Abstract
Background
To date, only few studies have examined the prognostic factors of community-acquired pneumonia (CAP) defined according to the latest criteria, which excludes healthcare-associated pneumonia (HCAP). Therefore, we aimed to investigate the factors that affect prognosis, and evaluate the usefulness of existing pneumonia severity scores for predicting the prognosis of CAP.
Methods
We retrospectively analyzed patients with CAP, excluding HCAP, who were enrolled prospectively between April 2007 and February 2016. Four patients who used macrolides other than azithromycin (AZM) were excluded. We used age, sex, comorbidities, laboratory findings and antimicrobial therapy as prognostic variables. The primary outcome was 30-day mortality and secondary outcome was ICU admission. We also performed receiver operating characteristic curve analysis of Pneumonia Severity Index (PSI), Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) severe criteria, CURB-65 and A-DROP pneumonia severity scores.
Results
Among 1834 CAP patients, mean age was 73.5 ± 14.3 years; 1281 (69.8%) were men; and 30-day mortality was 6.7% (122/1834). In total, 1830 patients were analyzed. Multivariate analysis identified age [Odds Ratio (OR): 1.04, 95% Confidence Interval (CI): 1.02–1.07], chronic obstructive pulmonary disease (COPD) [OR: 1.77, 95% CI: 1.13–2.76], malignancy (OR: 2.25, 95% CI: 1.25–4.06), body temperature (OR: 0.81, 95% CI: 0.67–0.99), respiratory rate (OR: 1.04, 95% CI: 1.01–1.07), PaO2/FiO2 ≤ 250 (OR: 3.15, 95% CI: 1.93–5.14), Alb (OR: 0.27, 95% CI: 0.19–0.39), BUN (OR: 1.01, 95% CI: 1.00–1.02), and mechanical ventilation (OR: 2.99, 95% CI: 1.75–5.12) as prognostic factors. AZM and β-lactam combination therapy significantly reduced 30-day mortality (OR: 0.50, 95% CI: 0.26–0.97). Areas under the curve of PSI, IDSA/ATS severe criteria, CURB-65 and A-DROP were 0.759, 0.746, 0.754 and 0.764, respectively.
Conclusions
Increasing age, presence of COPD and malignancy as comorbidities, hypothermia, tachypnea, PaO2/FiO2 ratio ≤250 mmHg, low Alb level, high BUN level and mechanical ventilatory support predict a worse prognosis; AZM combination therapy should be considered for CAP, excluding HCAP. All four pneumonia severity scores are useful for assessing the severity of CAP defined by the latest criteria.
Trial registration
UMIN-CTR UMIN000004353. Registered 7 October 2010. Retrospectively registered.
Electronic supplementary material
The online version of this article (doi:10.1186/s12890-017-0424-4) contains supplementary material, which is available to authorized users.
Keywords: Azithromycin, Combination therapy, Community-acquired pneumonia, Prognosis, Severity score
Background
Worldwide, several severity scoring systems have been used to guide decisions on the site of care and to assess the prognosis of community-acquired pneumonia (CAP). Examples of such scoring systems are the Pneumonia Severity Index (PSI) [1], severe pneumonia criteria by the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) (IDSA/ATS severe pneumonia criteria) [2], CURB-65 by the British Thoracic Society [3] and A-DROP by the Japanese Respiratory Society [4]. In the 2005 IDSA/ATS guidelines [5], healthcare-associated pneumonia (HCAP) was defined as being associated with a greater risk of antimicrobial-resistant infections and worse prognosis than CAP.
Although these pneumonia severity scoring indices incorporate a variety of prognostic factors for CAP, including patient characteristics, such as age, sex, comorbidities, vital signs and laboratory findings, the prognostic factors are distinct from severity scoring indices. All these pneumonia severity scores mentioned above were established before the definition of HCAP was proposed. Although many reports have investigated the prognostic factors and utility of these scoring systems for assessing the severity of CAP, only few have evaluated them in terms of CAP defined by the latest criteria, which excludes HCAP. In this study, we aimed to investigate the prognostic factors of CAP defined by the latest criteria, including not only patient characteristics, vital signs and laboratory findings, but also initial antibiotic therapy as prognostic factors; we also evaluated whether pre-existing pneumonia severity scoring systems are useful for predicting prognosis in CAP excluding HCAP.
Methods
Study design and setting
This study retrospectively analyzed hospitalized CAP patients who were enrolled in a prospective, observational, cohort study at Kurashiki Central Hospital between April 2007 and February 2016. CAP was diagnosed in accordance with the IDSA/ATS guidelines as [2]: presence of at least one of the clinical symptoms of cough, sputum, fever, dyspnea, and pleuritic chest pain, plus at least more than one finding of coarse crackles on auscultation or elevated inflammatory biomarkers, in addition to a new infiltrate on chest radiography. We enrolled consecutively hospitalized patients diagnosed with pneumonia in this cohort. Exclusion criteria were: age <15 years, acquired immune deficiency syndrome, hospital-acquired pneumonia, and HCAP [5]. This study was performed as part of a clinical study for pneumonia (UMIN000004353) after October 2010 and was approved by the institutional review board of Kurashiki Central Hospital (approval number 2235). All patients gave their informed consent to participate in this study.
In all the patients, severity of pneumonia was assessed on admission with the use of PSI [1], IDSA/ATS severe pneumonia criteria [2], CURB-65 score [confusion, urea >7 mmol/L, respiratory rate ≥30 breaths per minute, low blood pressure (systolic <90 mmHg or diastolic ≤60 mmHg), and age ≥65 y] [3] and A-DROP score [age ≥70 years in men or age ≥75 years in women, blood urea nitrogen ≥21 mg · dL−1 or dehydration, oxyhemoglobin saturation measured by pulse oximetry ≤90% or partial pressure of oxygen in arterial blood ≤60 mmHg, confusion, or systolic blood pressure ≤90 mmHg] [4].
All patients received antimicrobial agents at the discretion of the physician in charge and in accordance with the recommendations of the CAP guidelines of the Japanese Respiratory Society [4]. Blood tests and chest X-ray images were examined to assess the effectiveness of the antimicrobials. Basically, patients were treated in the intensive care unit (ICU) if they needed mechanical ventilatory support and/or vasopressor drugs.
Microbiological investigations
As far as possible, we tried to obtain samples of sputum and blood for cultures at the time of admission, and blood for measuring serum antibodies to detect the causative pathogens of CAP. A bacterial cause was identified if the following criteria were met: (1) positive sputum culture of more than 1+ on a qualitative test or 105 on a quantitative test, with reference to the sputum Gram stain; (2) positive blood culture (excluding contaminated normal skin flora); (3) positive pleural fluid culture; (4) positive urinary antigen test for Streptococcus pneumoniae and Legionella pneumophila; (5) seroconversion or four-fold increase in antibodies for Mycoplasma pneumoniae and Chlamydophila pneumoniae; and (6) ≥1:320 on a single antibody test for M. pneumoniae PA antibody (FUJIREBIO, Tokyo, Japan) or a cut-off index of ≥2.0 on a C. pneumoniae IgM antibody test using the Hitazyme® assay (Hitachi Chemical, Tokyo, Japan).
Prognostic variables
In this study, we assessed age, sex, smoking status, comorbidities, vital signs and laboratory findings that influenced prognosis. Comorbidities included chronic heart disease, chronic obstructive pulmonary disease (COPD), diabetes mellitus, cerebrovascular disease, malignant disease, chronic kidney disease, and chronic liver disease. We diagnosed COPD using the Global Initiative for chronic obstructive lung disease (GOLD) definition [6], and patients who were already diagnosed and treated as COPD in other hospitals and had emphysema on chest tomography were also included. We defined malignant disease as one that was active at the time of admission or was diagnosed within one year of admission.
We did not evaluate disturbances in consciousness as a prognostic variable in this study, because it is included as a factor in severity scoring systems and because we did not assess consciousness separately in all patients. Also, we used a partial pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio of ≤250 mmHg as a surrogate indicator of oxygenation, since we did not perform arterial blood gas analysis in all patients. We estimated PaO2 from oxyhemoglobin saturation measured by pulse oximetry.
Previous reports have shown that CAP patients with bacteremia [7] or multilobar pneumonia [8] have a worse prognosis; therefore, we included these variables in the analysis.
For the treatment of CAP, some systematic reviews and meta-analyses of observational cohort studies have shown that combination therapy of macrolides and β-lactams (BLs) improve prognosis [9, 10]; however, this was opposed by two randomized controlled studies [11, 12]. In this study, we investigated the efficacy of macrolide combination therapy, fluoroquinolone (FQN) combination therapy, and BL monotherapy in terms of 30-day mortality.
The primary study outcome assessed was 30-day mortality and secondary outcome was direct admission to the ICU at the time of hospitalization. We also evaluated the usefulness of existing pneumonia severity scores, such as PSI, IDSA/ATS severe pneumonia criteria, CURB-65 and A-DROP, for predicting 30-day mortality in CAP excluding HCAP. We designated patients who were discharged within 30 days of admission and those who did not follow-up after discharge as survivors.
Statistical analysis
Continuous variables were expressed as mean and standard deviation (SD), whereas categorical variables were expressed as frequency (percentage), as appropriate. Continuous variables were analyzed by Student’s t-test and categorical variables were assessed with the chi-square test. Univariate analysis was performed for predicting 30-day mortality. Multivariate analysis using stepwise logistic regression analysis was conducted for all variables that were found to have a P value of ≤0.1 on univariate analysis. For antimicrobial therapy, azithromycin (AZM) was used in almost all patients who received macrolide combination therapy, except in 4 patients who were given clarithromycin (n = 3) and erythromycin (n = 1). Therefore, we excluded these 4 patients and analyzed macrolide combination therapy as AZM combination therapy. To assess the usefulness of existing pneumonia severity scores for predicting the prognosis of CAP, we performed Receiver Operating Characteristic (ROC) curve analysis with application of the Bonferroni correction for two-way comparisons of the AUCs of pneumonia severity scores. All statistical tests were two-tailed, and we considered a P value <0.05 as significant. Analyses were performed using R (version 3.0.3, Vienna, Austria).
Results
Patient characteristics
The baseline characteristics of the 1834 patients enrolled in this prospective cohort study are listed in Table 1. Males comprised about 70% of the study population. The most common comorbidity was chronic heart disease (30.2%), followed by COPD (24.0%). The 30-day mortality rate was 6.7% (122/1834).
Table 1.
Characteristics of patients with community-acquired pneumonia
| n (%) | |
|---|---|
| All patients | 1834 |
| Male | 1281 (69.8) |
| Age (y) | 73.5 ± 14.3 |
| Smoking status a | |
| Current | 292 (15.9) |
| Past | 850 (46.3) |
| Never | 692 (37.7) |
| Comorbidity | |
| Chronic heart disease | 554 (30.2) |
| COPDb | 440 (24.0) |
| Diabetes mellitus | 364 (19.8) |
| Cerebrovascular disease | 354 (19.3) |
| Malignancyc | 167 (9.1) |
| Chronic kidney disease | 136 (7.4) |
| Chronic liver disease | 111 (6.1) |
| Bacteremia | 93 (5.1) |
| Duration of hospitalization (days) | 15.4 ± 12.7 |
| ICU admission | 95 (5.2) |
| A-DROP (score) | |
| 0 | 206 (11.2) |
| 1 | 486 (26.5) |
| 2 | 575 (31.4) |
| 3 | 426 (23.2) |
| 4 | 126 (6.9) |
| 5 | 15 (0.8) |
| CURB-65 (score) | |
| 0 | 193 (10.5) |
| 1 | 554 (30.2) |
| 2 | 634 (34.6) |
| 3 | 329 (17.9) |
| 4 | 106 (5.8) |
| 5 | 18 (1.0) |
| PSI (score) | 103.4 ± 34.2 |
| PSI (class) | |
| I | 37 (2.0) |
| II | 211 (11.5) |
| III | 456 (24.9) |
| IV | 799 (43.6) |
| V | 331 (18.0) |
| IDSA/ATS severe criteria | |
| Yes | 585 (31.9) |
| No | 1249 (68.1) |
| In-hospital mortality | 132 (7.2) |
| 30-day mortality | 122(6.7) |
Data are presented as mean ± SD or n (%)
Abbreviations: A-DROP age ≥70 years in men or age ≥75 years in women, blood urea nitrogen ≥21 mg · dL−1 or dehydration, oxyhemoglobin saturation measured by pulse oximetry ≤90% or partial pressure of oxygen in arterial blood ≤60 mmHg, confusion or systolic blood pressure ≤90 mmHg, ATS American Thoracic Society, COPD chronic obstructive pulmonary disease, CURB-65 confusion, urea >7 mmol/L, respiratory rate ≥30 breaths/min, low blood pressure (systolic <90 mmHg or diastolic ≤60 mmHg), and age ≥65 years, ICU intensive care unit, IDSA Infectious Diseases Society of America, PSI Pneumonia Severity Index
aCurrent: Patients who are current smokers and have been smoking more than 100 cigarettes in their entire life, Past: Patients who quit smoking more than a month ago and have been smoking more than 100 cigarettes in their entire life, Never: Patients who have not smoked in the past month and have not smoked more than 100 cigarettes in their entire life
bWe diagnosed COPD using the GOLD definition [6]. Patients who were already diagnosed and treated as COPD in other hospitals and had emphysema on chest tomography were also included
cThis included malignant disease that was active at the time of admission or was diagnosed within one year of admission
Etiology of community-acquired pneumonia
In this study, causative pathogens were identified in 854 patients (46.6%); of them, 107 were infected with more than two microorganisms. Of the 1834 patients, we performed sputum tests in 1652 (90.1%), blood culture in 1756 (95.7%), and urinary antigen tests in 1691 (92.2%) patients. Additional file 1: Table S1 shows the distribution of the causative pathogens identified by these tests. In seven patients, performance of these tests was not feasible. There were 107 patients with multiple bacterial etiologies; therefore, the cumulative infection rate was over 100%. The most common causative microorganism was S. pneumoniae (22.2%), followed by Haemophilus influenzae (7.0%). The prevalence of multidrug-resistant pathogens was 2.4%; these included Pseudomonas aeruginosa (1.8%), extended-spectrum β-lactamase (ESBL) producing Escherichia coli (0.1%), and methicillin-resistant Staphylococcus aureus (0.5%).
Initial antibiotic therapy
Table 2 shows the initial antimicrobial agents in all patients. Of the 1248 patients on monotherapy, 1181 patients (94.6%) were treated with BLs. Three hundred seventy-seven patients received AZM and BLs combination therapy; AZM was given orally at 500 mg/day for 3 days in 182 patients, as a single dose of 2 g/day in 159 patients, and intravenously at 500 mg/day in 32 patients. In patients on FQN combination therapy with BLs (n = 119), pazufloxacin (73.1%) was most prescribed, followed by levofloxacin (22.7%).
Table 2.
Initial antibiotic agents in all patients with community-acquired pneumonia
| n = 1834 | |||||
|---|---|---|---|---|---|
| Monotherapy | Combination therapy | ||||
| n = 1248 | n = 586 | ||||
| Macrolidesa | FQNb | TC | LCM | ||
| n = 377 | n = 119 | n = 82 | n = 8 | ||
| Penicillins | 933 | 199 | 47 | 61 | 2 |
| Cephalosporins | 223 | 163 | 23 | 19 | 6 |
| Carbapenems | 25 | 7 | 41 | 2 | 0 |
| Fluoroquinolones | 49 | 8 | ― | 0 | 0 |
| Macrolides | 12 | ― | 8 | 0 | 0 |
| Tetracycline | 3 | 0 | 0 | ― | 0 |
| Lincomycin | 1 | 0 | 0 | 0 | ― |
| Oseltamivir | 2 | 0 | 0 | 0 | 0 |
Abbreviations: FQN fluoroquinolone, TC tetracycline, LCM lincomycin
aOral azithromycin (500 mg/day) for 3 days in 182, oral azithromycin (2 g/day) single dose in 159, azithromycin (500 mg/day) injection in 32, oral erythromycin in 1, and oral clarithromycin in 3 patients
bCiprofloxacin in 4, tosufloxacin in 1, pazufloxacin in 87, and levofloxacin in 27 patients
Prognostic factors for 30-day mortality in community-acquired pneumonia
The characteristics of survivors and non-survivors are shown in Table 3. In univariate analysis, age, COPD and malignancy as comorbidities, vital signs such as body temperature, heart rate, respiratory rate and PaO2/FiO2 ≤ 250, laboratory findings such as albumin (Alb), blood urea nitrogen (BUN), creatinine (Cr), hematocrit (Ht) and C-reactive protein (CRP) levels, multilobar pneumonia, bacteremia, AZM or FQN combination therapy, and treatment including mechanical ventilatory and vasopressor drug support were significant prognostic factors in patients hospitalized with CAP. In multivariate analysis, age [Odds Ratio (OR): 1.04, 95% Confidence Interval (CI): 1.02–1.07], COPD [OR: 1.77, 95% CI: 1.13–2.76], malignant disease (OR: 2.25, 95% CI: 1.25–4.06), body temperature (OR: 0.81, 95% CI: 0.67–0.99), respiratory rate (OR: 1.04, 95% CI: 1.01–1.07), PaO2/FiO2 ≤ 250 (OR: 3.15, 95% CI: 1.93–5.14), Alb (OR: 0.27, 95% CI: 0.19–0.39), BUN (OR: 1.01, 95% CI: 1.00–1.02), AZM combination therapy (OR: 0.50, 95% CI: 0.26–0.97) and mechanical ventilation (OR: 2.99, 95% CI: 1.75–5.12) were significant prognostic factors for CAP (Table 4).
Table 3.
Characteristics of community-acquired pneumonia survivors and non-survivors
| Survivors | Non-survivors | Univariate | |
|---|---|---|---|
| n = 1708 | n = 122 | P value | |
| Male | 1187 (69.5) | 92 (75.4) | 0.20 |
| Age (y) | 73.2 ± 14.4 | 78.8 ± 10.4 | <0.001 |
| Smoking statusa | 0.26 | ||
| Current | 273 (16.0) | 19 (15.6) | |
| Past | 780 (45.7) | 68 (55.7) | |
| Never | 655 (38.3) | 35 (28.7) | |
| Comorbidity | |||
| Chronic heart disease | 509 (29.8) | 45 (36.9) | 0.12 |
| COPDb | 393 (23.0) | 46 (37.7) | <0.001 |
| Diabetes mellitus | 340 (19.9) | 24 (19.7) | 1.00 |
| Cerebrovascular disease | 325 (19.0) | 27 (22.1) | 0.47 |
| Malignancy | 148 (8.7) | 19 (15.6) | 0.02 |
| Chronic kidney disease | 125 (7.3) | 11 (9.0) | 0.47 |
| Chronic liver disease | 100 (5.9) | 10 (8.2) | 0.39 |
| Vital signs | |||
| Body temperature (°C) | 37.9 ± 1.1 | 37.4 ± 1.2 | <0.001 |
| Systolic blood pressure (mmHg) | 129 ± 26 | 126 ± 30 | 0.19 |
| Heart rate (beats/min) | 98 ± 19 | 103 ± 25 | 0.004 |
| Respiratory rate (breaths/min) | 23 ± 7 | 28 ± 9 | <0.001 |
| PaO2/FiO2 ≤ 250 (mmHg)c | 554 (32.4) | 92 (75.4) | <0.001 |
| Laboratory examinations | |||
| Alb (g/dL) | 3.2 ± 0.6 | 2.6 ± 0.6 | <0.001 |
| BUN (mg/dL) | 21.8 ± 14.5 | 34.5 ± 22.2 | <0.001 |
| Cr (mg/dL) | 1.01 ± 0.8 | 1.27 ± 1.2 | 0.001 |
| Na (mmol/L) | 136.7 ± 4.2 | 137.2 ± 6.3 | 0.25 |
| Ht (%) | 37.1 ± 5.5 | 35.6 ± 6.5 | 0.004 |
| Plt (×104/μL) | 22.5 ± 10.2 | 22.8 ± 10.2 | 0.75 |
| WBC (×103/μL) | 12.3 ± 6.2 | 12.1 ± 6.4 | 0.75 |
| CRP (mg/L) | 125 ± 94 | 164 ± 108 | <0.001 |
| Multilobar pneumonia | 939 (55.0) | 99 (81.1) | <0.001 |
| Bacteremia | 73 (4.3) | 20 (16.4) | <0.001 |
| β-lactam monotherapy | 1109 (64.9) | 72 (59.0) | 0.22 |
| AZM combination | 354 (20.7) | 12 (9.8) | 0.005 |
| FQN combination | 81 (4.7) | 30 (24.6) | <0.001 |
| MINO combination | 79 (4.6) | 3 (2.5) | 0.37 |
| Mechanical ventilation | 99 (5.8) | 40 (32.8) | <0.001 |
| Vasopressor drug usage | 60 (3.5) | 26 (21.3) | <0.001 |
| ICU admission | 67 (3.9) | 28 (23.0) | <0.001 |
| A-DROP (score) | <0.001 | ||
| 0 | 204 (11.9) | 1 (0.8) | |
| 1 | 478 (28.0) | 7 (5.7) | |
| 2 | 545 (31.9) | 28 (23.0) | |
| 3 | 372 (21.8) | 54 (44.3) | |
| 4 | 97 (5.7) | 29 (23.8) | |
| 5 | 12 (0.7) | 3 (2.5) | |
| CURB-65 (score) | <0.001 | ||
| 0 | 192 (11.2) | 0 (0) | |
| 1 | 540 (31.6) | 12 (9.8) | |
| 2 | 594 (34.8) | 39 (32.0) | |
| 3 | 294 (17.2) | 35 (28.7) | |
| 4 | 74 (4.3) | 32 (26.2) | |
| 5 | 14 (0.8) | 4 (3.3) | |
| PSI (score) | 100.7 ± 32.3 | 140.9 ± 37.2 | <0.001 |
| PSI (class) | <0.001 | ||
| I | 36 (2.1) | 0 (0) | |
| II | 210 (12.3) | 1 (0.8) | |
| III | 447 (26.2) | 8 (6.6) | |
| IV | 750 (43.9) | 47 (38.5) | |
| V | 265 (15.5) | 66 (54.1) | |
| IDSA/ATS severe criteria | <0.001 | ||
| Yes | 490 (28.7) | 95 (77.9) | |
| No | 1218 (71.3) | 27 (22.1) | |
Data are presented as mean ± SD or n (%)
Abbreviations: A-DROP age ≥70 years in men or age ≥75 years in women, blood urea nitrogen ≥21 mg · dL−1 or dehydration, oxyhemoglobin saturation measured by pulse oximetry ≤90% or partial pressure of oxygen in arterial blood ≤60 mmHg, confusion, or systolic blood pressure ≤90 mmHg, Alb Albumin, ATS American Thoracic Society, AZM azithromycin, BUN Blood urea nitrogen, COPD chronic obstructive pulmonary disease, Cr Creatinine, CRP C-reactive protein, CURB-65 confusion, urea >7 mmol/L, respiratory rate ≥30 breaths/min, low blood pressure (systolic <90 mmHg or diastolic ≤60 mmHg), and age ≥65 years, FiO 2 fraction of inspired oxygen, FQN fluoroquinolone, Ht Hematocrit, ICU intensive care unit, IDSA Infectious Diseases Society of America, MINO minomycin, Na Sodium, PaO 2 partial pressure of arterial oxygen, Plt Platelet, PSI Pneumonia Severity Index, WBC white blood cell
aCurrent: Patients who are current smokers and have been smoking more than 100 cigarettes in their entire life, Past: Patients who quit smoking more than a month ago and have been smoking more than 100 cigarettes in their entire life, Never: Patients who have not smoked in the past month and have not smoked more than 100 cigarettes in their entire life.
bWe diagnosed COPD according to the GOLD definition [6]. Patients who were already diagnosed and treated as COPD in other hospitals and had emphysema on chest tomography were also included.
cArterial blood gas analyses were not performed in 513 of the survivors and 12 of the non-survivors; in these patients, arterial oxygen partial pressure was estimated from oxyhemoglobin saturation measured by pulse oximetry.
Table 4.
Univariate and multivariate analysis of prognostic factors for 30-day mortality
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Prognostic factors | OR | 95% CI | P value | OR | 95% CI | P value |
| Age | 1.04 | 1.02–1.06 | <0.001 | 1.04 | 1.02–1.07 | <0.001 |
| COPD | 2.03 | 1.38–2.97 | <0.001 | 1.77 | 1.13–2.76 | 0.01 |
| Malignancy | 1.94 | 1.16–3.26 | 0.01 | 2.25 | 1.25–4.06 | 0.007 |
| Body temperature (°C) | 0.65 | 0.55–0.77 | <0.001 | 0.81 | 0.67–0.99 | 0.045 |
| Heart rate (beats/min) | 1.01 | 1.00–1.02 | 0.004 | |||
| Respiratory rate (breaths/min) | 1.08 | 1.06–1.11 | <0.001 | 1.04 | 1.01–1.07 | 0.008 |
| PaO2/FiO2 ≤ 250 (mmHg) | 6.39 | 4.18–9.76 | <0.001 | 3.15 | 1.93–5.14 | <0.001 |
| Alb (g/dL) | 0.22 | 0.16–0.30 | <0.001 | 0.27 | 0.19–0.39 | <0.001 |
| BUN (mg/dL) | 1.03 | 1.02–1.04 | <0.001 | 1.01 | 1.00–1.02 | 0.04 |
| Cr (mg/dL) | 1.27 | 1.10–1.47 | 0.001 | |||
| Ht (%) | 0.95 | 0.92–0.99 | 0.004 | |||
| CRP (mg/L) | 1.04 | 1.02–1.06 | <0.001 | |||
| Multilobar pneumonia | 3.53 | 2.22–5.60 | <0.001 | |||
| Bacteremia | 4.39 | 2.58–7.49 | <0.001 | |||
| AZM combination | 0.42 | 0.23–0.77 | 0.005 | 0.50 | 0.26–0.97 | 0.04 |
| FQN combination | 6.55 | 4.10–10.5 | <0.001 | |||
| Mechanical ventilation | 7.93 | 5.16–12.2 | <0.001 | 2.99 | 1.75–5.12 | <0.001 |
| Vasopressor drug usage | 7.44 | 4.49–12.3 | <0.001 | |||
Abbreviations: Alb Albumin, AZM azithromycin, BUN Blood urea nitrogen, CI confidence interval, COPD chronic obstructive pulmonary disease, Cr Creatinine; CRP C-reactive protein, FiO 2 fraction of inspired oxygen, FQN fluoroquinolone, Ht Hematocrit, OR odds ratio, PaO 2 partial pressure of arterial oxygen
Predictive factors for ICU admission in community-acquired pneumonia
In univariate analysis, vital signs such as body temperature, heart rate, systolic blood pressure, respiratory rate and PaO2/FiO2 ≤ 250, laboratory findings such as Alb, BUN, Cr and CRP value, multilobar pneumonia, bacteremia and treatment with mechanical ventilation and vasopressor drugs were significant predictive factors for ICU admission on the day of hospitalization. Of these, body temperature (OR: 0.62, 95% CI: 0.47–0.81, P < 0.001), respiratory rate (OR: 1.05, 95% CI: 1.00–1.09, P = 0.04), PaO2/FiO2 ≤ 250 (OR: 9.34, 95% CI: 3.62–24.1, P < 0.001), CRP (OR: 1.04, 95% CI: 1.01–1.07, P = 0.009), mechanical ventilation (OR: 18.4, 95% CI: 8.65–39.1, P < 0.001) and vasopressor drug usage (OR: 7.40, 95% CI: 3.29–16.6, P < 0.001) were significant predictive factors in multivariate analysis.
Usefulness of existing pneumonia severity scores for predicting 30-day mortality
The values of areas under the curve (AUC) in ROC analysis for prediction of 30-day mortality were 0.759 (95% CI: 0.721–0.796), 0.746 (95% CI: 0.707–0.784), 0.754 (95% CI: 0.713–0.794) and 0.764 (95% CI: 0.726–0.802) for PSI class, IDSA/ATS severe pneumonia criteria, CURB-65 and A-DROP scores, respectively (Fig. 1). There were no significant differences in the predictive ability of each pneumonia severity score.
Fig. 1.
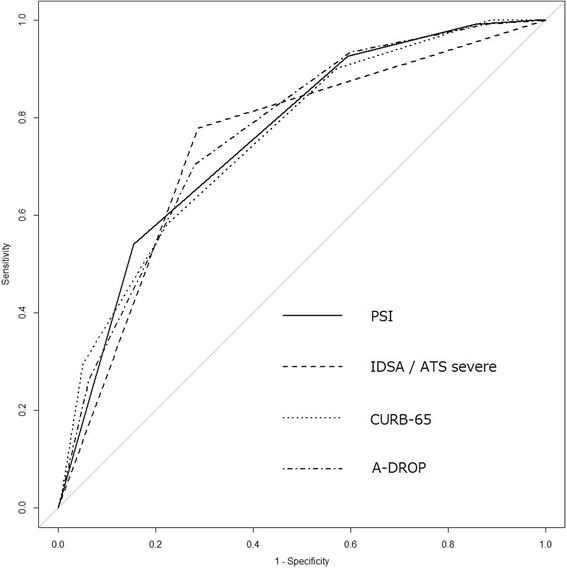
ROC curve analysis of ability of pneumonia severity scores to predict 30-day mortality in cases of community acquired pneumonia (CAP), excluding healthcare-associated pneumonia (HCAP). The AUCs of PSI, IDSA/ATS severe pneumonia criteria, CURB-65 and A-DROP were 0.759 (95% CI: 0.721–0.796), 0.746 (95% CI: 0.707–0.784), 0.754 (95% CI: 0.713–0.794) and 0.764 (95% CI: 0.726–0.802), respectively. In comparisons of the AUCs of these four pneumonia severity scores with each other, the P value was 1.0 in all comparison groups adjusted by the Bonferroni method (PSI vs. IDSA/ATS severe pneumonia criteria, PSI vs. CURB-65, PSI vs. A-DROP, IDSA/ATS severe pneumonia criteria vs. CURB-65, IDSA/ATS severe pneumonia criteria vs. A-DROP, and CURB-65 vs. A-DROP). A-DROP, age ≥70 years in men or age ≥75 years in women, blood urea nitrogen ≥21 mg · dL−1 or dehydration, oxyhemoglobin saturation measured by pulse oximetry ≤90% or partial pressure of oxygen in arterial blood ≤60 Torr, confusion, or systolic blood pressure ≤90 mmHg; ATS, American Thoracic Society; AUC, Area under the curve; CURB-65: confusion, urea >7 mmol/L, respiratory rate ≥30 breaths/min, low blood pressure (systolic <90 mmHg or diastolic ≤60 mmHg), and age ≥65 years; IDSA, Infectious Diseases Society of America; PSI, Pneumonia Severity Index; ROC, Receiver Operating Characteristic
Discussion
In this study, we showed that increasing age, presence of COPD and malignancy as comorbidities, low body temperature, tachypnea, PaO2/FiO2 ≤ 250, low Alb levels, high BUN levels and the need for mechanical ventilatory support were predictors of a poor prognosis in CAP patients. We also found that AZM combination therapy with BLs was a predictor of good prognosis, and that the existing pneumonia severity indices have a good predictive ability for the prognosis of CAP. The results of this study are important because the study population comprised patients who were diagnosed as CAP based on the new criteria, which excludes those with HCAP.
All four pneumonia severity scores, including PSI [1], IDSA/ATS severe pneumonia criteria [2], CURB-65 [3] and A-DROP [4] were previously shown to be useful for predicting prognosis in patients diagnosed with CAP based on the old criteria, which includes HCAP patients. Although some previous reports have investigated the prognostic factors and usefulness of existing pneumonia severity scores in predicting the prognosis of CAP [13, 14], our report is valuable because patient vital signs and antibiotic therapy, especially macrolide combination therapy, were not evaluated in previous studies.
Age is included as a prognostic factor in the PSI [1], CURB-65 [3] and A-DROP scoring systems [4]. Our study showed that increasing age is also an important prognostic factor for CAP defined according to the latest criteria, which exclude HCAP. As for the comorbidities, Restrepo et al reported that CAP patients with COPD showed significantly higher 30-day mortality (HR 1.32; 95% CI 1.01–1.74) and 90-day mortality (HR 1.34; 95% CI 1.02–1.76) than those without COPD [15]. Molinos et al also showed that COPD was an independent poor prognostic factor in CAP, after adjusting for age (OR 2.62; 95% CI 1.08–6.39) [16]. In this study, we showed similar results. Therefore, our study underscores the importance of pneumococcal vaccination in the prevention of pneumonia in COPD patients, as recommended by the Global Initiative for Chronic Obstructive Lung Disease for stable COPD [6]. In fact, Maruyama et al [17] reported in their study that the 23-valent pneumococcal vaccine significantly reduced pneumococcal pneumonia by 63.8% (95% CI 32.1–80.7; P = 0.0015) and all-cause pneumonia by 44.8% (95% CI 22.4–60.8; P = 0.0006). Malignancy is also a known prognostic factor for CAP, with a score of 30 points in PSI [1]. Tashiro and colleagues reported that the presence of malignancy is a poor prognostic factor for CAP in patients aged 18–64 years [18]. We also showed that malignancy is a prognostic factor for CAP in patients aged over 15 years.
All four pneumonia severity scores include vital signs as prognostic factors, although they are different from pneumonia severity indices. Respiratory status is included in all pneumonia severity scores and respiratory rate is adopted in CURB-65 [3], while respiratory failure is adopted in PSI [1], IDSA/ATS severe pneumonia criteria [2] and A-DROP [4]. A PaO2/FiO2 ratio of ≤250 is one of the prognostic factors in IDSA/ATS severe pneumonia criteria. Our study showed that both tachypnea and PaO2/FiO2 ≤ 250 are poor prognostic factors. Hypothermia is adopted as a prognostic factor in PSI (body temperature less than 35 °C or more than 40 °C, 15 points) and IDSA/ATS severe pneumonia criteria (body temperature less than 36 °C); our study showed similar results. However, since the cut-off values of these factors for predicting prognosis are unknown, further studies are needed to determine these.
Regarding laboratory findings, our data indicated that Alb and BUN were poor prognostic factors of CAP. BUN is included in all four pneumonia severity scores, while Alb is not included. Previous reports showed that low levels of Alb are a poor prognostic factor in CAP [19, 20] and in both CAP and HCAP [21]. Our study also supported these findings, which suggests that, in future, Alb should be included as a prognostic factor in existing pneumonia severity indices.
Recently, some systematic reviews and meta-analysis indicated that compared with BL monotherapy, macrolide combination therapy reduced CAP mortality rate [9, 10]. However, the studies included in these reviews were all observational in design. On the other hand, two randomized controlled trials [11, 12] did not demonstrate a reduced mortality rate with macrolide combination therapy. Therefore, the efficacy of macrolide combination therapy in reducing mortality in CAP is controversial. Previous reports that assessed the usefulness of macrolide therapy in patients with CAP, including some cases of HCAP, included erythromycin, clarithromycin, and AZM. In this study, we showed that AZM combination therapy with BLs reduced mortality rate in CAP patients, excluding HCAP patients.
Macrolides, including AZM, have anti-inflammatory properties and immunomodulating effects, such as regulation of neutrophil chemotaxis, decreased pro-inflammatory cytokine production, and regulation of adhesion molecule expression [22, 23]. AZM demonstrated anti-inflammatory and antivirulent characteristics in mouse and human studies on P. aeruginosa [24, 25]. An experimental study on pneumococcal pneumonia in mice showed that AZM combination therapy with ampicillin was effective in downregulating lung inflammation and accelerating bacterial clearance [26]. These effects of AZM, in addition to its antibacterial properties, may have brought about the reduction of mortality in CAP.
In systematic reviews and meta-analyses, the AUC of summary ROC curves for predicting 30-day mortality with PSI and CURB-65 in CAP were reported as 0.81 and 0.80, respectively [27]. Shindo et al. [28] reported that A-DROP was as useful in assessing the severity of CAP as CURB-65, and their AUCs for predicting 30-day mortality were 0.846 and 0.835, respectively. Compared to previous reports, the results of our study indicated mild low AUCs for all pneumonia severity scores.
However, since the AUC was about 0.75 for each scoring system, we believe that the existing pneumonia severity scores are useful for predicting the prognosis of CAP defined by recent criteria.
This study has certain limitations. First, since it was performed at a single center, the applicability of our results to other areas or countries is uncertain. Regardless, the present study analyzed over 1800 CAP patients, which is a large number of patients. Second, our study had some missing data. We did not include disturbance of consciousness as a prognostic variable because we could not assess it in all patients as a separate item. Although arterial blood gas analysis was not performed in 513 patients from among the survivors and 12 patients from among non-survivors, oxyhemoglobin saturation, as measured by pulse oximetry, was ≥90% in almost all these patients. Hence, we estimated that the PaO2/FiO2 ratio in these patients was at least >250. Analysis of disturbance of consciousness and PaO2/FiO2 ratio as separate prognostic factors might have revealed different results. Finally, although AZM combination therapy was associated with a good prognosis in CAP, the best formulation for a particular population is unclear. In this study, 91.6% of patients received AZM in the oral form; therefore, oral AZM may be sufficient, at least for its anti-inflammatory effect. However, it is important to note that use of AZM combination therapy in all CAP patients may increase antimicrobial resistance and cost. Therefore, the CAP population that would benefit from AZM combination therapy should be determined in future randomized controlled trials.
Conclusions
Age, COPD and malignant disease as comorbidities, hypothermia, tachypnea, PaO2/FiO2 ≤ 250, low Alb level, high BUN level and treatment including mechanical ventilation are poor prognostic factors for CAP defined by the new criteria. All existing pneumonia severity scores, such as PSI, IDSA/ATS severe pneumonia criteria, CURB-65 and A-DROP are useful in predicting the prognosis of patients with CAP, excluding HCAP. AZM combination therapy with BLs reduced the 30-day mortality across all severities of CAP. Although AZM seemed to be a good choice of therapy, it is important to determine the population who will best benefit from it, while minimizing antibiotic resistance and high treatment cost.
Acknowledgements
The authors would like to thank all their colleagues who recruited and treated the CAP patients.
Funding
No financial support was received for this study.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon request.
Authors’ contributions
AI served as the principal author, had full access to all data in the study, and takes responsibility for the integrity and accuracy of the data and data analysis. AI, TI, and NT contributed to study conception and design; AI, TI, YW, AY, YI, and HT contributed to acquisition of data; AI, TI, NT, and HT contributed to analysis and interpretation of data; AI, TI, NT, YW, AY, YI, and HT contributed to drafting and revision of the manuscript and approval of the final version to be submitted for consideration for publication.
Competing interests
TI has received honoraria from Pfizer, Japan, Inc. The other authors have no conflict of interest to disclose.
Consent for publication
All patient data were anonymized, so consent for publication was not required.
Ethics approval and consent to participate
This study was approved by the institutional review board of Kurashiki Central Hospital (approval number 2235). All patients gave their informed consent to participate in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Alb
Albumin
- ATS
American Thoracic Society
- AZM
Azithromycin
- BLs
β-lactams
- BUN
Blood urea nitrogen
- CAP
Community-acquired pneumonia
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- Cr
creatInine
- CRP
C-reactive protein
- FiO2
Fraction of inspired oxygen
- FQN
Fluoroquinolone
- HCAP
Healthcare-associated pneumonia
- Ht
Hematocrit
- ICU
Intensive care unit
- IDSA
Infectious diseases society of America
- OR
Odds ratio
- PaO2
Partial pressure of arterial oxygen
- PSI
Pneumonia severity index
Additional file
Distribution of causative microorganisms of community-acquired pneumonia. (DOCX 14 kb)
Contributor Information
Akihiro Ito, Phone: +81-86-422-0210, Email: ai12306@kchnet.or.jp.
Tadashi Ishida, Email: ishidat@kchnet.or.jp.
Hironobu Tokumasu, Email: tokumasu.hironobu@kchnet.or.jp.
Yasuyoshi Washio, Email: yw13539@kchnet.or.jp.
Akio Yamazaki, Email: ay14809@kchnet.or.jp.
Yuhei Ito, Email: yi14402@kchnet.or.jp.
Hiromasa Tachibana, Email: tcbnh9@gmail.com.
References
- 1.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–50. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 2.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim WS, Eerden van der MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–82. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyashita N, Matsushima T, Oka M. Japanese Respiratory Society. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med. 2006;45(7):419–28. doi: 10.2169/internalmedicine.45.1691. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 7.Torres A, Cillóniz C, Ferrer M, Gabarrús A, Polverino E, Villegas S, et al. Bacteraemia and antibiotic-resistant pathogens in community acquired pneumonia: risk and prognosis. Eur Respir J. 2015;45(5):1353–63. doi: 10.1183/09031936.00152514. [DOI] [PubMed] [Google Scholar]
- 8.Mannu GS, Loke YK, Curtain JP, Pelpola KN, Myint PK. Prognosis of multi-lobar pneumonia in community-acquired pneumonia: a systematic review and meta-analysis. Eur J Intern Med. 2013;24(8):857–63. doi: 10.1016/j.ejim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Nie W, Li B, Xiu Q. β-lactam/macrolide dual therapy versus β-lactam monotherapy for the treatment of community-acquired pneumonia in adults: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(6):1441–6. doi: 10.1093/jac/dku033. [DOI] [PubMed] [Google Scholar]
- 10.Sligl WI, Asadi L, Eurich DT, Tjosvold L, Marrie TJ, Majumdar SR. Macrolides and mortality in critically ill patients with community-acquired pneumonia: a systematic review and meta-analysis. Crit Care Med. 2014;42(2):420–32. doi: 10.1097/CCM.0b013e3182a66b9b. [DOI] [PubMed] [Google Scholar]
- 11.Garin N, Genné D, Carballo S, Chuard C, Eich G, Hugli O, et al. β-lactam monotherapy vs. β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894–1901. doi: 10.1001/jamainternmed.2014.4887. [DOI] [PubMed] [Google Scholar]
- 12.Postma DF, Werkhoven van CH, Elden van LJ, Thijsen SF, Hoepelman AI, Kluytmans JA, CAP-START Study Group et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372(14):1312–23. doi: 10.1056/NEJMoa1406330. [DOI] [PubMed] [Google Scholar]
- 13.Gorelik O, Izhakian S, Barchel D, Almoznino-Sarafian D, Tzur I, Swarka M, et al. Changes in red cell distribution width during hospitalization for community-acquired pneumonia: clinical characteristics and prognostic significance. Lung. 2016;194(6):985–95. doi: 10.1007/s00408-016-9942-8. [DOI] [PubMed] [Google Scholar]
- 14.Gorelik O, Izhakian S, Barchel D, Almoznino-Sarafian D, Tzur I, Swarka M, et al. Prognostic significance of platelet count changes during hospitalization for community-acquired pneumonia. Platelets. 2016;29:1–7. doi: 10.1080/09537104.2016.1219032. [DOI] [PubMed] [Google Scholar]
- 15.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28(2):346–51. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 16.Molinos L, Clemente MG, Miranda B, Alvarez C, Busto del B, Cocina BR, et al. Community-acquired pneumonia in patients with and without chronic obstructive pulmonary disease. J Infect. 2009;58(6):417–24. doi: 10.1016/j.jinf.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004. doi: 10.1136/bmj.c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tashiro M, Fushimi K, Takazono T, Kurihara S, Miyazaki T, Tsukamoto M, et al. A mortality prediction rule for non-elderly patients with community-acquired pneumonia. BMC Pulm Med. 2016;16:39. doi: 10.1186/s12890-016-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Kim J, Kim K, Jo YH, Rhee J, Kim TY, et al. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care. 2011;26(3):287–94. doi: 10.1016/j.jcrc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Viasus D, Garcia-Vidal C, Simonetti A, Manresa F, Dorca J, Gudiol F, et al. Prognostic value of serum albumin levels in hospitalized adults with community-acquired pneumonia. J Infect. 2013;66(5):415–23. doi: 10.1016/j.jinf.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Goto Y, et al. Risk factors for 30-day mortality in patients with pneumonia who receive appropriate initial antibiotics: an observational cohort study. Lancet Infect Dis. 2015;15(9):1055–65. doi: 10.1016/S1473-3099(15)00151-6. [DOI] [PubMed] [Google Scholar]
- 22.Culic O, Erakovic V, Parnham MJ. Anti-inflammatory effects of macrolide antibiotics. Eur J Pharm. 2001;429(1–3):209–29. doi: 10.1016/S0014-2999(01)01321-8. [DOI] [PubMed] [Google Scholar]
- 23.Amsden GW. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(1):10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]
- 24.Imperi F, Leoni L, Visca P. Antivirulence activity of azithromycin in Pseudomonas aeruginosa. Front Microbiol. 2014;5:178. doi: 10.3389/fmicb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. JAMA. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 26.Majhi A, Kundu K, Adhikary R, Banerjee M, Mahanti S, Basu A, et al. Combination therapy with ampicillin and azithromycin in an experimental pneumococcal pneumonia is bactericidal and effective in down regulating inflammation in mice. J Inflamm. 2014;11(1):5. doi: 10.1186/1476-9255-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmers JD, Singanayagam A, Akram AR, Mandal P, Short PM, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. 2010;65(10):878–83. doi: 10.1136/thx.2009.133280. [DOI] [PubMed] [Google Scholar]
- 28.Shindo Y, Sato S, Maruyama E, Ohashi T, Ogawa M, Imaizumi K, et al. Comparison of severity scoring system A-DROP and CURB-65 for community-acquired pneumonia. Respirology. 2008;13(5):731–5. doi: 10.1111/j.1440-1843.2008.01329.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon request.


