Abstract
The distribution and localization of cholinesterase in Phaseolus aureus, Glycine max, and Pisum sativum is described. The enzyme is present in roots, leaves, stems, root callus tissue, root cells suspension cultures, and root nodules. Cholinesterase in roots is found primarily in the cell wall. In cell fractionation experiments, at least 95% of the cholinesterase activity is associated with cell wall material. The enzyme can be solubilized by salt solutions, whereas Triton X-100 and sodium deoxycholate solubilize relatively small amounts of the enzyme. Cytochemical techniques have been employed to show the presence of cholinesterase activity at the cell surface and in the cell wall of certain cells of the root.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W. N. Beta-fructofuranosidase from grape berries. II. Solubilization of a bound fraction. Biochim Biophys Acta. 1966 Oct 17;128(1):124–129. doi: 10.1016/0926-6593(66)90148-2. [DOI] [PubMed] [Google Scholar]
- Brewin N. J., Northcote D. H. Partial purification of a cyclic AMP phosphodiesterase from soybean callus. Isolation of a non-dialysable inhibitor. Biochim Biophys Acta. 1973 Aug 17;320(1):104–122. doi: 10.1016/0304-4165(73)90171-2. [DOI] [PubMed] [Google Scholar]
- Chang C. W., Bandurski R. S. Exocellular Enzymes of Corn Roots. Plant Physiol. 1964 Jan;39(1):60–64. doi: 10.1104/pp.39.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu T. H., Dolly J. O., Barnard E. A. Solubilization from skeletal muscle of two components that specifically bind -bungarotoxin. Biochem Biophys Res Commun. 1973 Mar 5;51(1):205–213. doi: 10.1016/0006-291x(73)90529-9. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., Seifert S., O'Brien R. D. Properties of lubrol-solubilized acetylcholine receptor from Torpedo electroplax. Arch Biochem Biophys. 1972 May;150(1):210–218. doi: 10.1016/0003-9861(72)90028-8. [DOI] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Villemez C. L. Solubilization of a mannose-polymerizing enzyme from Phaseolus aureus. Biochem J. 1972 Jun;128(2):243–252. doi: 10.1042/bj1280243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Wang J. H. Solubilization of a specific tetrodotoxin-binding component from garfish olfactory nerve membrane. Biochemistry. 1972 Nov 21;11(24):4565–4569. doi: 10.1021/bi00774a022. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J. Evidence for the regulation of phytochrome-mediated processes in bean roots by the neurohumor, acetylcholine. Plant Physiol. 1970 Dec;46(6):768–777. doi: 10.1104/pp.46.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen E. F., Jang R. Binding of Enzymes to Avena Coleoptile Cell Walls. Plant Physiol. 1960 Sep;35(5):567–574. doi: 10.1104/pp.35.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
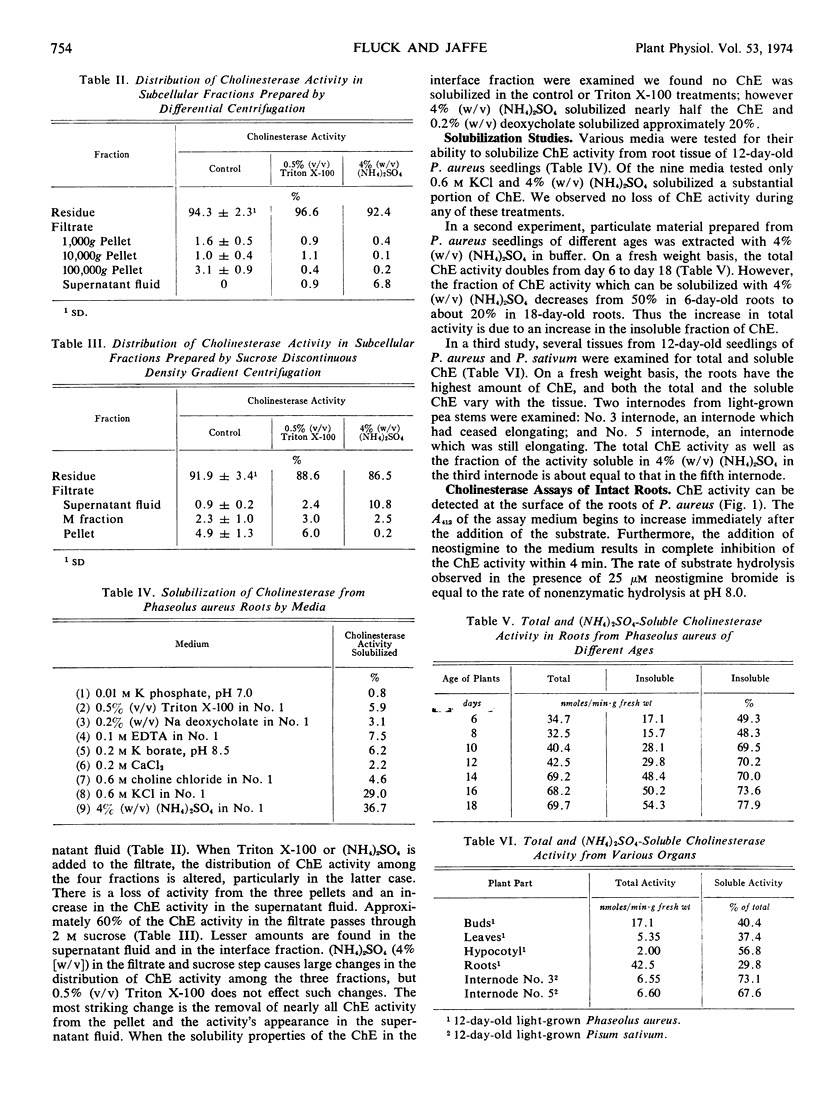
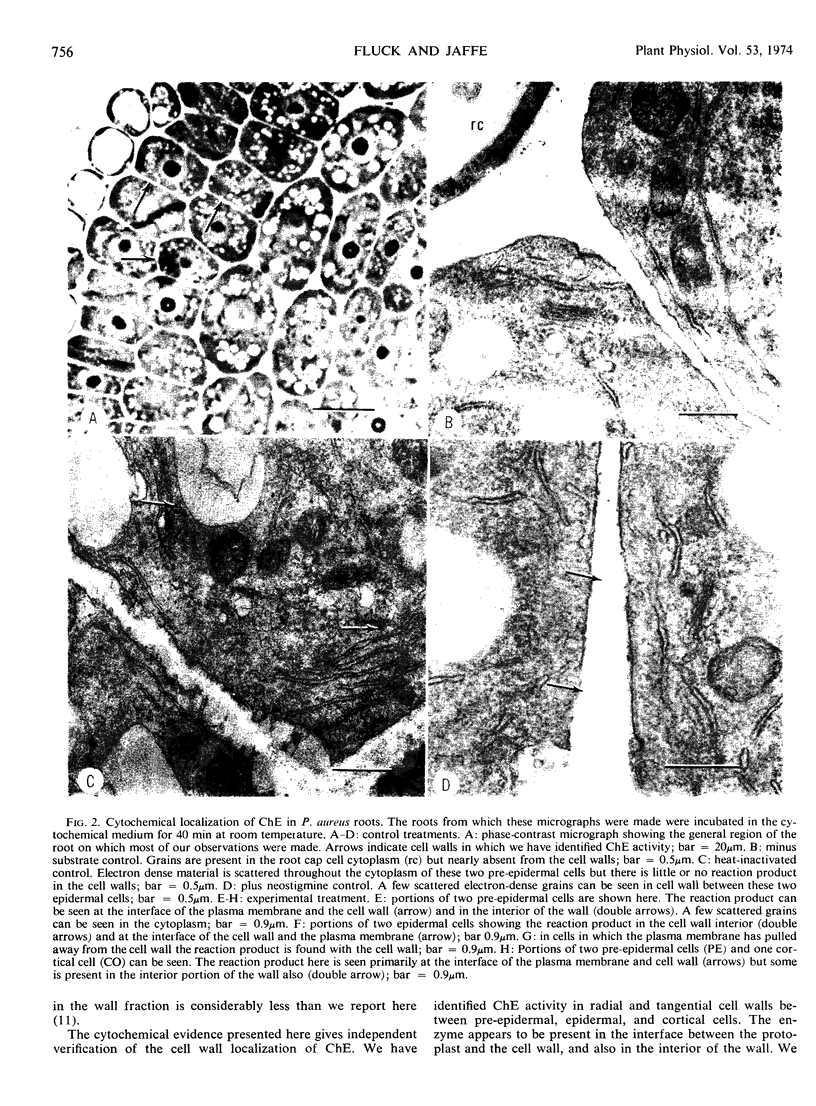
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- MANDELS G. R. The properties and surface location of an enzyme oxidizing ascorbic acid in fungus spores. Arch Biochem Biophys. 1953 Jan;42(1):164–173. doi: 10.1016/0003-9861(53)90249-5. [DOI] [PubMed] [Google Scholar]
- McFarland B. H., Inesi G. Solubilization of sarcoplasmic reticulum with Triton X-100. Arch Biochem Biophys. 1971 Aug;145(2):456–464. doi: 10.1016/s0003-9861(71)80005-x. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Nevins D. J. Relation of glycosidases to bean hypocotyl growth. Plant Physiol. 1970 Sep;46(3):458–462. doi: 10.1104/pp.46.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riov J., Jaffe M. J. Cholinesterases from plant tissues: I. Purification and characterization of a cholinesterase from mung bean roots. Plant Physiol. 1973 Mar;51(3):520–528. doi: 10.1104/pp.51.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]




