Abstract
Previous in vitro studies have established a pre-transfer proofreading mechanism for editing of homocysteine by bacterial methionyl-, isoleucyl-, and valyl-tRNA synthetases. The unusual feature of the editing is the formation of a distinct compound, homocysteine thiolactone. Now, two-dimensional TLC analysis of 35S-labeled amino acids extracted from cultures of the bacterium Escherichia coli reveals that the thiolactone is also synthesized in vivo. In E. coli, the thiolactone is made from homocysteine in a reaction catalyzed by methionyl-tRNA synthetase. One molecule of homocysteine is edited as thiolactone per 109 molecules of methionine incorporated into protein in vivo. These results not only directly demonstrate that the adenylate proofreading pathway for rejection of misactivated homocysteine operates in vivo in E. coli but, in general, establish the importance of error-editing mechanisms in living cells.
Full text
PDF
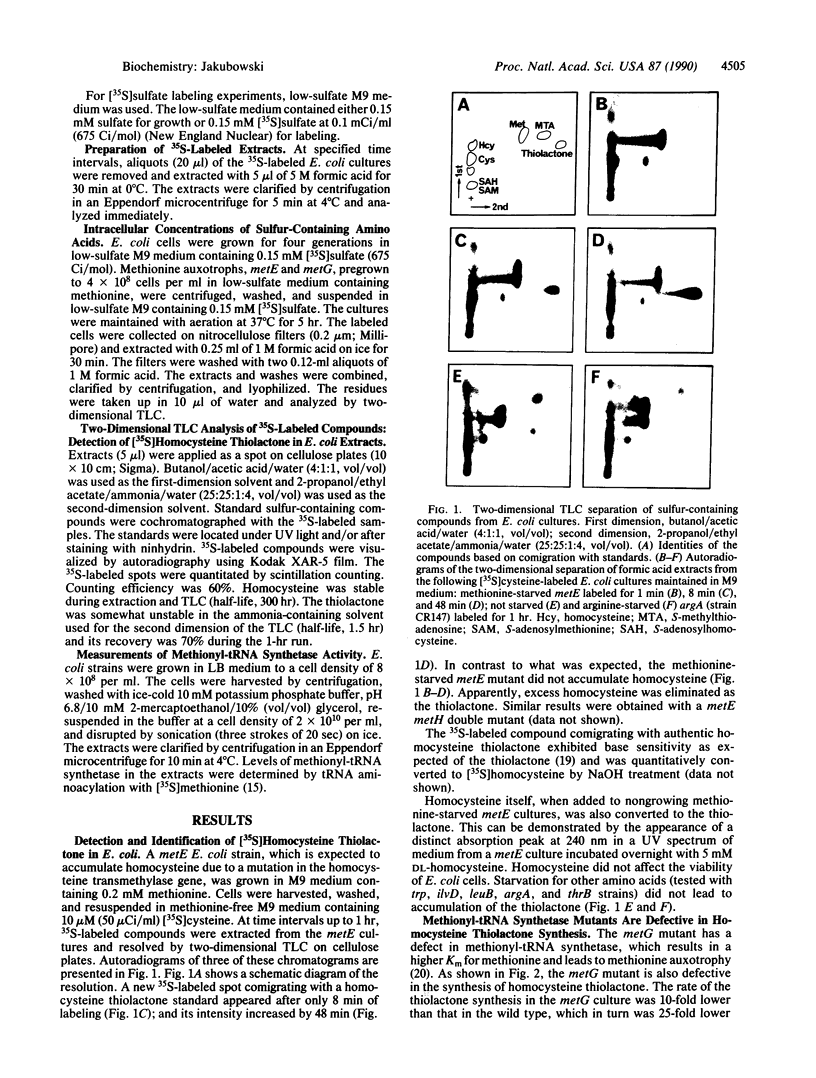
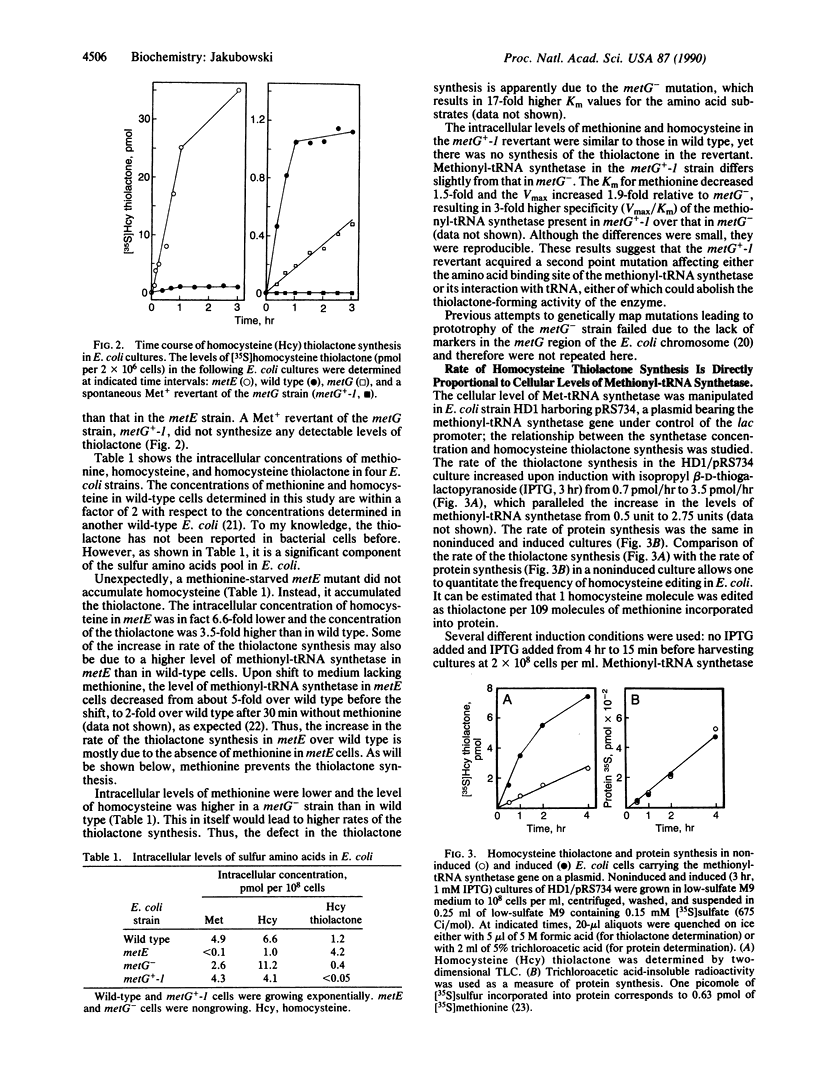
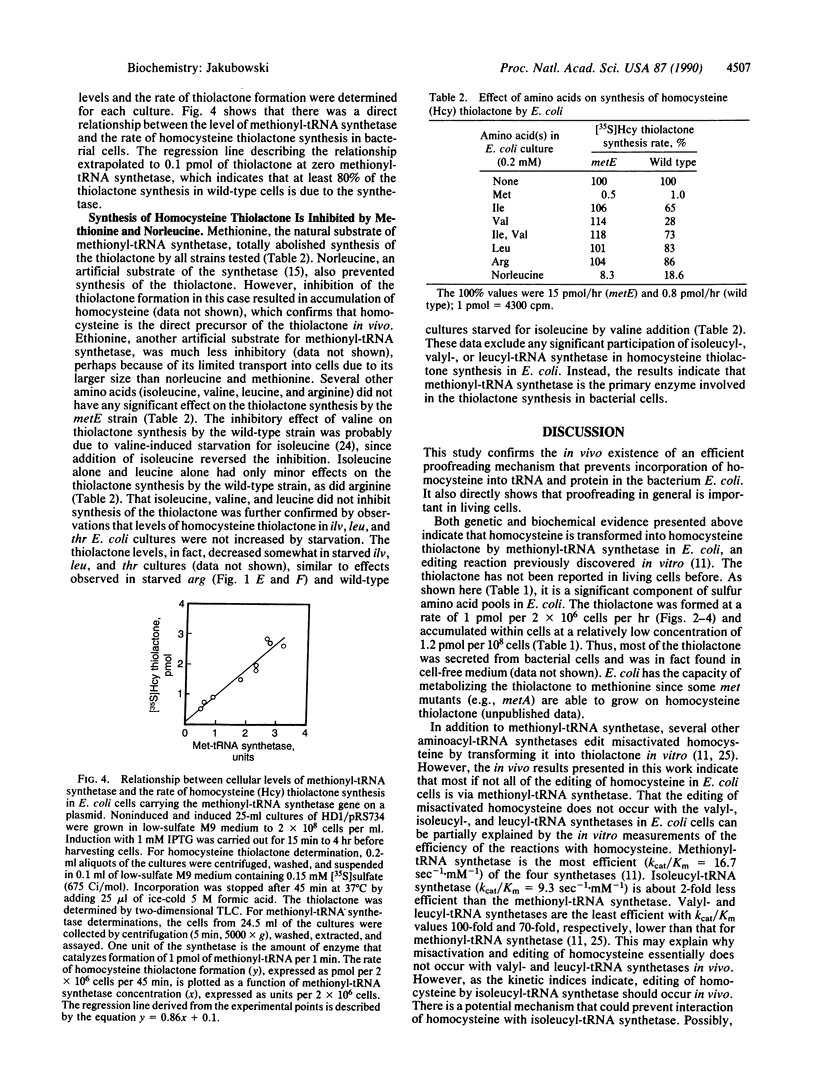
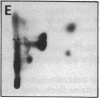
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Mechanism of repression of methionine biosynthesis in Escherichia coli. I. The role of methionine, s-adenosylmethionine, and methionyl-transfer ribonucleic acid in repression. Mol Gen Genet. 1973 Jul 16;123(4):299–324. doi: 10.1007/BF00433648. [DOI] [PubMed] [Google Scholar]
- Baldwin A. N., Berg P. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J Biol Chem. 1966 Feb 25;241(4):839–845. [PubMed] [Google Scholar]
- Barker D. G., Ebel J. P., Jakes R., Bruton C. J. Methionyl-tRNA synthetase from Escherichia coli. Primary structure of the active crystallised tryptic fragment. Eur J Biochem. 1982 Oct;127(3):449–457. [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J. Role of methionine in the regulation of the synthesis of serine hydroxymethyltransferase in Escherichia coli. J Biol Chem. 1984 Jul 10;259(13):8402–8406. [PubMed] [Google Scholar]
- Eldred E. W., Schimmel P. R. Rapid deacylation by isoleucyl transfer ribonucleic acid synthetase of isoleucine-specific transfer ribonucleic acid aminoacylated with valine. J Biol Chem. 1972 May 10;247(9):2961–2964. [PubMed] [Google Scholar]
- Englisch S., Englisch U., von der Haar F., Cramer F. The proofreading of hydroxy analogues of leucine and isoleucine by leucyl-tRNA synthetases from E. coli and yeast. Nucleic Acids Res. 1986 Oct 10;14(19):7529–7539. doi: 10.1093/nar/14.19.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Dingwall C. Establishing the misacylation/deacylation of the tRNA pathway for the editing mechanism of prokaryotic and eukaryotic valyl-tRNA synthetases. Biochemistry. 1979 Apr 3;18(7):1238–1245. doi: 10.1021/bi00574a019. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Kaethner M. M. Enzyme hyperspecificity. Rejection of threonine by the valyl-tRNA synthetase by misacylation and hydrolytic editing. Biochemistry. 1976 Jul 27;15(15):3342–3346. doi: 10.1021/bi00660a026. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J., Yamane T., Yue V., Coutts S. M. Direct experimental evidence for kinetic proofreading in amino acylation of tRNAIle. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1164–1168. doi: 10.1073/pnas.73.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski H., Fersht A. R. Alternative pathways for editing non-cognate amino acids by aminoacyl-tRNA synthetases. Nucleic Acids Res. 1981 Jul 10;9(13):3105–3117. doi: 10.1093/nar/9.13.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski H. Valyl-tRNA synthetase form yellow lupin seeds: hydrolysis of the enzyme-bound noncognate aminoacyl adenylate as a possible mechanism of increasing specificity of the aminoacyl-tRNA synthetase. Biochemistry. 1980 Oct 28;19(22):5071–5078. doi: 10.1021/bi00563a021. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. Valyl-tRNA synthetase from yellow lupin seeds. Instability of enzyme-bound noncognate adenylates versus cognate adenylate. FEBS Lett. 1978 Nov 15;95(2):235–238. doi: 10.1016/0014-5793(78)81001-1. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORRIS A. T., BERG P. MECHANISM OF AMINOACYL RNA SYNTHESIS: STUDIES WITH ISOLATED AMINOACYL ADENYLATE COMPLEXES OF ISOLEUCYL RNA SYNTHETASE. Proc Natl Acad Sci U S A. 1964 Aug;52:330–337. doi: 10.1073/pnas.52.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57(5):587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- Starzyk R. M., Burbaum J. J., Schimmel P. Insertion of new sequences into the catalytic domain of an enzyme. Biochemistry. 1989 Oct 17;28(21):8479–8484. doi: 10.1021/bi00447a031. [DOI] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]
- Yamane T., Hopfield J. J. Experimental evidence for kinetic proofreading in the aminoacylation of tRNA by synthetase. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2246–2250. doi: 10.1073/pnas.74.6.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. Phenylalanyl-tRNA synthetase and isoleucyl-tRNA Phe : a possible verification mechanism for aminoacyl-tRNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1915–1919. doi: 10.1073/pnas.69.7.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]