Abstract
Male sterility is a valuable trait for plant breeding and hybrid seed production. The dominant male-sterile gene Ms2 in common wheat has facilitated the release of hundreds of breeding lines and cultivars in China. Here, we describe the map-based cloning of the Ms2 gene and show that Ms2 confers male sterility in wheat, barley and Brachypodium. MS2 appears as an orphan gene within the Triticinae and expression of Ms2 in anthers is associated with insertion of a retroelement into the promoter. The cloning of Ms2 has substantial potential to assemble practical pipelines for recurrent selection and hybrid seed production in wheat.
The dominant male-sterile gene Ms2 has facilitated the release of hundreds of wheat-breeding lines and cultivars in China. Here Ni et al. describe the cloning of the Ms2 gene, which appears as an orphan gene in the Triticinae, and show that Ms2 can be used to confer male sterility in wheat, barley and Brachypodium.
In the last 48 years, the world's population has doubled to 7.3 billion people1. Cereal production similarly increased from 1.2 billion tons in 1969 to 2.8 billion tons in 2014 (FAOSTAT data). The increase in cereal production is basically due to the green revolution2 and the development of hybrid crops3. However, ca. 793 million people still lack sufficient food4, and the global population is projected to exceed 9.7 billion in 2050 (ref. 1). This rapid growth in population challenges the global food supply in which wheat and rice predominate with wheat providing 20% of humanity's calories.
Plant male sterility has been documented in 617 species or species crosses5. Genetically, male sterility is classified as either cytoplasmic male sterility (CMS) controlled by extranuclear genes or genic male sterility (GMS) controlled by nuclear genes6. Plant male sterility genes are particularly useful for cereal breeding and hybrid seed production. A three-line hybrid rice that uses the CMS trait was introduced in China after 1974 (ref. 7). However, CMS systems typically confer a cytoplasmic penalty on heterosis. In 1995, a two-line hybrid rice was introduced with an environmentally sensitive GMS (EGMS). This hybrid produced 5–10% more yield than the three-line hybrids7. If a male sterility gene were cloned, genetic modification could be used to develop improved methods for improved hybrid production in major crops8,9.
Although heterosis in wheat results in yield increases of 3.5–15%, hybrid wheat is grown on <0.2% of the global acreage10. There are over 70 male-sterile cytoplasm systems in wheat11, but most are used infrequently10 because of the yield penalty and a paucity of effective fertility-restorer genes. Nearly all hybrid wheat in Europe (>0.2 mha) uses chemically-induced male sterility10, which adds cost and furthermore, results in inferior hybrids with either poor germination or reduced seedling vigour12. Future adoption of hybrid wheat depends on the availability of a more practical male sterile/restorer system.
In wheat, there are at least five GMS genes and three EGMS genes13. The premier Ms2 gene, discovered in 1972 in Taigu county, China, appeared as a spontaneous mutation in common wheat ‘223' (refs 14, 15). Ms2 confers GMS, and all wheat lines with Ms2 are called ‘Taigu genic male-sterile wheat' (Taigu). Ms2 confers 100% male sterility regardless of genetic background, phytohormones and environmental conditions16,17. Ms2 confers genetically dominant male sterility in hexaploid and tetraploid wheat18, and hexaploid and octaploid triticale19,20,21. Other than male sterility, Ms2 has no other phenotypes, and Taigu pistils are readily cross-pollinated. Consequently, Ms2 seems suitable for developing hybrids and for recurrent selection in multiple autogamous crops22. In the late 1980s, Liu et al.23 developed a dwarf male-sterile wheat with a tight linkage between the dominant dwarf gene Rht-D1c and Ms2 (RMs2). By 2010, RMs2-based systems had produced 42 wheat cultivars planted on a total of 12.3 mha, with an increased grain yield of 5.6 million tons. Today both Ms2- and RMs2-based recurrent selection systems are widely used in wheat-breeding programs in China during the recurrent selection phase. New wheat varieties are often bred in a ten or more generation process that includes recombination of parental lines (which is greatly assisted by male sterility in an autogamous crop), selection of recombinants during the ‘recurrent selection' phase, followed by five or more generations of selfing.
In this study, we report the map-based cloning of wheat male-sterile gene Ms2, and the Ms2-based transcriptome and interaction network. The cloned Ms2 could be used to improve global food security by facilitating breakthroughs in plant breeding and hybrid seed production.
Results
Ms2 male-sterile phenotype in Taigu lines
Taigu lines have small anthers (Fig. 1) and abnormal microsporogenesis (Supplementary Fig. 1). In wild-type LM15, anthers grew continuously from the S1 to S4 stages, appearing green initially and yellow-green at maturity. In LM15RMs2, sterile anthers ceased growth at S2 when meiosis occurs, remained the same size through the S3 time period, and decreased in size during the S4 time period. Taigu sterile anthers were initially pale green but faded to pale white or greyish by the end of the S4 time period. At the S4 stage, fertile anthers of wild-type wheat bore germinable pollen; Taigu lines have a ‘non-pollen type' male sterility17 (see trinucleate microspores in Supplementary Fig. 1). Although late-season Taigu flowers may have more normally sized anthers16, they are aberrant and any pollen that is produced is non-viable (Supplementary Fig. 2).
Figure 1. Typical anther growth in Taigu lines.
(a) Wild-type male-fertile LM15. (b) Male-sterile Taigu LM15RMs2. LM15RMs2 is characterized by diminutive stamens and wild-type pistils. Anther growth was divided into four developmental stages (S1 to S4). Codes in parentheses indicate a comparable Zadok's scale37. The experiment was repeated three times. Scale bars, 0.5 mm.
The Ms2 gene is mapped to a 0.05-cM interval
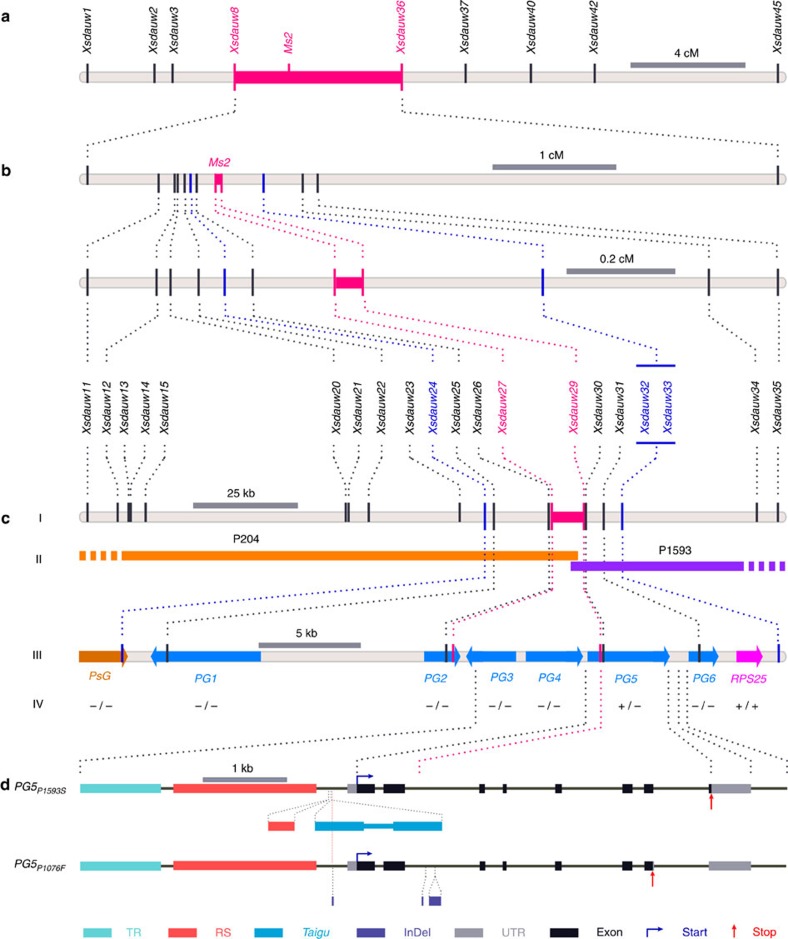
We used synthetic hexaploid wheat (Supplementary Table 1) to enrich for D-genome SNPs. On the basis of the wheat 90k SNP chip, Taigu lines had 5.1% and 14.2% SNPs with SW7 and SW41, respectively (Supplementary Fig. 3), which was sufficient for mapping and cloning Ms2. We mapped Ms2 using four BC1F1 populations, including popA, popD, popE and popF (Supplementary Table 1). Using the Aegilops (Ae.) tauschii map24, we developed nine PCR markers and mapped Ms2 to the Xsdauw8-Xsdauw36 interval in popA (Fig. 2a, Supplementary Fig. 4 and Supplementary Data 1). Although Ms2 is 4 cM distal to Xsdauw36 in popA, it co-segregates with Xsdauw36 in popE and popF (Supplementary Fig. 4), possibly because the RMs2 locus interferes with chromosome recombination. We screened the Ms2-based populations for recombinants in the Xsdauw2-Xsdauw42 region. Using the physical map of Ae. tauschii (Supplementary Fig. 5), we designed close markers, mapped Ms2 to the 0.6 cM Xsdauw20–Xsdauw32 interval using 3,487 popD plants (Supplementary Fig. 4), and precisely mapped Ms2 to the 0.05 cM Xsdauw27-Xsdauw29 interval using 3,826 popA-2 plants (Fig. 2b; Supplementary Fig. 4). After screening 7,441 gametes in popA and popD, we linked Xsdauw20-Xsdauw27-Ms2-Xsdauw29-Xsdauw32.
Figure 2. Map-based cloning of Ms2.
(a,b) Maps based on popA. (c) Physical maps of the Ms2 region: (I) markers; (II) Ms2-associated BAC clones; (III) gene, pseudogene (PsG) or potential genes (PG) in the Xsdauw24-Xsdauw32 interval; (IV) mRNA detected (+) or not (−) in S2-stage anthers of LM15RMs2/LM15 (PG5 additionally tested in Ms2-isogenic lines of CSRMs2/CS and XY6Ms2/XY6; RT-PCR data is provided in Supplementary Fig. 18). (d) Genomic structure of PG5P1593S and PG5P1076F. Primers for RT-PCR were described in the Supplementary Data 2. InDel, insertion or deletion; RS, repetitive sequence; Start and Stop, respective codons; Taigu, a class 1 LTR retrotransposon; TR, tandem repeat; UTR, untranslated-region.
We constructed a bacterial artificial chromosome (BAC) library of LM15RMs2, which contained 706,176 BAC clones for a 5.1-fold coverage of the wheat genome (Supplementary Table 2). We assembled physical maps of the MS2 region in LM15RMs2 (Supplementary Fig. 6) and primarily analysed the Xsdauw24-Xsdauw32 interval (ca. 33 kb). Xsdauw24 is in a pseudogene (PsG, related to AK331827 and AK368680 in GenBank) and Xsdauw32 is near ribosomal protein S25 (RPS25, GenBank CJ502521). Six potential genes (PG1 to PG6; Fig. 2c) were predicted between PsG and RPS25 using the Fgenesh programme (v2.6)25. By reverse-transcription PCR (RT-PCR), PG5 mRNA could be detected in immature spikes of Taigu lines CSRMs2, LM15RMs2 and XY6Ms2, but not in male-fertile CS, LM15 and XY6 (Fig. 2c). In contrast, the ribosomal protein S25 (RPS25) mRNA was detected in developing flowers of both Taigu and male-fertile lines; we did not detect expression of the other hypothetical genes (Fig. 2c). Thus, PG5 was the strongest candidate for Ms2. PG5 has two alleles: the P1593-type PG5 (PG5P1593S; GenBank KX585234) that is completely linked to male sterility in Taigu lines and the P1076-type PG5 (PG5P1076F; GenBank KX585235) from male-fertile LM15. Although the mRNA of PG5P1076F was not detected in LM15, it is expressed somewhat in the S2-stage anthers of LM15RMs2, albeit at 23% (mean) ±8.8% (one standard error of the mean, n=3) of the mRNA of PG5P1593S in LM15RMs2 as indicated by RNA-seq reads (158 reads of PG5P1593S versus 28 reads of PG5P1076F), 5′- and 3′-terminal cDNA clones (10 clones of PG5P1593S versus 4 clones of PG5P1076F) and internal cDNA clones (199 clones of PG5P1593S versus 22 clones of PG5P1076F). Both PG5P1593S and PG5P1076F have eight exons. PG5P1593S encodes a 293-aa protein (31.5 kDa, pI 5.7) with no annotated domains or protein homologues in plants except wheat.
The PG5P1593S and PG5P1076F alleles of LM15RMs2 have seven SNPs in the coding region; the first six SNPs cause six amino acid changes and the seventh encodes a stop codon in PG5P1076F (Supplementary Data 3). We found three structural variations in a 750-bp non-repetitive region upstream of the start codon. In contrast to PG5P1076F, PG5P1593S has a long terminal-repeat (LTR) retrotransposon integrated at nucleotide position −314..−310 (Fig. 2d, Table 1 and Supplementary Fig. 7). This LTR retroelement represents a new family of the terminal-repeat retrotransposons in miniature (TRIM), which we named Taigu_P1593-1 (Taigu; GenBank KX585234). Taigu_P1593-1 is associated with two identical LTRs (direct repeats, 582 bp each), a 630-bp internal domain, and a 5-bp target site duplication (TSD). We postulated that the Taigu retroelement acts as a transcription enhancer that activates transcription of PG5P1593S rather than an inactivator of a repressive cis-element in the promoter region.
Table 1. Haplotype analysis of the MS2 gene in common wheat and Ae. tauschii.
| Haplotypes |
Markers, location and genotypes* |
Number of lines |
|||||
|---|---|---|---|---|---|---|---|
| HT1 | HT2 | HT3 | HT4 | Ae† | Ta‡ | Subtotal | |
| −673..−364 | −314..−310 | 902..905 | 3404 | ||||
| CS group | |||||||
| A1 | A | A | A | A | 7 | 60 | 67 |
| A2 | A | C§ | A | A | 0 | 3 | 3 |
| A3 | A | D‖ | A | A | 0 | 1 | 1 |
| LM15 group | |||||||
| B1 | B | B | B | B | 31 | 392 | 423 |
| B2 | B | D | B | B | 1 | 0 | 1 |
| B3 | B | — | B | B | 20 | 0 | 20 |
| B4 | B | B | — | B | 0 | 3 | 3 |
| B5 | B | B | B | — | 0 | 4 | 4 |
| Hybrid group | |||||||
| H1 | A | A | A | B | 0 | 1 | 1 |
| H2 | A | E¶ | B | B | 7 | 0 | 7 |
| H3 | A | D | B | B | 5 | 0 | 5 |
| H4 | B | B | A | A | 1 | 0 | 1 |
| H5 | B | B | B | A | 1 | 1 | 2 |
| H6 | B | — | B | A | 3 | 0 | 3 |
| H7 | B | B | A | B | 1 | 4 | 5 |
| Null group | |||||||
| N1 | — | — | — | A | 20 | 0 | 20 |
| N2 | — | — | — | B | 2 | 0 | 2 |
| N3 | — | — | — | — | 7 | 0 | 7 |
| Sizes# | |||||||
| A | 679 bp | 237 bp | 360 bp | 260 bp | NA | NA | NA |
| B | 384 bp | 258 bp | 511 bp | 237 bp | NA | NA | NA |
| Number of lines (subtotal and total) | 106 | 469 | 575 | ||||
NA, not applicable.
*We use two periods (..) to separate the starting and ending nucleotide position (Supplementary Data 3). Regular PCR was performed to genotype the haplotype (HT). Chinese Spring (CS) genotypes are denoted by ‘A', and those of LM15 by ‘B'. New genotypes are marked by other letters. A dash symbol ‘—' indicates a negative PCR reaction, possibly caused a target region deletion or low primer efficiency.
†Ae. tauschii and synthetic hexaploid wheat.
‡Triticum aestivum (common wheat).
§C=2,037 bp.
‖D=209 bp.
¶E=197 bp.
#Major diagnostic bands in base pairs (bp).
PG5 P1593S confers plant male sterility
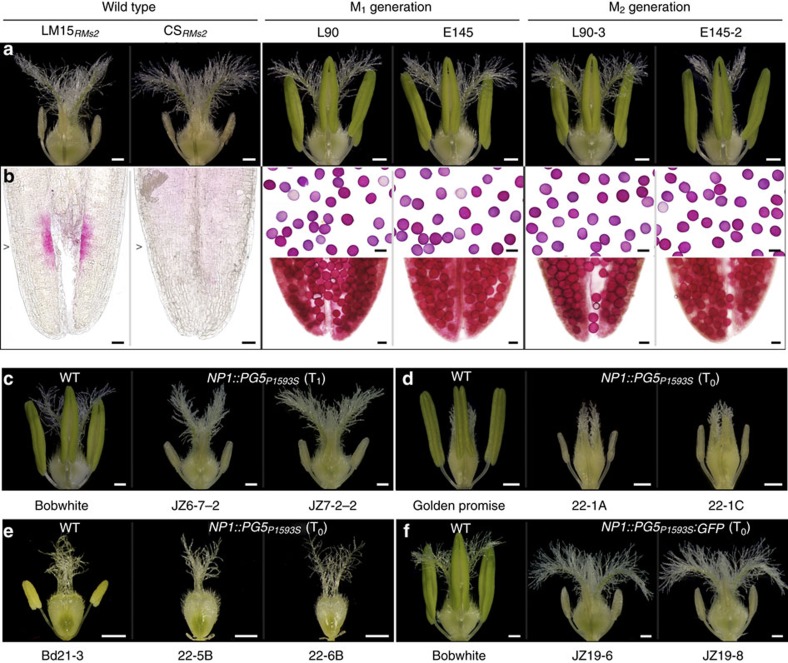
Ms2 is dominant for male sterility (MS), in keeping with the dominant expression of PG5P1593S in Taigu lines; all Taigu lines are heterozygous for Ms2 because two Taigu lines cannot cross. We chemically mutagenized LM15RMs2 and CSRMs2 to determine if any EMS mutants had (1) a male-fertile (MF) phenotype and if so, (2) a variation in their PG5P1593S that would explain the transformation from MS to MF. We generated 2,266 M1 plants and examined 37,471 spikes, of which 254 had MF anthers and viable pollen (Fig. 3a,b). Among all the EMS-treated LM15RMs2 M1 sampled (n=1,200), 2.9% of the primary tillers had a TILLING mutation in PG5P1593S, compared to a 40% mutation rate in PG5P1593S in the induced MF tillers (n=20). A χ2 test negated the independence between PG5P1593S mutations and male fertility in LM15RMs2 (χ2=97.1, df=1, P=6.5 × 10−23), that is, EMS-induced mutation(s) in PG5P1593S are associated with the loss of male sterility. Among all the EMS-treated CSRMs2 M1 sampled (n=1,066), we inspected 34,333 spikes and identified 234 MF spikes in 149 M1 plants. On average, each CSRMs2 M1 plant produced 32 tillers, but only one to eight tillers were MF, indicating a common phenomenon of chimeracism in the M1 generation (Supplementary Fig. 8). We selected 178 MF M1 tillers and examined the length of PG5P1593S using the HT5 marker (Supplementary Data 4); 29% had either partial or complete deletion in PG5P1593S. We then selected 111 of the MF M1 tillers and sequenced the first two exons of PG5P1593S; 23% had point mutations that caused either a residue change or a truncation in the PG5P1593S protein (Supplementary Data 5). We then selected 99 MF M1 to represent the range of mutations, selfed them, and examined the M2 (Fig. 3a,b); all were male-fertile, and all had inherited the mutation from the M1. Among 48 MF M2 plants with the PG5P1593S gene, 42 had mutations in the full-length cDNA of PG5P1593S, including 34 missense mutations, 3 nonsense mutations and 5 splice site mutations (Supplementary Data 5). Thus, PG5P1593S likely confers male sterility in Taigu lines.
Figure 3. PG5P1593S confers male-sterility in Taigu.
(a) Mature flowers from LM15RMs2 and CSRMs2 with diminutive anthers, and CSRMs2 mutants with normally-sized anthers. (b) Alexander's staining of anthers (lower) and pollen (upper, except when no pollen was produced). Wheat lines are the same as in (a). (c–f) Flowers in wild-type (WT, on left) and transgenic wheat (c,f), barley (d) and Brachypodium (e). Male-sterile transgenic plants at the T1 (c) and T0 (d–f) generations were positive for the native PG5P1593S gene (PC976; c–e) and the PG5P1593S:GFP gene (PC973; f). Scale bars, 500 μm (a,c–f) and 50 μm (b).
To further corroborate the function of PG5P1593S, we performed genetic complementation with plasmid PC976 (Supplementary Table 3), which contained a 10,592-bp genomic fragment (−5578..4078..+936) that included the presumed promoter (5,578 bp), PG5P1593S (4,078 bp) and the terminator region (936 bp) of the gene. PC976 was introduced into wheat, barley and Brachypodium (Fig. 3c–e, Supplementary Fig. 9 and Supplementary Data 6). Among 40 putative T0 plants, the expression of PG5P1593S was only detected in three wheat, three barley, and ten Brachypodium plants, all of which were the only male-sterile plants in the T0 generation. Transformants with PG5P1593S remained male-sterile in the T1 generation (Fig. 3c; Supplementary Data 6). We also assembled a genomic PG5P1593S and a green fluorescence protein (GFP) cDNA (PC973; Supplementary Table 3), which was introduced into wheat (Supplementary Data 6). Among 29 putative T0 plants, the only six male-sterile plants all expressed PG5P1593S:GFP (Fig. 3f; Supplementary Data 6). This showed that the C-terminal GFP tag did not compromise PG5P1593S protein expression or function. Thus, transgenic studies proved that PG5P1593S confers male sterility in wheat, barley and Brachypodium, and that the 10,592-bp fragment encodes for both function and proper spatiotemporal expression of Ms2. In conjunction with fine mapping and mutagenesis, we conclude that PG5P1593S controls male sterility in Taigu lines, and that PG5P1593S is the dominant Ms2 gene.
We tried to overexpress the cDNA of PG5P1593S (PC970), PG5P1076F (PC971) and PG5P1593S:GFP (PC972) using the maize ubiquitin promoter in wheat, barley and Brachypodium (Supplementary Table 3; Supplementary Data 6). In total, we treated 3,474 immature embryos and generated 35 putative transgenic plants, but did not recover any actual transgenics. It is possible that excessive PG5P1593S, PG5P1076F and PG5P1593S:GFP caused cell death and prevented regeneration of transgenic plants. Despite some nucleotide differences between PG5P1593S and PG5P1076F, PG5P1076F appears to be functionally equivalent to PG5P1593S, because the overexpression of either PG5P1593S or PG5P1076F apparently prevents generation of transgenic plants. We postulate that the key difference between the MS and MF alleles is that the MS allele is expressed and the MF allele is not.
Spatiotemporal expression pattern of Ms2
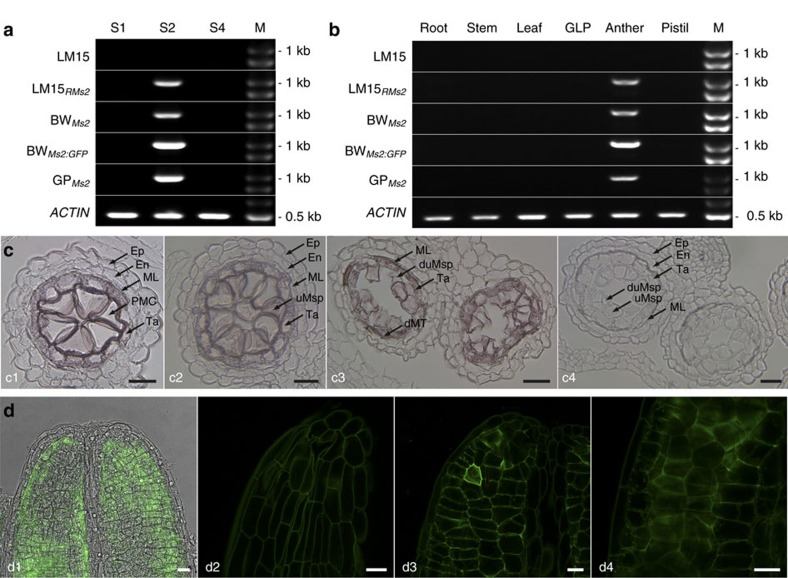
We did not detect MS2 mRNA in vegetative and reproductive tissues in male-fertile wheat by RT-PCR (Fig. 4a,b). In Taigu lines, Ms2 (=PG5P1593S) expression was not detected in early and late in anther development, but was detected in anthers at the S2 stage when meiosis occurs (Fig. 4a). We did not detect MS2 mRNA in other tissues (Fig. 4b). In Taigu lines, the unexpected transcription of ms2 (=PG5P1076F) was also limited to the S2-stage anthers. The transgenic Ms2 in wheat and barley also was detected at the S2 stage in anthers (Fig. 4a,b). Because the PG51593S:GFP construct in wheat confers male sterility, the 936-bp terminator region is not required for induction of male sterility.
Figure 4. Spatiotemporal expression of Ms2.
(a,b) Ms2 expression in anthers. BWMs2, BWMs2:GFP, and GPMs2 are male-sterile transgenic plants JZ7-2_2, JZ19-6, and 22-1A, respectively. GLP was pooled from glumes, lemma and palea. (c) In situ hybridization of Ms2 mRNA in cross sections of LM15RMs2 stamens. Hybridization with antisense probes was detected in pollen mother cells (PMC, c1), early uninucleate microspores (c2), and late uninucleate microspores (c3); hybridization with sense probes was negative in uninucleate microspores (c4). (d) Ms2:GFP in optical sections of an undissected male-sterile anther (from JZ19-6) at the PMC stage. (d1) A superimposed laser scanning confocal and light micrograph showing GFP across the end of an anther. GFP was detected in the middle layer (d2), tapetum (d3) and PMC (d4). The experiment was repeated three times. En, endothecium; Ep, epidermis; ML, middle layer; Ta, tapetum; uMsp, uninucleate microspore; dMT, degenerated middle layer and tapetum; duMsp, degenerated uninucleate microspore; M, the 250 bp DNA Ladder (GENEray, Shanghai, China). Scale bars, 25 μm (c) and 20 μm (d).
By in situ hybridization, Ms2 mRNA was detected in Taigu lines in the middle layer, tapetum, pollen mother cells and uninucleate microspores, but not in the endothecium, epidermis and other tissues (Fig. 4c). Ms2:GFP is functionally equivalent to the native Ms2 protein in conferring male sterility (Fig. 3f; Supplementary Data 6). The distribution of Ms2:GFP and the Ms2 mRNA also were indistinguishable (Fig. 4d). In mesophyll protoplasts of tobacco, the GFP:Ms2 appeared to have a distribution consistent with cytoplasmic and rough endoplasmic reticulum localization, however, there was no detectable green fluorescence in cells with the Ms2:GFP construct (Supplementary Fig. 10). The reason for this currently remains unclear.
MS2 appears within the Triticeae
MS2 homologues only are present in some Triticeae species, including Ae. tauschii, Triticum urartu and T. aestivum (Supplementary Fig. 11; Supplementary Table 4). The lack of close homologues in Hordeum vulgare suggested that MS2 originated between 2.6 and 8.9 Myr ago26. Afterwards, MS2 apparently duplicated, resulting in at least two paralogues in each chromosome of the homoeologous group 4 in common wheat and the ancestral species of Ae. tauschii and T. urartu (Supplementary Table 4). Some regions only share similarity with one or a few pseudo-exons, which have additional truncations, inversions and duplications. Interestingly, five pseudo-exons in WGSC_4898666 match one cDNA entry (GenBank AK331503), and two pseudo-exons in WGSC_3034403 match another cDNA entry (GenBank BE500370). We propose that the MS2-like regions may have undergone dynamic exon shuffling.
We compared the Xsdauw7-Xsdauw35 interval in the D-genome in eleven accessions, including seven common wheat, three synthetic wheat and Ae. tauschii. Two major haplogroups were identified in common wheat: Chinese Spring (CS) and LM15 groups (Supplementary Data 7). We then compared MS2 in nine accessions (Supplementary Fig. 12; Supplementary Data 3). In the CS haplogroup, Ms2 has a Taigu retroelement (inserted at nucleotide position −314..−310), but the male-fertile ms2 does not; the only difference between these two alleles is the Taigu retroelement. Taigu has two identical LTRs (direct repeats, 582 bp), suggesting a recent formation of Ms2 within the CS lineage. There were seven exonic SNPs between CS and LM15; SNP3404 caused a premature stop codon (L284*) in the LM15 group. Both Ae. tauschii and common wheat had two additional variants: one in the promoter (at nucleotide position −673..−364) and one in the second intron (at nucleotide position 902..905).
We further genotyped 575 accessions using four haplotype markers, which target polymorphic sites located at nucleotide positions −673..−364, −314..−310, 902..905 and 3404 (Table 1; Supplementary Data 3, 4 and 8). The MS2 gene is associated with at least 18 haplotypes, three in the CS group (A1-A3; 71 lines), five in the LM15 group (B1-B5; 451 lines), seven in the hybrid group (H1-H7; 24 lines), and three in the null group (N1-N3; 29 lines) (Table 1; Supplementary Data 8). Overall, 74% and 12% of our assessions are in haplotypes B1 in the LM15 and A1 in the CS groups, respectively. Common wheat and Ae. tauschii, contain both A1 and B1 haplotypes, suggesting these haplotypes diverged before the formation of common wheat, and were inherited independently in common wheat. Sixty-eight per cent of our Ae. tauschii accessions had SNP3404. All the remaining haplotypes are unique to either Ae. tauschii or common wheat, and apparently evolved from the major haplotypes via mutations, recombinations and/or deletions. All null group accessions are in Ae. tauschii, illustrating a common phenomenon of partial or complete deletion of MS2 DNA in the D-genome ancestor.
Of the four regions investigated, we compared the number of accessions in three genotype groups (A, B, and a ‘non-AB' group, Table 1) of all materials except for those in the null group (n=546). A χ2 revealed no significant difference among HT1, HT3 and HT4, but HT2 was associated with a higher number of ‘non-AB' accessions than those in either HT1, HT3 or HT4 (χ2>35.2, df=2, P<2.2 × 10−8). Therefore, the polymorphic site −314..−310 (HT2) is the most variable and might be a region that is more likely to be involved in genetic change. We conclude that Ms2 (haplotype A2) was formed when the retrotransposon Taigu inserted into the A1 haplotype.
Here, we propose that a Ms2 allele (Ms2O261S) originated in ‘O261,' a line in PopA that has an intragenic recombination in MS2. Ms2O261S inherited its promoter and first two exons from the dominant Ms2 in XY6Ms2, but the rest of the gene comes from a recessive ms2 in SW41 (Supplementary Data 3). Despite having a large portion of the recessive ms2 allele and the T3404A mutation, O261 is sterile, which suggests that the hybrid Ms2O261S gene confers male sterility and that nucleotide differences between MS and MF alleles in the third to last exons have no effect on MS2 function. Therefore, we propose that the retroelement insertion acts as a molecular switch that turns on MS2 expression and leads to functionalization of the gene.
Putative interaction network of Ms2
To reveal the effect of Ms2 on the transcriptome, we compared the S2-stage mRNA from anthers of LM15 and LM15RMs2, using flag leaves and pistils as controls. Based on the wheat genome annotation (IWGSC1.0+popseq.28), we detected 72,499 active genes in anthers, leaves, and/or pistils (Supplementary Fig. 13a). After removing minimally-expressed genes, we extracted 7,294 differently expressed genes (DEGs) between fertile and sterile anthers (adjusted P value <0.01). Using higher stringency, we then extracted 2,991 sterile-anther-enriched genes (SAEGs) and 939 fertile-anther-enriched genes (FAEGs) (Supplementary Fig. 13b). Among them, 1,907 SAEGs were in the gene ontology (GO) database and had 127 significant GO terms, and 537 FAEGs had 59 significant GO terms (Supplementary Data 9).
In the FAEG group, genes with the GO terms ‘metabolism' (GO:0008152, P=4 × 10−10; hereafter in this paragraph: the P value is based on the Fisher's statistical test, and a FDR-adjusted P value is also indicated in the Supplementary Data 9), ‘photosynthesis' (GO:0015979, P=4 × 10−76), and ‘translation' (GO:0006412, P=5 × 10−14) were overrepresented. Among molecular function GO terms, the ‘structural constituent of ribosome' was also significantly overrepresented in the FAEG group (GO:0003735, P=2 × 10−20). The cellular component GO terms ‘macromolecular complex', ‘membrane', ‘thylakoid', ‘ribosome' and ‘intracellular non-membrane-bounded organelle' were overrepresented. In the SAEG group, there were also many genes involved in metabolism (GO:0008152, P=2 × 10−16), oxidation reduction (GO:0055114, P=9 × 10−13), and protein modification (GO:0006464, P=5 × 10−21). Other GO terms overrepresented among SAEG genes include ‘cell wall organization' (GO:0071554, P=3 × 10−10) and ‘cell wall macromolecule catabolic process' (GO:0016998, P=9 × 10−16). Among molecular function GO terms, ‘catalytic activity' (GO:0003824, P=3 × 10−26) was overrepresented and genes predicted to encode oxidoreductase, transferase, and chitinase were among this group. ‘Extracellular region' (GO:0005576, P=2 × 10−6) was the only significantly overrepresented cellular component GO term among SAEG genes.
We screened for MS2-interacting proteins in the S2-stage spikes using yeast two-hybrids (Y2H) analysis, and identified 312 positive clones encoding for 142 proteins (Supplementary Fig. 13c,d; Supplementary Data 10). Analysis indicated four significant GO terms ‘nucleotide binding' (GO:0000166, P=0.00001; hereafter in this paragraph: the P value is based on the Central Limit Theorem, and a FDR-adjusted P value is also indicated in the Supplementary Data 9), ‘purine nucleotide binding' (GO:0017076, P=0.00001), ‘ribonucleotide binding' (GO:0032553, P=0.00001), and ‘purine ribonucleotide binding' (GO:0032555, P=0.00001) for 17 entries (Supplementary Fig. 13e; Supplementary Data 9 and 10), including eight for the eukaryotic elongation factor (eEF1A), which accounted for 25% of the 312 positive clones, the eukaryotic initiation factor (eIF-4A), and the GTP-binding protein (GTPase) (Supplementary Data 10). Among the other interacting proteins, there were two eukaryotic elongation factors (eEF1B) and seven ribosomal proteins. Using pairwise Y2H, we then demonstrated that wild-type MS2 appears to form homodimers and hetrodimers, and that wild-type MS2 interacts with full-length proteins of interest (Supplementary Figs 13f and 14).
Discussion
Common wheat is a hexaploid species, which has 124,201 annotated genes, 80% repetitive sequences, and a 17-gigabase haploid genome27. Consequently, map-based positional cloning in common wheat is complicated and time consuming. There are three dominant GMS genes reported in wheat: Ms2, Ms3, and Ms4 (ref. 13); Ms2 is on chromosome arm 4DS14. The wheat D genome (originally from Ae. tauschii) has low DNA diversity, which complicated positional cloning of genes in this genome. Synthetic hexaploid wheat (derived from Ae. tauschii) has considerable diversity when compared to the D genome in common wheat. However, the MS2 region is partially or completely deleted in 40% of Ae. tauschii accessions tested here. Consequently, the particular selection of synthetic wheat was critical for the cloning of the Ms2 gene. For example, the deletion of the Ms2 region (X25-Xsdauw30, Supplementary Data 7) in the synthetic wheat SW7 impeded further mapping of MS2 within the Xsdauw20–Xsdauw32 interval. The RMs2 linkage has a tandem segmental duplication over a 1 Mb region28, which apparently was responsible for our exceptionally low recombination in the Ms2 region among the RMs2-based populations (popE and popF, Supplementary Fig. 4). Regardless, low recombination complicates positional cloning, as indicated with the male-sterile gene Ms3 (ref. 29). By selecting the regular Taigu line XY6Ms2 and the synthetic wheat SW41 as parents, we started with sufficient variation in the D genomes and produced progeny with recombination in the MS2 region, which facilitated successful cloning of the Ms2 gene.
Taigu_P1593-1 (Taigu) represents a new family of non-autonomous LTR retrotransposon. First, Taigu_P1593-1 is a TRIM retroelement30. Taigu_P1593-1 is associated with two identical LTRs and a perfect TSD, suggesting a recent origin of this retroelement. Second, the Taigu retroelements only emerged in the Triticeae lineage; there are 50 repetitive sequences in common wheat and 28 repetitive sequences in Ae. tauschii that are similar to part or to a complete Taigu element. Third, there are no significant hits of Taigu in the Triticeae repeat sequence database31. The insertion of Taigu into the MS2 promoter results in Ms2 expression in anthers of Taigu lines. There is one somewhat analagous case in Citrus sinensis32. We do not know whether the Taigu retroelement acts as a transcription enhancer or its insertion destroys a repressive cis-element. In LTR-type retrotransposons, the 5′ LTR usually contains a promoter for producing template RNAs30. The retrotransposon/LTR-based promoters can directly induce gene expression32 or can play a role in activating ‘sleeping' genes33. LTR retrotransposons also can have finely tuned responses to a diverse array of external stimuli, and can act as dispersed regulatory modules that can respond to external stimuli and activate adjacent plant genes34. If there were a repressive cis-element in the promoter region of the MS2 gene, other sequence variants of the cis-element would likely also activate the MS2 gene. For example, the H2 and H3 haplotypes (e.g., ‘CIae 30' and ‘AL8/78', Supplementary Data 8) are associated with a 49-bp deletion in close vicinity (7 bp) to the insertion site of the Taigu retroelement, but accessions of the H2 and H3 haplotypes are male fertile. Consequently, we hypothesize that the Taigu retroelement acts as a transcription enhancer and activates the transcription of the MS2 gene.
In China, the Ms2 gene has been widely used during recurrent selection in conventional breeding of wheat35. The cloning of the dominant Ms2 could boost recurrent selection in wheat globally and enable additional applications for population improvement and gene pyramiding in autogamous crops22. For instance, any transformation-amenable wheat can be engineered to carry the Ms2 gene, which will facilitate a Ms2-based recurrent selection in the ‘engineered Taigu' (eTaigu) line (Supplementary Fig. 15). Although eTaigu lines would be classified as either cisgenic or transgenic, the final cultivars would have male fertility restored and be Ms2-free. Consequently, use of the Ms2 gene may be more readily accepted by the public. Here, we also demonstrated that Ms2 confers male sterility in barley and Brachypodium, in addition to wheat. The range of crops in which the transgenic Ms2 could function is unknown. Nonetheless, the potential for cereal and perhaps other crop improvement is great.
Hybrid corn and rice cultivars are renowned for their higher yield and other superior traits compared to inbreed lines. Despite interest by wheat breeders in hybrids, the development of wheat hybrids globally has been limited by a lack of tools. Cloned Ms2 offers a straightforward method to use cisgenics or transgenics transiently in either wheat or barley breeding programs. Here, we propose a high-throughput hybrid production system using any wheat that can be transformed with Ms2 plus an aleurone-specific gene for pigmented kernels (MSC for Ms2 colour wheat) (Supplementary Fig. 16). The MSC system has two potential applications: (1) screening for desirable heterosis groups, and (2) hybrid seed production for either specific heterosis groups or, for example, for plants with new combinations of disease resistance genes.
This work sheds light on plant male sterility. In wild-type anthers, the middle layer and tapetum (Supplementary Fig. 1) eventually degenerate via programmed cell death36, but not until after the pollen mother spores undergo meiosis. In Taigu lines, the middle layer starts to degenerate as early as the sporogenous cell stage, and any uninucleate microspores that are produced collapse; the collape is correlated with the expression of Ms2 in the middle layer and the male reproductive cells.
In conclusion, we report molecular characterization of a male sterility gene in wheat. The current Ms2 gene has played pivotal roles in wheat breeding, enabling the release of hundreds of breeding lines/cultivars in common wheat. Remarkably, Ms2 homologues are only found in wheat and its close relatives, and are absent from other crops outside the Triticeae tribe. The cloned Ms2 gene provides high value for breeding and producing hybrid wheat, and potentially for other major crops.
Methods
Plant materials and growth conditions
We used 18 plant accessions and four BC1F1 populations, including those of common wheat (Triticum aestivum L., 2n=6x=42) and synthetic hexaploid wheat (SHW, 2n=6x=42) (Supplementary Table 1). Additional germplasm was used for MS2 diversity (Supplementary Data 8). Plants were grown in the greenhouse with a 16 h photoperiod and a daytime and nighttime temperature of 22–25 °C and 15–20 °C, respectively, in the growth chamber with a 16 h photoperiod and a constant temperature of 23 °C, or in the research farm of the Shandong Agricultural University, Taian, China.
‘Langdon', ‘LDNDIC521-2B', and three SHW lines (SW7, SW8 and SW41) were received from Dr J.D. Faris at the USDA-ARS, Fargo, ND, USA. The remaining SHW lines were provided by Dr T. Payne at the International Maize and Wheat Improvement Center, El Batán, Texcoco, México. All Ae. tauschii accessions except for ‘AL8/78' were provided by Dr H.E. Bockelman at the USDA-ARS, Aberdeen, ID, USA.
Anther development in common wheat
We inspected wheat anthers at four stages: when the auricle distance between the flag and penultimate leaves was from −3 to −5 cm (S1≈a Zadoks scale Z38, ref. 37); when the auricle distance was from 3 to 5 cm (S2≈Z41); when the first awn was visible in an undissected spike (S3≈Z49); and when all of the first inflorescence was visible (S4≈Z59). Anthers and pollen were either stained directly using the Alexander protocol38 which dyes viable pollen either red or pink, or embedded using a modified protocol for semi-thin sections39. For semi-thin sections, anthers were fixed in glutaraldehyde (3% glutaraldehyde in 0.2 M phosphate buffer, pH 7.4), dehydrated using an increasing gradient of ethanol (30–100%), cleared using an increasing gradient of xylene (25–100% in ethanol), infiltrated with paraffin, and cut into 8 μm thick sections. Anther sections were then dewaxed, rehydrated, stained in 0.02% ruthenium red and dehydrated using an increasing gradient of ethanol (30–100%). The dehydrated anther sections were washed with an increasing gradient of xylene (50–100% in ethanol), air-dried, and mounted in neutral balsam for future use. Anther, pollen and their sections were examined under an Olympus SZX16 stereo microscope (Olympus, Shinjuku, Tokyo, Japan) or a Nikon ECLIPSE Ni microscope (Nikon, Shinagawa, Tokyo, Japan). Images were recorded with a Digital Sight DS-Fi camera (Nikon), and processed using the NIS Elements 4.0 (Nikon).
Polymorphic status among parental lines
We used the wheat 90 K iSelect SNP array40 to genotype six hexaploid lines (CSRMs2, LM15RMs2, XY6Ms2, SW7, SW8 and SW41; genome=AABBDD), two tetraploid wheat (Langdon and LDNDIC521-2B; genome=AABB), and two diploid accessions (CIae 9 and PI 569536; genome=DD). To extract the A and B-specific SNP markers, we discarded non-specific or low quality SNP markers by performing a three-step elimination: (1) those with any missing data or any heterozygous genotypes within Langdon or LDNDIC521-2B; (2) those with any genotype data within CIae 9 or PI 569536; and (3) those with any missing data or any heterozygous genotypes within the six parental lines. We used a similar procedure to extract the genome D-specific SNP markers, but step one was for CIae 9 or PI 569536 and step two was for Langdon or LDNDIC521-2B. Using genome-specific SNP markers (5,975 in AB and 1,563 in D), we calculated the proportion of polymorphic SNP markers for 15 pairwise comparisons between the parental lines.
Precise mapping of the Ms2 gene
To map the Ms2 gene, we used three Taigu lines (CSRMs2, LM15RMs2 and XY6Ms2) and three SHW lines (SW7, SW8 and SW41) (Supplementary Table 1). The three SHW lines share the same durum donor41. We started of a 3 × 3 factorial backcross between Taigu and SHW lines. The same SHW line was used as the recurrent male parent for each backcross. In total, we prepared eight BC1F1 populations; the XY6Ms2/SW8 failed to produce seed.
We initially developed nine PCR markers using the Ae. tauschii map24, and mapped the Ms2 gene to the Xsdauw8-Xsdauw36 interval, which aligns to the AT4D3406-AT4D3410 region in Ae. tauschii (Supplementary Fig. 4). AT4D3406 and AT4D3410 anchor to the physical contigs ctg16527 and ctg10366, respectively, of which ctg10366 appears to span most of the AT4D3406-AT4D3410 interval (Supplementary Fig. 5). We selected overlapping BAC clones for sequencing and marker development. As a result, we designed a distal marker Xsdauw11 on RI298G16, and a proximal marker Xsdauw35 on MI225K13 (Supplementary Figs 4 and 5). Coincidently, both Xsdauw11 and Xsdauw35 anchor to the physical contig ctg10366, defining a physical map of MS2. We then used 13 overlapping BACs (Supplementary Fig. 5) to narrow down the MS2 interval. During the process, we developed 19 markers in the Xsdauw11-Xsdauw35 interval and precisely mapped the MS2 gene between Xsdauw27 and Xsdauw29. Genetic maps were constructed using the regression algorithm in JoinMap 4.0 (Kyazma B.V., Wageningen, Netherlands). The recombination frequency was transformed into centimorgans (cM) using the Kosambi function.
Physical map of the Ms2 region in Taigu
Using standard prototols42,43, we constructed a BAC library of LM15RMs2. Briefly, high-molecular weight genomic DNA was extracted from leaf tissues, partially digested by the restriction enzyme HindIII, and ligated into the BAC vector pIndigoBAC536-S43. The ligation product was transformed into the ElectroMAX DH10B T1 phage-resistant cells (Invitrogene, Carlsbad, CA, USA) and screened on the LB medium with 12.5 mg l−1 chloramphenicol, 80 mg l−1 X-gal and 100 mg l−1 IPTG. White colonies were individually picked into 384-well microtiter plates. In total, 706,176 BAC clones were arranged in 1,839 384-well plates, representing a 5.1-fold coverage of the wheat genome (∼17 Gb) (Supplementary Table 2).
We developed a PCR-based screening of the LM15RMs2 BAC library. In brief, a 384-well stock was duplicated and pooled to extract a primary DNA pool using the ZR BAC DNA Miniprep Kit (Zymo Research Corporation, Irvine, CA, USA). A super DNA pool was then prepared by mixing ten primary DNA pools. In total, we prepared 1,839 primary DNA pools and 184 super DNA pools. We screened the BAC library using Xsdauw11, Xsdauw20, Xsdauw25, Xsdauw33 and Xsdauw35, and recovered twelve BAC clones in this region (Supplementary Fig. 6). We then used Xsdauw26 and X26 to determine whether these BAC clones were derived from a ms2 or Ms2-associated chromosome.
High-throughput sequencing was done by the Berry Genomics Company (Beijing, China). The BAC DNA was processed into a paired-end (PE) DNA library. In brief, DNA was acoustically fragmented using the Covaris instrument (Covaris, MA, USA), end-repaired and 3′ adenylated using the NEBNext Sample Reagent Set (New England Biolabs, Ipswich, MA, USA), ligated to Illumina adaptors, and separated on a 2% agarose gel to select fragments about 400–500 bp. Adaptor specific primers were used to amplify the ligation products. The final library was evaluated by quantitative RT-PCR with the StepOne Plus Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). PE reads (150 bp) were obtained using the Illumina HiSeq2500 (Illumina, San Diego, CA, USA).
Using raw reads, we eliminated adaptors, low quality PE reads (half or more bases of any PE reads with a quality value Q≤5 or unknown bases accounting for over 10% of any PE reads), and then removed any vector or E. coli. sequence using the cross_match tool in Phrap44. We then performed a de novo assembly on clean reads (ca. 4.5 Gb in total for all BACs) using the ABySS 1.5.2 programme45. Specific BAC clones were further assembled to cover the Ms2 and ms2 regions. In the Xsdauw24-Xsdauw32 interval, we identified several Ms2 candidates and compared their transcription in Taigu and transgenic plants (Figs 2c and 4a,b; Supplementary Figs 9a, 17 and 18).
PG5 P1593S and PG5 P1076F transcripts in LM15 RMs2
We estimated the relative expression of PG5P1593S and PG5P1076F in LM15RMs2 by comparing their frequency in 5′- and 3′-terminal cDNA clones, internal cDNA clones and RNA-Seq reads.
For 5′- and 3′-terminal cDNA clones, we synthesized the S2-stage spike cDNA of LM15RMs2 using the RevertAid Frist Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA), and conducted the rapid amplification of cDNA ends (RACE) to recover the 5′ and 3′ untranslated-regions of PG5 using the SMARTer RACE cDNA Amplification Kit (Clontech, Mountain View, CA, USA). The 5′ RACE utilized the PG5-specific primers P100 and P101, as well as the 3′ RACE with P102 and P103 (Supplementary Data 2). PCR products were cloned into the pMD18-T vector (Takara, Dalian, China) and sequenced individually. For internal cDNA clones, we first amplified the PG5 cDNA using the conserved primers P104 and P100 (Supplementary Data 2), and cloned the PCR products into the pMD18-T vector. Each PCR clone was again amplified using P104 and P100, and was differentiated by a cleavage amplification polymorphism sequence (CAPS) that uses the restriction enzyme TaqI (New England Biolabs). PCR products of the PG5P1593S clone were cut into two bands (398 bp and 42 bp), but only one band (440 bp) in the PG5P1076F clone, which was scored in a 1.5% Agrose gel (Supplementary Fig. 18). Specific RNA-seq reads of PG5P1593S and PG5P1076F were identified as describled in the Method section: RNA sequencing and GO analysis.
Mutagenesis and mutation screening
We used backcrossed seeds of LM15RMs2/LM15 (the LM15RMs2 set) and the BC1F1 seeds of CSRMs2/2*SW7 and CSRMs2/2*SW8 (the CSRMs2 set) for chemical mutagenesis. Using ethyl methane sulfonate (EMS), we prepared the mutant populations. Briefly, lots of 400 seeds (M0) were soaked in 100 ml ethyl methane sulfonate (EMS, Sigma-Aldrich, St Louis, MO, USA) solution (87.4 μM in water), incubated on a shaker at 150 r.p.m. at 25 °C for 10 h, and washed with running water at room temperatures for 4 h. After germination, vigorous seedlings with roots were grown in greenhouse and field conditions. Because the populations of mutagenized seeds had an expected 1:1 segregation of dwarf, male-sterile RMs2:wild-type male-fertile rms2, at the flag-leaf stage, we screened the M1 plants for a dwarf phenotype (≤60 cm) and the presence of a PG5P1593S allele using the HT5 marker (Supplementary Data 4) and discarded those that were tall (>60 cm) and had no PG5P1593S. Twelve hundred plants in the LM15RMs2 set and 1,066 plants in the CSRMs2 set were retained for mutation screening.
For the LM15RMs2 set, genomic DNA was prepared from the main-stem flag leaf using the Sarkosyl method46. The DNA samples were pooled four-fold and organized into a 96-well format. Flag-leaf DNA was also prepared from a main stem or tiller that produced a male-fertile spike; samples were pooled one-fold with the genomic DNA of LM15RMs2. For the CSRMs2 set, the flag-leaf DNA was only prepared from a main stem or tiller that produced a male-fertile spike, and samples were pooled one-fold with the genomic DNA of CSRMs2.
Positive DNA pools with a mutation in the PG5P1593S gene were identified using a modified TILLING approach47. We first screened for positive DNA pools, which involved two PCR reactions. A long-range PCR was performed to amplify a 5,925-bp fragment (between primers P133 and P138; Supplementary Data 2) by using the KOD FX kit (Toyobo Co., Osaka, Japan). The PCR product was diluted 500 times using ddH2O and then used as a template to amplify the following three regions: (1) exons 1–2 between P133 and P134, (2) exons 3–4 between P135 and P136 and (3) exons 6–8 between P137 and P138 (Supplementary Data 2). PCR products were then subjected to a slow denaturing and re-annealing to enhance heteroduplex formation. The PCR heteroduplexes were digested with celery juice extract (CJE)48. Positive DNA pools were those associated with one or more specifically cleaved PCR products (Supplementary Fig. 8). For two-fold pools, a positive DNA pool directly anchored to a specific DNA sample. For four-fold pools, each DNA of a positive pool was mixed with LM15RMs2 to prepare a two-fold DNA pool, which was further used to identify a mutant. Specific M1 mutants with a mutation in the PG5P1593S gene were selected (Supplementary Fig. 8). The base change was identified by sequencing a specific PCR product in M1 or M2 plants and was confirmed using PCR markers (Supplementary Figs 8 and 17; Supplementary Data 4 and 5).
Plant genetic transformation
For genetic transformation, we prepared four types of plasmids: (1) native expression of PG5, (2) native expression of PG5:GFP, (3) overexpression of PG5 and (4) overexpression of PG5:GFP. For native expression, we used a 10,592-bp genomic fragment (−5578..4078..+936) of the PG5P1593S gene, which includes the native promoter (NP1, 5,578 bp), the genomic coding region (4,078 bp) and the native terminator (NT, 936 bp). We isolated a 10,592-bp fragment from LM15RMs2 (or BAC clones) using PCR primers P139 and P140 (Supplementary Data 2), and assembled the plant expression construct PC976 (NP1::PG5P1593S) (Supplementary Table 3). By using the first 9,653 bp that lacks the stop codon and NT, we then assembled the tag construct PC973 (NP1::PG5P1593S:GFP) (Supplementary Table 3).The KOD FX kit was used for PCR amplification. To overexpress the PG5 gene, we prepared the PG5P1593S- and PG5P1076F-type cDNA from LM15RMs2 using the PCR primers P149 to P151 (Supplementary Data 2), and assembled three plant expression constructs: PC970 (Ubi::PG5P1593S), PC971 (Ubi::PG5P1076F), and PC972 (Ubi::PG5P1593S:GFP) (Supplementary Table 3). These binary constructs have both BAR and hygromycin selection markers on their T-DNA. Plasmid DNA was used for biolistic bombardment in wheat. For Agrobacterium-mediated transformation of barley and Brachypodium, we introduced the binary constructs into the Agrobacterium strain AGL1.
Using standard methods on plant transformation and tissue culture, we performed biolistic bombardment in wheat49,50 and Agrobacterium-mediated transformation in barley51 and Brachypodium52. For PC976, we bombarded 2,742 immature embryos of wheat ‘Bobwhite', infected 500 immature embryos of ‘Golden Promise' and 100 immature embryos of ‘Bd21-3′ using AGL1 cells harbouring PC976, and generated putative transgenic plants (Supplementary Data 6). For PC973, we bombarded 2,102 immature embryos of Bobwhite and generated putative transgenic plants (Supplementary Data 6). For overexpression, we infected 500 immature embryos of Golden Promise and 200 immature embryos of Bd21-3 with each of three constructs (PC970, PC971 and PC972), bombarded 1,374 immature embryos of Bobwhite for PC972, and generated putative transgenic plants (Supplementary Data 6).
Regular PCR was used to confirm transgene integration: P121 and P122 for BAR, P133 and P141 for PG5P1593S and PG5P1593S:GFP (Supplementary Figs 9 and 17; Supplementary Data 2). For the PG5P1593S positive transgenic plants, a 36-cycle RT-PCR was used to confirm transcription of the transgene and the ACTIN control; we used PCR primers P129 and P130 for PG5P1593S, P129 and P151 (or P152) for PG5P1593S:GFP, P142 and P143 for ACTIN (Supplementary Data 2). The same ACTIN primers were used for both wheat, barley and Brachypodium, but only those from LM15RMs2 are shown in Fig. 4 and Supplementary Fig. 17.
Cellular localization of the MS2 protein
To study cellular localization, we used four constructs: PC972 (Ubi::Ms2:GFP) and PC983 (35S::GFP:Ms2), and their respective controls PC134 (Ubi::GFP) and pMDC43 (35S::GFP) (Supplementary Table 3). Each construct was stored in Agrobacterium strain AGL1. We performed transient expression using the tobacco relative, Nicotiana benthamiana Domin. Four to six week old tobacco plants were co-infiltrated with an equal mixture of two Agrobacterium suspensions, one with the target construct and one with the 35S-driven p19 suppressor53; suspensions were made using 10 mM MES (pH 5.7), 10 mM MgCl2 and 100 μM acetosyringone. To prepare mesophyll cells, we removed the abaxial epidermis of the infected leaf tissue (3 mm × 10 mm), and soaked the remaining tissue in a fresh enzyme solution (0.6 M mannitol, 1.3% cellulase, 0.75% macerozyme, 20 mM MES (pH 5.7), 0.1% BSA and 2 mM CaCl2; Sangon Biotech, Shanghai, China) for 2 h at room temperature. The protoplast suspension was inspected under a Leica TCS SP5 II laser scanning confocal microscope (Leica Microsystems, Wetzlar, Hesse, Germany). Three views were captured for each target cell: (1) a brightfield, (2) a GFP channel and (3) a chlorophyll autofluorescence (ChlAF).
RNA sequencing and GO analysis
We collected four types of samples from plants grown in greenhouses to the S2 stage: fertile anthers of LM15, sterile anthers of LM15RMs2, pistils of LM15 and LM15RMs2, and flag leaves of LM15 and LM15RMs2. Tissues were stored in liquid nitrogen during collection and then at −80 °C for storage. There were three biological replicates per tissue-genotype.
We extracted total RNA using TRIzol (Invitrogen) and evaluated the RNA integrity with an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). The Berry Genomics Company prepared the sequencing libraries (ca. 500 bp per insert) and performed high-throughput sequencing (125 bp PE reads) using HiSeq2500. We pre-processed raw reads using the FastQC algorithm (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), and then performed quality trimming and adaptor removal by Trimmomatic54. In total, we retrieved 484.25 million clean pairs (Supplementary Table 5), and used them for mapping and transcript assembly. For the PG5 gene, specific reads were indicated by a perfect match to PG5P1593S or PG5P1076F of LM15RMs2 using the STAR programme55 and a customized Perl script.
On the basis of the current wheat genome27, clean PE reads were mapped to a repeat-masked data set using the STAR programme55. The PE reads were divided into three groups: (1) unique mapped reads—those mapped to a unique location in the masked genome, (2) multiple mapped reads—those mapped to at least two locations in the masked genome, (3) unmapped reads—those absent in the masked genome or those mapped to repeats (Supplementary Table 6). Unique mapped reads were retrieved from the STAR-derived BAM files using Perl scripts.
Based on the wheat genome (IWGSC1.0+popseq.28, http://plants.ensembl.org), we assembled the unique mapped reads using htseq-count script (v.0.6.1) with the union mode in the HTSeq package56, and quantified their normalized transcript counts using the DESeq package (v.1.26.0)57. There were 79,153 genes with at least one nonzero count value in the four types of samples. To remove false positives and poorly-expressed genes27, we defined a count cutoff value of 1.06 (Supplementary Table 7). The count values less than 1.06 were assigned a zero value; 72,499 expressed genes were retained (Supplementary Table 7). Between male-fertile (MF) and male-sterile (MS) anthers, we determined 7,294 differentially expressed genes (DEGs) by controlling the false discovery rate (FDR<0.01) for multiple testing in DESeq (v.1.26.0)57 and using the ajdusted P value (Padj<0.01). Furthermore, we divided the anther-related DEGs into two groups: (1) sterile-anther-enriched genes (SAEG): those associated with higher transcription in sterile anthers compared to the fertile anthers; (2) fertile-anther-enriched genes (FAEG): those associated with higher transcription in fertile anthers compared to the sterile anthers. The following rules were used to define the final list in each group: (1) genes active in only one type of anther with a count value >the 10th percentile (3.29) of the cleaned data (Supplementary Table 7); (2) genes active in both MF and MS anthers but the relative amount was >10 fold in either MF or MS anthers. By this way, we extracted 2,991 and 939 genes in the final SAEG and FAEG lists, respectively.
The 3,930 anther-enriched DEGs were analysed for GO frequencies. All genes were first assigned identities with the agriGO toolkit58, and then compared to the Triticum aestivum transcript (v2.2) using the singular enrichment analysis (SEA). To prepare heat maps of the anther-enriched DEGs, their expression values were first lg transformed (normalized count +1), and then organized in an expression matrix. A hclust() function and ‘average' method in R was used to cluster the expression matrix using the correlation distance. We then created heatmaps using the Heatmap.2 of the R package gplots (http://www.inside-r.org/packages/cran/gplots/docs/heatmap.2).
Yeast two-hybrid analysis
Both Ms2 (=PG5P1593S) and ms2 (=PG5P1076F) were targeted for Y2H analyses. The coding sequence of each gene was amplified from LM15RMs2 using PCR primers P153 and P150 (Supplementary Data 2), cloned into the pENTR/D-TOP vector (Invitrogen), and recombined into the Gateway-compatible pGADT7 (AD, prey) and pGBKT7 (BD, bait) vectors59. Four Y2H constructs were prepared including PC986 (ADH1::AD:Ms2) and PC987 (ADH1::BD:Ms2), PC988 (ADH1::AD:ms2), and PC989 (ADH1::BD:ms2) (Supplementary Table 3). Similarly, we prepared the AD and/or BD plasmids for four PG5P1593S cDNA variants and eight MS2-interacting protein genes (Supplementary Table 3).
The Y2H assay included the use of the double dropout medium (DDO: SD/-Trp/-Leu), the triple dropout medium (TDO: SD/-Trp/-Leu/-His), and the quadruple dropout medium (QDO: SD/-Trp/-Leu/-His/-Ade) (Clontech). We first performed autoactivity tests (Supplementary Fig. 14). We then constructed a cDNA library and performed the Y2H screening using standard methods60. The cDNA library was prepared on the S2-stage spikes of LM15RMs2. Aliquots of 1 ml with a titre value of 9 × 107 c.f.u. ml−1 were prepared and stored in a −80 °C freezer. Both Ms2 and ms2, as bait proteins, were used to screen the cDNA library on the QDO and TDO media, respectively. Positive clones from the library screening were tested for their autoactivity and pairwise interactions with Ms2 and ms2. Selected clones were sequenced using a T7 primer (Supplementary Data 2). The identity and function of the cDNA clones were predicted by searching the NCBI database and the pfam database. In total, we identified 142 MS2-interacting protein genes (Supplementary Data 10).
We tested the interaction between MS2 and eight representative full-length interactors, which included the eukaryotic elongation factors eEF1A, five ribosomal proteins (RPS2, RPS9, RPS25, RPL15, and RPL17), the GTP-binding protein (GTPase), and the ubiquitin-conjugating enzymes Ubc4B (Supplementary Table 3; Supplementary Data 10). We also tested the pairwise interaction between wild-type Ms2 and its four single-residue variants, including Ms2L44F, Ms2T109M, Ms2A153V and Ms2E248K (Supplementary Data 5), which confer male-sterility in EMS mutants. During pairwise interactions, wXb12 (PC332) and wXb12IP2 (PC324) were used as a positive control; wXb12 and wRAR1 (PC322) were used as a negative control (Supplementary Table 3)59.
For MS2-interacting proteins, we performed GO analysis using SEA and the parametric analysis of gene set enrichment (PAGE)58 and prepared a heat map (Supplementary Fig. 13d). Both a protein ID and its count in the cDNA library screening were submitted for the PAGE analysis.
mRNA in situ hybridization
Tissue preparation and in situ hybridization was performed according to a standard protocol61. LM15RMs2 anthers at different stages were harvested and fixed in FAA (10% formaldehyde:5% acetic acid:50% alcohol) for 12 h at 4 °C. Probe templates were amplified from the Ms2 cDNA using PCR primers P146 and P147. Both sense and antisense RNA probes were prepared independently using the digoxigenin RNA labelling kit (Roche Diagnostics, Mannheim, Germany).
MS2 diversity in Triticeae species
To assess genetic diversity of the MS2 gene, we genotyped 575 germplasm, including 469 common wheat lines, 36 SHW lines and 70 Ae. tauschii accessions (Table 1; Supplementary Data 8). For the MS2 gene, we developed four haplotype markers (HT1, HT2, HT3 and HT4; Supplementary Data 4) which target the polymorphic sites located at nucleotide positions −673..−364, −314..−310, 902..905 and 3404, respectively (Supplementary Data 3). The first three haplotype markers were amplified for 38 cycles with annealing temperatures of 67, 63, and 64 °C, respectively. HT4 was amplified for 35 cycles with an annealing temperature of 62 °C, and its PCR product was cleaved using the restriction enzyme AluI (New England Biolabs). Their PCR products were separated on a 1.5–2% agarose gel.
Data availability
The DNA sequence data have been deposited in GenBank: KX533929 (Ms2 cDNA), KX533930 (ms2 cDNA), KX585234 (Ms2-associated BAC sequence) and KX585235 (ms2-associated BAC sequence). The RNA-seq data have been deposited in the NCBI Sequence Read Archive under accession number SRP092366. Other data supporting the findings of this study are either within the article and the Supplementary Information or available on request from the corresponding author. Plant transformation vectors are available with a material transfer agreement. For overseas requests, transgenic plants and Taigu lines will be provided if an export permit is issued by the Chinese Ministry of Agriculture on a case-by-case basis.
Additional information
How to cite this article: Ni, F et al. Wheat Ms2 encodes for an orphan protein that confers male sterility in grass species. Nat. Commun. 8, 15121 doi: 10.1038/ncomms15121 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables and Supplementary References
PCR markers used in linkage analysis
Other PCR primers used in this study
Haplotypes of the MS2 gene
PCR markers used in haplotype analysis
Structural variations and point mutations in the PG5P1593S gene in male-fertile mutants
Putative transgenic T0 and T1 plants with the PG5P1593S gene
Haplotypes of the 2-cM MS2 region in common wheat and Ae. tauschii
Germplasm used for the MS2 haplotype analyses
Enriched GO terms in male-sterile anthers (SAEG & Y2H) and male-fertile anthers (FAEG) in the S2 stage
MS2 interacting-proteins from cDNA library screening
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31471161 and 31110103917) and the Natural Science Foundation of Shandong Province, China (JQ201107). We thank Drs Cathy M. Wilson and Allan Caplan for reviewing the manuscript and Dr Lei Ge for help with microscopy.
Footnotes
Shandong Agricultural University has filed patent applications (201510303817.0 and PCT/IB2016/000537) on behalf of F.N., J.Q., B.L., S.W. and D.F. regarding the gene and its promoter. The remaining authors declare no competing financial interests.
Author contributions D.F. conceived the project; M.-C.L., L.E., H.W., X. Zhang, C.C. and L.S. contributed ideas and resources, F.N., J.Q., Q.H., B.L., Y.W., S.W., F.C., C.Z. and X. Zhao performed the experiments; D.F. and F.N. analysed the data; D.F., F.N. and L.E. wrote the manuscript; and all authors discussed the results and the manuscript.
References
- United-Nations. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables (Department of Economic and Social Affairs-Population Division, United Nations, 2015).
- Pingali P. L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl Acad. Sci. USA 109, 12302–12308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D. et al. Utilization of crop heterosis: a review. Euphytica 197, 161–173 (2014). [Google Scholar]
- FAOSTAT. FAO Statistical Pocketbook 2015: World Food and Agriculture Food and Agriculture Organization of the United Nations (2015). [Google Scholar]
- Kaul M. L. H. (ed.) Male Sterility in Higher Plants 1005Springer-Verlag (1988). [Google Scholar]
- Chen L. & Liu Y.-G. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 65, 579–606 (2014). [DOI] [PubMed] [Google Scholar]
- Ma G.H. & Yuan L.P. Hybrid rice achievements, development and prospect in China. J. Integr. Agric 14, 197–205 (2015). [Google Scholar]
- Chang Z. et al. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl Acad. Sci. USA 113, 14145–14150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. et al. Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnol. J. 14, 1046–1054 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longin C. et al. Hybrid breeding in autogamous cereals. Theor. Appl. Genet. 125, 1087–1096 (2012). [DOI] [PubMed] [Google Scholar]
- Singh S. P., Srivastava R. & Kumar J. Male sterility systems in wheat and opportunities for hybrid wheat development. Acta Physiol. Plant. 37, 1713 (2015). [Google Scholar]
- Adugna A., Nanda G. S., Singh K. & Bains N. S. A comparison of cytoplasmic and chemically-induced male sterility systems for hybrid seed production in wheat (Triticum aestivum L.). Euphytica 135, 297–304 (2004). [Google Scholar]
- McIntosh R. A. et al. in The 12th International Wheat Genetics Symposium (Yokohama, 2013).
- Liu B. & Deng J. A dominant gene for male sterility in wheat. Plant Breed. 97, 204–209 (1986). [Google Scholar]
- Deng J. & Gao Z. The use of a dominant male-sterile gene in wheat breeding. Acta Agron. Sin. 6, 85–98 (1980). [Google Scholar]
- Zhang Y., Deng J. & Ji F. A study for stability of male sterility of Taigu genic male sterile wheat. Shanxi Agric. Sci. 6, 5–8 (1987). [Google Scholar]
- Deng J. & Gao Z. Discovery and determination of a dominant male-sterile gene and its importance in genetics and wheat breeding. Sci. Sin. (Ser. B) 25, 508–516 (1982). [Google Scholar]
- Ji F. et al. A report on the breeding of dominant male-sterile durum (T. durum) and bearded wheat (T. dicoccum). Sci. Agric. Sin. 29, 23–27 (1996). [Google Scholar]
- Ji F. & Deng J. Further study on the inheritance of the genic male-sterile wheat of taigu and the production of the dominant male-sterile octoploid triticale. Sci. China Chem. 28, 608–617 (1985). [Google Scholar]
- Ji F. & Deng J. Utilization of the dominant male-sterile gene Ta1 in Triticale breeding program II. Production and utilization of dominant male-sterile octoploid Triticale. Hereditas 8, 15–18 (1986). [Google Scholar]
- Ji F., Deng J., Li S. & Cheng C. A primary report on breeding of dominant male-sterile hexaploid Triticale. Hereditas 11, 1–4 (1989). [Google Scholar]
- Sorrells M. E. & Fritz S. E. Application of a dominant male-sterile allele to the improvement of self-pollinated crops. Crop Sci. 22, 1033–1035 (1982). [Google Scholar]
- Liu B. & Yang L. Breeding of dwarfing-sterile wheat and its potential values in wheat breeding. Chin. Sci. Bull. 36, 1562–1564 (1991). [Google Scholar]
- Luo M.-C. et al. A 4-gigabase physical map unlocks the structure and evolution of the complex genome of Aegilops tauschii, the wheat D-genome progenitor. Proc. Natl Acad. Sci. USA 110, 7940–7945 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyev V., Kosarev P., Seledsov I. & Vorobyev D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 7, S10–S10 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton C. P. et al. Sequencing of chloroplast genomes from wheat, barley, rye and their relatives provides a detailed insight into the evolution of the Triticeae tribe. PLoS ONE 9, e85761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWGSC. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345, 1251788 (2014). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. A tandem segmental duplication (TSD) in green revolution gene Rht-D1b region underlies plant height variation. New Phytol. 196, 282–291 (2012). [DOI] [PubMed] [Google Scholar]
- Qi L. L. & Gill B. S. High-density physical maps reveal that the dominant male-sterile gene Ms3 is located in a genomic region of low recombination in wheat and is not amenable to map-based cloning. Theor. Appl. Genet. 103, 998–1006 (2001). [Google Scholar]
- Havecker E. R., Gao X. & Voytas D. F. The diversity of LTR retrotransposons. Genome Biol. 5, 1–6 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T., Matthews D. E. & Keller B. TREP: a database for Triticeae repetitive elements. Trends Plant Sci. 7, 561–562 (2002). [Google Scholar]
- Butelli E. et al. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 24, 1242–1255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K. & Yoshida H. Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. Plant J. 57, 413–425 (2009). [DOI] [PubMed] [Google Scholar]
- Grandbastien M.-A. LTR retrotransposons, handy hitchhikers of plant regulation and stress response. Biochim. Biophys. Acta Gene Regul. Mech. 1849, 403–416 (2015). [DOI] [PubMed] [Google Scholar]
- Yang L. et al. in Induced Plant Mutations in The Genomics Era (ed. Shu, Q. Y.) 370–372 (Food and Agriculture Organization of the United Nations, 2009).
- Papini A., Mosti S. & Brighigna L. Programmed-cell-death events during tapetum development of angiosperms. Protoplasma 207, 213–221 (1999). [Google Scholar]
- Zadoks J. C., Chang T. T. & Konzak C. F. A decimal code for the growth stages of cereals. Weed Res. 14, 415–421 (1974). [Google Scholar]
- Alexander M. P. Differential staining of aborted and nonaborted pollen. Biotech. Histochem. 44, 117–122 (1969). [DOI] [PubMed] [Google Scholar]
- Chang F., Zhang Z., Jin Y. & Ma H. in Flower Development, Vol. 1110 (eds Riechmann, J. L. & Wellmer, F.) 203–216 (Springer, 2014).
- Wang S. et al. Characterization of polyploid wheat genomic diversity using a high-density 90 000 single nucleotide polymorphism array. Plant Biotechnol. J. 12, 787–796 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen T. L., Xu S. S. & Harris M. O. Stem rust, tan spot, stagonospora nodorum blotch, and hessian fly resistance in Langdon durum–Aegilops tauschii synthetic hexaploid wheat lines. Crop Sci. 48, 1062–1070 (2008). [Google Scholar]
- Luo M. & Wing R. An improved method for plant BAC library construction. in Plant Functional Genomics, Vol. 236 (ed. Grotewold, E.) 3–19 (Humana Press, 2003). [DOI] [PubMed]
- Shi X., Zeng H., Xue Y. & Luo M. A pair of new BAC and BIBAC vectors that facilitate BAC/BIBAC library construction and intact large genomic DNA insert exchange. Plant Methods 7, 33 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing B., Hillier L., Wendl M. C. & Green P. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 8, 175–185 (1998). [DOI] [PubMed] [Google Scholar]
- Simpson J. T. et al. ABySS: a parallel assembler for short read sequence data. Genome Res. 19, 1117–1123 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C. et al. Distribution, frequency and variation of stripe rust resistance loci Yr10, Lr34/Yr18 and Yr36 in Chinese wheat cultivars. J. Genet. Genomics 39, 587–592 (2012). [DOI] [PubMed] [Google Scholar]
- Uauy C. et al. A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol. 9, 115 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till B. J., Zerr T., Comai L. & Henikoff S. A protocol for tilling and ecotilling in plants and animals. Nat. Protoc. 1, 2465–2477 (2006). [DOI] [PubMed] [Google Scholar]
- Weeks J. T., Anderson O. D. & Blechl A. E. Rapid production of multiple independent lines of fertile transgenic wheat (Triticum aestivum). Plant Physiol. 102, 1077–1084 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B. et al. Characterization of FLOWERING LOCUS T1 (FT1) gene in Brachypodium and wheat. PLoS ONE 9, e94171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood W. A. et al. in Transgenic Wheat, Barley and Oats: Production and Characterization Protocols, Vol. 478 (eds Jones, D. H. & Shewry, R. P.) 137–147 (Humana Press, 2009). [DOI] [PubMed]
- Bragg J. N. et al. Generation and characterization of the Western Regional Research Center Brachypodium T-DNA insertional mutant collection. PLoS ONE 7, e41916 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin E. B., Lepage É., Matton D. P., De Crescenzo G. & Jolicoeur M. Transient expression of antibodies in suspension plant cell suspension cultures is enhanced when co-transformed with the tomato bushy stunt virus p19 viral suppressor of gene silencing. Biotechnol. Prog. 26, 1534–1543 (2010). [DOI] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M. & Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P. T. & Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S. & Huber W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Zhou X., Ling Y., Zhang Z. & Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38, W64–W70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D. et al. Comparative analysis of protein-protein interactions in the defense response of rice and wheat. BMC Genomics 14, 166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H. et al. The HSP90-RAR1-SGT1 based protein interactome in barley and stripe rust. Physiol. Mol. Plant Pathol. 91, 11–19 (2015). [Google Scholar]
- Zhao X. Y., Cheng Z. J. & Zhang X. S. Overexpression of TaMADS1, a SEPALLATA-like gene in wheat, causes early flowering and the abnormal development of floral organs in Arabidopsis. Planta 223, 698–707 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables and Supplementary References
PCR markers used in linkage analysis
Other PCR primers used in this study
Haplotypes of the MS2 gene
PCR markers used in haplotype analysis
Structural variations and point mutations in the PG5P1593S gene in male-fertile mutants
Putative transgenic T0 and T1 plants with the PG5P1593S gene
Haplotypes of the 2-cM MS2 region in common wheat and Ae. tauschii
Germplasm used for the MS2 haplotype analyses
Enriched GO terms in male-sterile anthers (SAEG & Y2H) and male-fertile anthers (FAEG) in the S2 stage
MS2 interacting-proteins from cDNA library screening
Data Availability Statement
The DNA sequence data have been deposited in GenBank: KX533929 (Ms2 cDNA), KX533930 (ms2 cDNA), KX585234 (Ms2-associated BAC sequence) and KX585235 (ms2-associated BAC sequence). The RNA-seq data have been deposited in the NCBI Sequence Read Archive under accession number SRP092366. Other data supporting the findings of this study are either within the article and the Supplementary Information or available on request from the corresponding author. Plant transformation vectors are available with a material transfer agreement. For overseas requests, transgenic plants and Taigu lines will be provided if an export permit is issued by the Chinese Ministry of Agriculture on a case-by-case basis.