Abstract
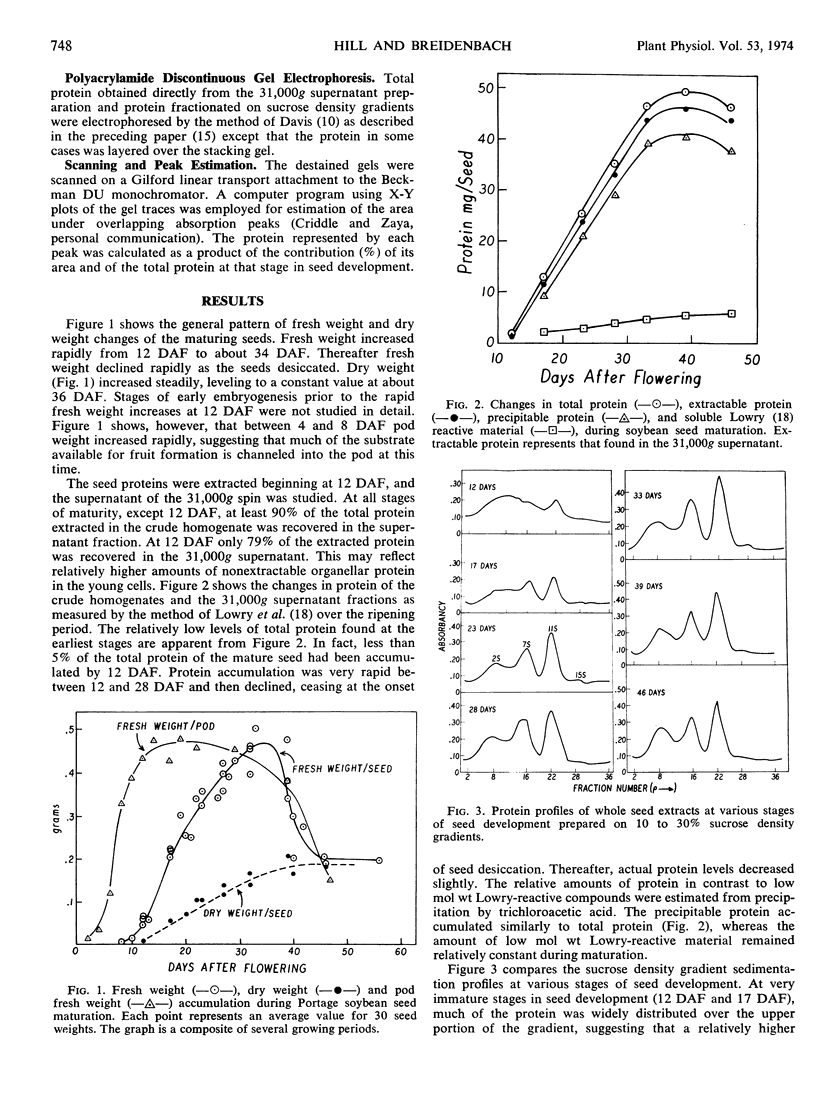
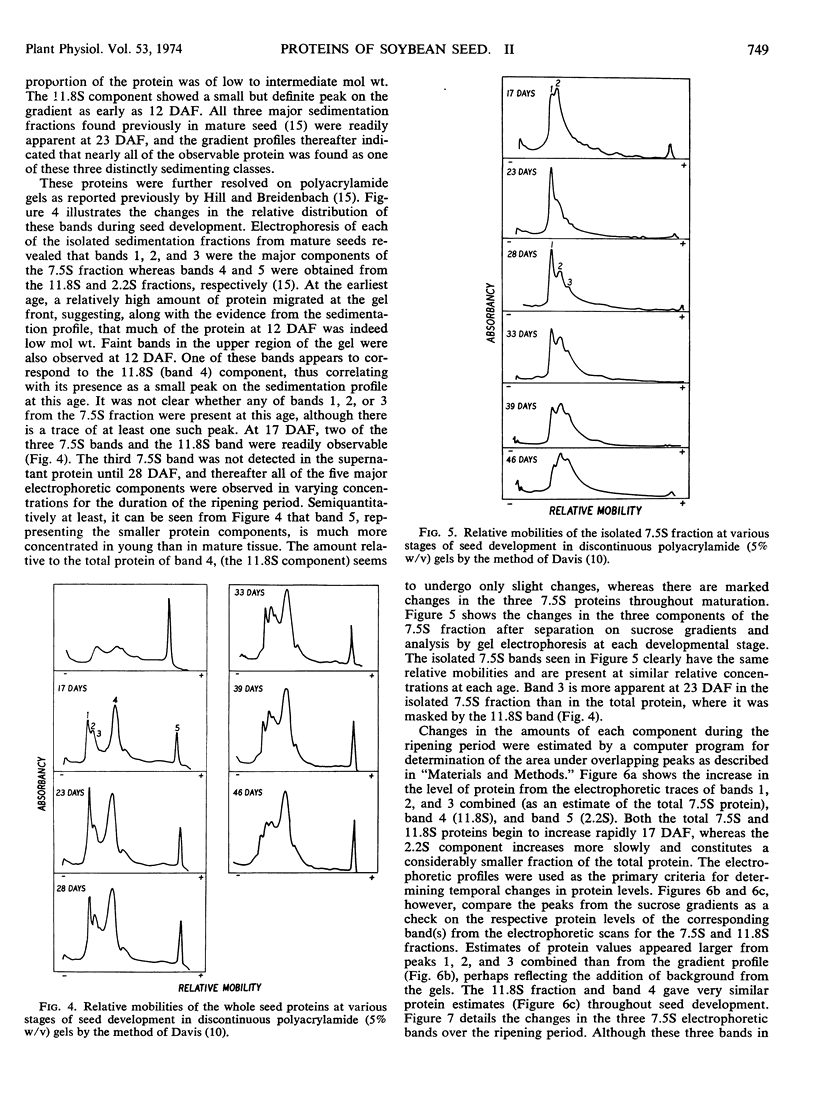
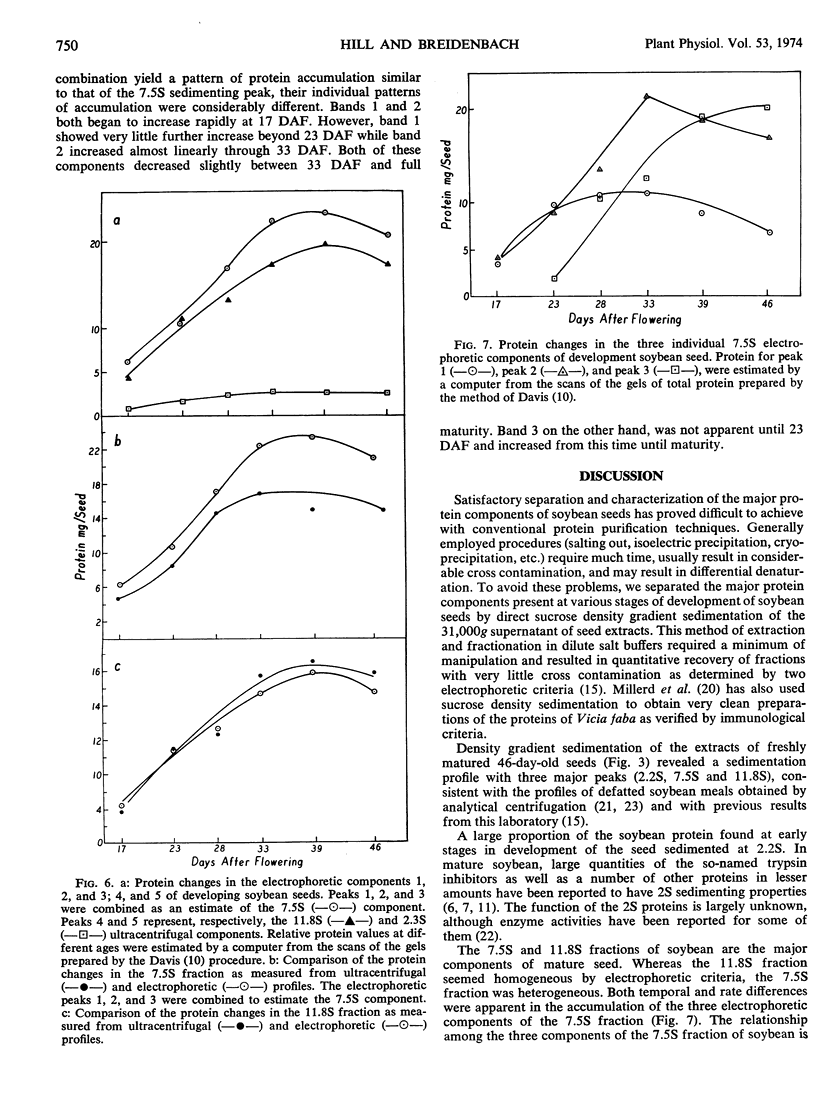
Fresh weight and dry weight as well as quantitative and qualitative protein changes in the developing soybean (Glycine max) seed were described from 12 days after flowering until maturity. The seed proteins were separated on sucrose density gradients into three major fractions, having average sedimentation coefficients of 2.2S, 7.5S, and 11.8S. The 2.2S sedimenting proteins predominated at very early stages of development (12 days after flowering) and decreased proportionately throughout maturation. The 7.5S and 11.8S components appeared to be synthesized later in maturity and in larger amounts than the 2.2S proteins. Electrophoretic studies on extracts from whole seeds and on isolated protein fractions confirmed the early abundance of proteins in the 2.2S fraction and revealed temporal differences in the accumulation of three components of the 7.5S fraction. The 11.8S sedimenting fraction appeared throughout seed development as a homogeneous protein which accumulated in the seed with a time course similar to that of the total 7.5S protein fraction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers L., Poulson R. Protein Synthesis in Cotyledons of Pisum sativum L: I. Changes in Cell-Free Amino Acid Incorporation Capacity during Seed Development and Maturation. Plant Physiol. 1972 Apr;49(4):476–481. doi: 10.1104/pp.49.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsimpoolas N., Leuthner E. The major pH 4.5 soluble proteins of soybean cotyledons. I. Separation by gel filtration, disc electrofocusing and immunoelectrophoresis. Biochim Biophys Acta. 1969 Jul 1;181(2):404–409. doi: 10.1016/0005-2795(69)90273-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Danielsson C. E. Seed globulins of the Gramineae and Leguminosae. Biochem J. 1949;44(4):387–400. doi: 10.1042/bj0440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge A. C., Anderson R. L., Wolf W. J. Polyacrylamide-gel electrophoresis of soybean whey proteins and trypsin inhibitors. Arch Biochem Biophys. 1966 Sep 9;115(3):495–504. doi: 10.1016/0003-9861(66)90068-3. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. A., Gunning B. E. Localization of legumin and vicilin in bean cotyledon cells using fluorescent antibodies. Nature. 1970 Oct 3;228(5266):81–82. doi: 10.1038/228081a0. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Breidenbach R. W. Proteins of soybean seeds: I. Isolation and characterization of the major components. Plant Physiol. 1974 May;53(5):742–746. doi: 10.1104/pp.53.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Millerd A., Simon M., Stern H. Legumin Synthesis in Developing Cotyledons of Vicia faba L. Plant Physiol. 1971 Oct;48(4):419–425. doi: 10.1104/pp.48.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF W. J., BRIGGS D. R. Ultracentrifugal investigation of the effect of neutral salts on the extraction of soybean proteins. Arch Biochem Biophys. 1956 Jul;63(1):40–49. doi: 10.1016/0003-9861(56)90007-8. [DOI] [PubMed] [Google Scholar]


