.mx.
Hugo Villamil-Ramírez, Email: hugo_villamil@hotmail.com.
Juan Antonio González-Barrios, Email: jantgonzalez69@gmail.com.
José Luis Merino-García, Email: joseluis_qfb@yahoo.com.mx.
María Rocío Thompson-Bonilla, Email: thompson_068@hotmail.com.
Diego Jarquin, Email: diego.jarquin@gmail.com.
Osvaldo Erik Sánchez-Hernández, Email: shoserik@hotmail.com.
Martha Eunice Rodríguez-Arellano, Email: marthaeunicer@yahoo.com.mx.
Carlos Posadas-Romero, Email: carlos.posadas@cardiologia.org.mx.
Gilberto Vargas-Alarcón, Email: gvargas63@yahoo.com.
Francisco Campos-Pérez, Email: loboluna28@gmail.com.
Manuel Quiterio, Email: mquitero@insp.mx.
Jorge Salmerón-Castro, Email: jorge.salmec@gmail.com.
Alessandra Carnevale, Email: acarnevale@inmegen.gob.mx.
Sandra Romero-Hidalgo, Email: sromero@inmegen.gob.mx.
References
- 1.Qi Q, Kilpeläinen TO, Downer MK, Tanaka T, Smith CE, Sluijs I, et al. FTO genetic variants, dietary intake and body mass index: insights from 177,330 individuals. Hum Mol Genet. 2014;23(25):6961–72. doi: 10.1093/hmg/ddu411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villalobos-Comparán M, Flores-Dorantes MT, Villarreal-Molina MT, Rodríguez-Cruz M, García-Ulloa AC, Robles L, et al. The FTO gene is associated with adulthood obesity in the Mexican population. Obesity. 2008;16(10):2296–301. doi: 10.1038/oby.2008.367. [DOI] [PubMed] [Google Scholar]
- 3.León-Mimila P, Villamil-Ramírez H, Villalobos-Comparán M, Villarreal-Molina T, Romero-Hidalgo S, López-Contreras B, et al. Impact of common genetic variants on childhood and adult obesity in the Mexican population. PLoS One. 2013;8(8):e70640. doi: 10.1371/journal.pone.0070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mejía-Benítez A, Klünder-Klünder M, Yengo L, Meyre D, Aradillas C, Cruz E, et al. Analysis of the contribution of FTO, NPC1, ENPP1, NEGR1, GNPDA2 and MC4R genes to obesity in Mexican children. BMC Med Genet. 2013;14:21. doi: 10.1186/1471-2350-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Loos RJ. Obesity genomics: assessing the transferability of susceptibility loci across diverse populations. Genome Med. 2013;5(6):55. doi: 10.1186/gm459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarreal-Molina T, Aguilar-Salinas CA, Rodríguez-Cruz M, Riaño D, Villalobos-Comparan M, Coral-Vazquez R, et al. The ATP-binding cassette transporter A1 R230C variant affects HDL cholesterol levels and BMI in the Mexican population: association with obesity and obesity-related comorbidities. Diabetes. 2007;56(7):1881–7. doi: 10.2337/db06-0905. [DOI] [PubMed] [Google Scholar]
- 7.Acuña-Alonzo V, Flores-Dorantes T, Kruit JK, Villarreal-Molina T, Arellano-Campos O, Hünemeier T, et al. A functional ABCA1 gene variant is associated with low HDL-cholesterol levels and shows evidence of positive selection in Native Americans. Hum Mol Genet. 2010;19(14):2877–85. doi: 10.1093/hmg/ddq173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarreal-Molina T, Posadas-Romero C, Romero-Hidalgo S, Antúnez-Argüelles E, Bautista-Grande A, Vargas-Alarcón G, et al. The ABCA1 gene R230C variant is associated with decreased risk of premature coronary artery disease: the genetics of atherosclerotic disease (GEA) study. PLoS One. 2012;7(11):e49285. doi: 10.1371/journal.pone.0049285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung YC, Yeo GS, O’Rahilly S, Coll AP. Obesity and FTO: changing focus at a complex locus. Cell Metab. 2014;20(5):710–8. doi: 10.1016/j.cmet.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Klöting N, Schleinitz D, Ruschke K, Berndt J, Fasshauer M, Tönjes A, et al. Inverse relationship between obesity and FTO gene expression in visceral adipose tissue in humans. Diabetologia. 2008;51(4):641–7. doi: 10.1007/s00125-008-0928-9. [DOI] [PubMed] [Google Scholar]
- 11.Wåhlén K, Sjölin E, Hoffstedt J. The common rs9939609 gene variant of the fat mass and obesity associated gene (FTO) is related to fat cell lipolysis. J Lipid Res. 2007;49(3):607–11. doi: 10.1194/jlr.M700448-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Zabena C, González-Sánchez JL, Martínez-Larrad MT, Torres-García A, Alvarez-Fernández-Represa J. The FTO obesity gene. Genotyping and gene expression analysis in morbidly obese patients. Obes Surg. 2009;19(1):87–95. doi: 10.1007/s11695-008-9727-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Zhang Y, Ma J, Guo F, Cao Q, Zhang Y, et al. The demethylase activity of FTO (Fat mass and obesity associated protein) is required for preadipocyte differentiation. PLoS One. 2015;10(7):e0133788. doi: 10.1371/journal.pone.0133788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Haan W, Bhattacharjee A, Ruddle P, Kang MH, Hayden MR. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J Lipid Res. 2014;55(3):516–23. doi: 10.1194/jlr.M045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero-Hidalgo S, Villarreal-Molina T, González-Barrios J, Canizales-Quinteros, Rodríguez-Arellano M, Yañez-Velazco LB. Carbohydrate intake modulates the effect of the ABCA1-R230C variant on HDL cholesterol concentrations in premenopausal women. J Nutr. 2012;142:278–83. doi: 10.3945/jn.111.152421. [DOI] [PubMed] [Google Scholar]
- 17.Velázquez-Cruz R, Jiménez-Ortega RF, Parra-Torres AY, Castillejos-López M, Patiño N, Quiterio M, et al. Analysis of association of MEF2C, SOST and JAG1 genes with bone mineral density in Mexican-Mestizo postmenopausal women. BMC Musculoskelet Disord. 2014;15:400. doi: 10.1186/1471-2474-15-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosoy R, Nassir R, Tian C, White PA, Butler LM, et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30(1):69–78. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are available within the manuscript and its supporting information documents.


