Abstract
Objectives
To identify geriatric obesity interventions that can guide clinical recommendations.
Design
Systematic review using Medline (PubMed), Cochrane Central Register of Controlled Trials, Web of Science, CINAHL, EMBASE (Ovid), and PsycINFO (Proquest) from January 1, 2005, to October 12, 2015, to identify English-language randomized controlled trials.
Participants
Individuals aged 60 and older (mean age ≥65) and classified as having obesity (body mass index ≥30 kg/m2).
Interventions
Behavioral weight loss interventions not involving pharmacological or procedural therapies lasting 6 months or longer.
Measurements
Two investigators performed the systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria and achieved a high concordance rate (97.3%) in summarizing the primary outcomes. The three primary outcomes were weight loss, physical performance, and quality of life.
Results
Of 5,741 citations, 19 were included. (Six studies were unique, and the remaining 13 were based on the same study population.) Duration ranged from 6 to 18 months (n=405 participants, age range 66.7–71.1). Weight loss in the intervention groups ranged from 0.5 to 10.7 kg (0.1–9.3%). Five studies had a resistance exercise program accompanying a dietary component. Greater weight loss was observed in groups with a dietary component than those with exercise alone. Exercise alone led to better physical function but no significant weight loss. Combined dietary and exercise components led to the greatest improvement in physical performance measures and quality of life and mitigated reductions in muscle and bone mass observed in diet-only study arms. Heterogeneous outcomes were observed, which limited the ability to synthesize the data quantitatively.
Conclusions
The evidence supporting geriatric obesity interventions to improve physical function and quality of life is of low to moderate quality. Well-designed trials are needed in this population.
Keywords: obesity, weight loss, interventions, systematic review
The epidemic of obesity, defined as a body mass index (BMI) of 30.0 kg/m2 or greater, is a public health concern for the rapidly growing segment of Americans aged 65 and older. Based on epidemiological surveys, approximately 30% of the population aged 65 and older is overweight (BMI 25.0–29.9 kg/m2), and 35.4% are obese1. Obesity is associated with illness and disease2, premature mortality3, impaired function4, and poor quality of life5. These poor health outcomes affect not only individuals’ lives, but also overall healthcare expenditures6. The American Society of Nutrition and the Obesity Society suggest that providers recommend weight loss to older adults (aged ≥65) with obesity who have functional impairments or metabolic complications7.
Preventing chronic disease, reducing the risk of cardiometabolic conditions, and achieving clinically significant weight loss are well-established population health objectives, but lifestyle-focused treatments are only moderately effective, result in modest weight loss, and are not usually customized for older adults8. Weight loss–induced sarcopenia and bone loss9 and changes in body composition that occur during the aging process10 are important to consider when addressing obesity in older adults to prevent accelerated disability11. Because weight loss alone is an inadequate target for geriatric obesity interventions, it is crucial to consider other outcomes, including mobility, quality of life, and physical function, when evaluating the effectiveness of lifestyle interventions.
Primary care is the cornerstone of chronic disease management; changes in the way obesity is treated in older adults must occur in this setting. In November 2011, the Centers for Medicare and Medicaid Services released a reimbursement mechanism focusing on intensive behavioral therapy to address obesity in Medicare beneficiaries. Although it provides a mechanism to encourage clinicians to address this condition, it has been highly underused12. Furthermore, this reimbursement strategy is not structured to address the specific features of geriatric obesity13. Although it supports frequent follow-up, it is based upon data largely collected from younger adults. Clinicians often are reluctant to recommend geriatric obesity interventions because the results of earlier observational studies were conflicting as to the effect of weight loss on mortality.14 A recent review based on randomized clinical trials demonstrated a 15% reduction in death from weight loss15, and in select individuals, intentional weight loss may have the potential to improve function and decrease morbidity.
The purpose of this review was to provide an updated evaluation of randomized controlled trials (RCTs) of geriatric obesity interventions in the context of this newly formulated benefit. This review focuses not only on weight loss as a primary outcome of behavioral (nonpharmacological, nonprocedural) interventions, but also on other geriatric-specific outcomes, including physical function, functional status, and quality of life, in older adults with obesity.
METHODS
A literature search was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews and metaanalyses.
Study Protocol
All English-language studies since January 1, 2005, were reviewed because previous reviews had systematically and comprehensively examined the obesity literature before this date. The search was performed on June 12, 2015, and updated on October 12, 2015, and April 5, 2016. The results of the combined search review are presented below. The electronic databases Medline (PubMed), Cochrane Central Register of Controlled Trials, Web of Science, CINAHL, EMBASE (Ovid), and PsycINFO (Proquest) were searched with the assistance of reference librarians (HBB, PJB). Index terms, text words, and concepts for older adults, obesity, and interventions were captured. Full details of the search and methodologies are available upon request. No search limits were applied, allowing all potentially relevant articles to be captured. Bibliographies of eligible articles and systematic reviews were searched manually for additional citations.
Selection Criteria
Records were reviewed using the following inclusion criteria: human subjects; English language; peer-reviewed journal article; behavioral weight loss intervention, defined as any weight loss intervention not involving pharmacological or procedural therapies (endoscopic treatments or bariatric surgery); all subjects aged 60 and older and mean study age per group 65 and older; RCTs; group mean BMI of 30.0 kg/m2 or greater or waist circumference (WC) 88 cm or greater in woman and 102 cm or greater in men16; and intervention duration of 6 months or longer. Conference abstracts, editorials, commentaries, correspondence, case reports, case series, literature reviews, and trials comparing surgical procedures or pharmaceutical weight loss therapies were excluded. Studies primarily assessing weight maintenance were excluded. Bibliographies of known systematic reviews were evaluated to identify additional studies that were not captured during screening review2, 17–23. Studies were initially included during first-level screening if titles or abstracts used the term “overweight” and did not list a mean BMI less than 30.0 kg/m2 to include studies in which the term “overweight” was used to refer to obese subjects (BMI ≥30.0 kg/m2). Studies were excluded on second-level screening if subjects did not meet the prespecified BMI or WC criteria and according to the above-noted exclusion criteria in a hierarchal manner. All non-English-language studies were excluded.
Methodological Quality Review
Before the full review was conducted, two investigators (RKM, LEG) performed a test review for quality assurance. They manually reviewed 150 records that were generated in a preliminary search; screening included title and abstract review only. Of the 150 records, the investigators disagreed on four (2.7% discordance rate), at which point a third investigator adjudicated for consensus (JAB).
The quality of included trials was independently rated using the Cochrane Collaboration’s tool for assessing the risk of bias, focusing on the following criteria: sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Two reviewers (RKM, LEG) working independently classified each trial as being of high, low, or unclear quality for each criterion, with adequate reliability to determine these elements. A third investigator (JAB) adjudicated for consensus.
Data Extraction
Five thousand seven hundred forty-one citations were identified in the initial search and imported into EndNote X7 software (Thomson Reuters, New York). Two investigators (RKM, LEG) manually reviewed record titles and abstracts using broad inclusion and exclusion criteria. Two levels of study screening were performed for study selection; first-level screening included title and abstract review, and second-level screening involved full-text article review. A third investigator (JAB) reconciled discrepancies between selected records before full-text review. Selected studies (n=395) were subject to full-text review and screened using the exclusion criteria hierarchy.
After full review, studies were separated based on the source study population. The parent study was defined as the original randomized trial, and kin studies were those based on the same study population.
Study-Level Outcomes
The primary outcome measures were weight loss and any measure of physical performance or quality of life. Physical function was broadly defined according to 6-minute walk test (6MWT), peak oxygen uptake (VO2peak), measures of muscle strength, or physical performance test (PPT). Each included study contained at least one of the aforementioned outcomes. Secondary self-reported or objective outcome measures that were considered included body composition, insulin resistance, bone mineral density, and cognitive function. Studies were not required to have an aforementioned geriatric-specific outcome. A standardized data collection form was used. The study site, participant characteristics (age, sex, BMI/WC), intervention groups and their descriptions, intervention duration, length of follow up, and main outcome measures were abstracted. Metaanalysis was considered, but the data were found to be too methodologically heterogeneous to perform such an analysis.
RESULTS
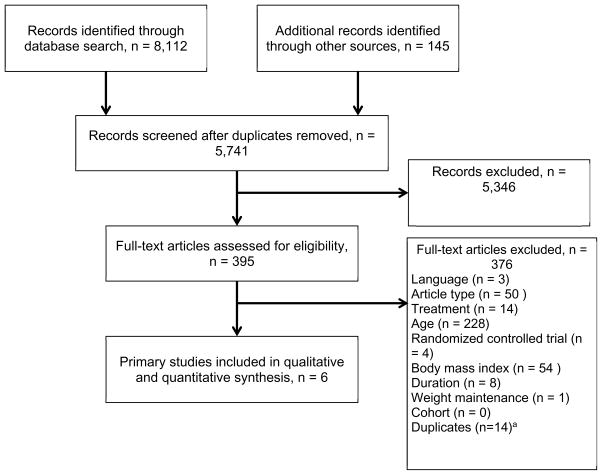
Of the 5,741 citations, 395 underwent full-text review. A flow diagram that outlines the systematic review process is provided in Figure 1. After the full inclusion and exclusion criteria were applied, bibliographies of existing systematic reviews reviewed, and adjudicated accordingly, 19 articles remained. The most common reasons for exclusion of articles were not English language, article type (abstract, review), treatment type (surgical, pharmacological), age younger than 60, not a RCT, BMI less than 30.0 kg/m2 (WC <88 cm in women, <102 cm in men), duration less than 6 months, or weight maintenance study. The results of the methodological assessment are presented in Table 1. Of the 19 final selected articles, six were parent studies (Tables 2 and 3, Supplemental Appendix 1), and 13 were kin studies (Supplemental Appendix 2). Decisions were deliberately made about the relationships between publications to maximize high-quality information without counting participants twice. In Table 3, only the primary outcomes are presented because the baseline characteristics are the same as those reported in the kin studies (Table 2).
Figure 1.
Flow diagram of study selection process for systematic review. aEight existing systematic reviews on the topic of behavioral weight loss in obese older adults before the review process were identified, and their bibliographies accounted for 145 articles (accounted for in the flow diagram as “additional records identified through other sources”). Duplicates from these 145 articles (n=14) were accounted for in box “Full-text articles excluded.”
Table 1.
Methodological Quality of the Included Randomized Controlled Studies—Cochrane Risk-of-Bias Tool
| Reference | Sequence Generation | Allocation Concealment | Blinding | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias | |||
|---|---|---|---|---|---|---|---|---|---|
| Participants | Healthcare Providers | Data Collectors | Outcome Assessors | ||||||
| Miller, 200627 | Unclear | Unclear | No | No | Unclear | Unclear | Unclear | Yes | Yes |
| Villareal, 200629 | Yes | Yes | No | No | Yes | Unclear | Yes | Yes | Yes |
| Frimel, 200826 | Unclear | Unclear | No | No | Unclear | Yes | Unclear | Yes | Yes |
| Davidson, 200925 | Unclear | Unclear | No | No | Yes | Yes | Unclear | Yes | Yes |
| Shah, 200924 | Yes | No | No | No | Unclear | Unclear | No | Unclear | Yes |
| Villareal, 20119 | Unclear | Unclear | No | No | Unclear | Yes | Yes | Yes | Yes |
| Fulfilling yes, %a | 33 | 17 | 0 | 0 | 33 | 50 | 33 | 83 | 100 |
Criteria for the author’s judgment of a summary assessment: “Yes” indicates a low risk of bias; “No” indicates a high risk of bias; “Unclear” indicates an uncertain risk of bias according to the Cochrane Collaboration tool criteria. The proportion fulfilling yes is determined by the number of ‘Yes” responses divided by the overall number of studies.
Table 2.
Baseline Characteristics of Included Studies (n=6)
| Reference | Primary Outcome | Participant Group | Duration | Intervention Description | n | White, % | Age, Mean ± Standard Deviation | Body Mass Index, kg/m2 | Waist Circumference, cm | |
|---|---|---|---|---|---|---|---|---|---|---|
| Miller, 200627 | Function, body composition | Older adults with knee osteoarthritis | 6 months | Weight stable | Bimonthly group meetings + newsletters | 43 | 86 | 69.3±0.9 | 34.3±3.9 | |
| Weight loss | 1,000-kcal/d deficit, dietician or exercise physiologist weekly group meetings, exercise program 3 d/wk (60 min/session), pedometer use and log | 44 | 81.8 | 69.7±0.9 | 34.9±4.9 | |||||
| Villareal, 200629 | Function, body composition, quality of life | Sedentary, mildly to moderately frail older adults | 26 weeks | Control | No treatment | 10 | 90 | 71.1±5.1 | 39±5.0 | |
| Diet and exercise | 750-kcal/d deficit diet, daily vitamin, weekly dietician group meetings, 3x/wk 90-minute group exercise (flexibility, strength, endurance, balance), educational materials | 17 | 83 | 69.4±4.6 | 38.5±5.3 | |||||
| Frimel, 200826 | Fat-free mass, lean mass | Older adults with mild to moderate frailty | 6 months | Diet | 750-kcal/d deficit diet, weekly dietician group meeting | 15 | NR | 70.3±4.8 | 36.9±4.9 | |
| Diet and exercise | 750-kcal/d deficit diet, 3x/wk 90-min/wk (30 minutes each low-impact aerobic and high-intensity resistance, 15 minutes balance) | 15 | NR | 68.7±4.3 | 36.7±5.1 | |||||
| Davidson, 200925 | Insulin resistance, functional limitations | Abdominally obese older adults | 6 months | Control | No exercise, healthy diet from dietician | 11 male | NR | 67.4±3.8 | 30.5±2.0 | 112.8±5.4 |
| 17 female | 66.7±3.7 | 30.0±3.4 | 104.9±7.4 | |||||||
| Resistance | 20 minutes, 9 muscle groups, 3x/wk, healthy diet from dietician | 15 male | NR | 67.4±5.7 | 30.1±2.6 | 111.0±5.4 | ||||
| 21 female | 67.6±4.2 | 29.2±3.7 | 104.3±8.5 | |||||||
| Aerobic | 30-minutes moderate-intensity treadmill walking, 5x/wk, healthy diet from dietician | 17 male | NR | 68.8±6.0 | 29.9±3.0 | 113.0±7.9 | ||||
| 20 female | 69.1±6.5 | 29.2±3.0 | 104.2±10.4 | |||||||
| Combined exercise | 50 minutes, 9 muscle groups, 30 minutes moderate-intensity treadmill walking 3x/wk, healthy diet from dietician | 14 male | NR | 67.1±4.5 | 31.1±3.1 | 114.1±8.3 | ||||
| 21 female | 67.5±5.1 | 29.7±3.3 | 102.8±9.6 | |||||||
| Shah, 200924 | Intrahepatic fat content | Older adults with BMI ≥30.0 kg/m2 | 6 months | Diet | 500–1,000-kcal/d deficit (adjusted to maintain 0.4–0.9-kg loss/wk), weekly meeting with dietitian (60-minute sessions) | 9 | NR | 68.6±1.1 | NR | |
| Diet and exercise | Diet arm with 90 minutes group exercise training sessions 3x/wk | 9 | NR | 68.5±1.3 | NR | |||||
| Villareal, 20119 | Physical function | Sedentary older adults | 52 weeks | Control | Monthly staff visits, general diet material, 1,500 mg calcium, 1,000 IU vitamin D/d | 27 | 81 | 69±4 | 37.3±4.7 | |
| Diet | 500–750-kcal/d deficit, 1 g protein/kg per day, dietician weekly group meetings and weigh-in, food diary, 1,500 mg calcium, 1,000 IU vitamin D/d | 26 | 88 | 70±4 | 37.2±4.5 | |||||
| Exercise | Weight maintenance diet, 3x/wk 90 minutes group exercise (aerobic, resistance, flexibility, balance), 1,500 mg calcium, 1,000 IU vitamin D/d | 26 | 81 | 70±4 | 36.9±5.4 | |||||
| Diet and exercise | Combination of diet and exercise arms | 28 | 89 | 70±4 | 37.2±5. | |||||
NR=not reported.
Table 3.
Outcome Measures of Included Studies
| Reference | Group | Weight, kg | Weight Change, kg | P-Value | Change in Physical Function Domain | Quality of Life | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Aerobic Capacity | Muscle Strength or Pain | Physical Performance Test | Functional Status | |||||
| Mean±SD (%) | ||||||||||
| Miller, 200627 | Weight stable | 97.5±15.9 | 98.9±2.9 | 0.0±0.7 (0) | <.01 | 6MWT: 10.5±6.3 m | WOMAC: −1.7±2.2 | NR | NR | NR |
| Weight loss | 98.1±17.3 | 89.8±2.7 | −8.3±0.8 (−8.7) | 6MWT: 72.8±10.3 m | WOMAC: −11.2±2.4 | NR | NR | NR | ||
| p<.01 | p<.01 | p=NR | p=NR | p=NR | ||||||
| Villareal, 200629 | Control | 103.2±19.8 | 103.9±21.3 | 0.7±2.7 (0.5) | <.001 | VO2peak: 0.3±1.1j | Extension: 2.2±7.3 feet/pound | 0.1±1.0r | FSQ: −0.2±3.9r | 2.5±26.4s |
| Diet and exercise | 99.7±13.6 | 91.5±15.4 | −8.2±5.7 (−8.4) | VO2peak: 1.7±1.6j | Extension: 9.1±8.0 feet/pound | 2.6±2.5r | FSQ: 2.9±3.7r | 23.2±20.9s | ||
| p=.02 | p=.04 | p=.02 | p=.02 | p=.03 | ||||||
| Frimel, 200826 | Diet | 102.9±14.6 | NR | −10.7±4.5 (−10.6) | .52 | NR | NR | NR | NR | NR |
| Diet and exercise | 97.3±13.5 | NR | −9.7±4.0 (−10.0) | NR | NR | NR | NR | NR | ||
| p=NR | p=.04 | p=NR | p=NR | p=NR | ||||||
| Davidson, 200925 | Control | NR | NR | 0.28±0.37 | NR | VO2peak: −0.1±0.25a | Extensor: NR | NR | −1.01±0.12b | NR |
| Resistance | NR | NR | −0.64±0.37 | NR | VO2peak: 1.1±2.9a | Extensor: 29.1±19.5 kgc | NR | 0.17±0.12bd | NR | |
| Aerobic | NR | NR | −2.77±0.33 | <.05de | VO2peak: 3.9±3.0afg | Extensor: NR | NR | −0.01±0.10bd | NR | |
| Combined exercise | NR | NR | −2.31±0.33 | <.05de | VO2peak: 3.7±4.4afg | Extensor: 28.7±21.0 kgb | NR | 0.52±0.10bdh | NR | |
| Shah, 200924 | Diet | 106±6.0 | 97.3±5.8 | −9.2±1.6 (−8.7)t | .001 | VO2peak: −0.01±0.02a | Strength: −6±23 pounds | NR | NR | NR |
| Diet and exercise | 95.4±3.4 | 87.1±4.5 | −8.3±1.7 (−8.7)t | .001 | VO2peak: 0.21±0.01a | Strength: 105±20 pounds | NR | NR | NR | |
| p=.04 | p=.04 | |||||||||
| Villareal, 20119 | Control | 101.0±16.3 | NR | −0.1±.3.5i (−0.1)t | NR | VO2peak: −0.9±1.5ij | 1RM: −6±101 poundsi | 0.2±1.8i | FSQ: −0.2±2.4i | NR |
| Diet | 104.1±15.3 | NR | −9.7±5.4i (−9.3)t | <.001k | VO2peak: −0.7±2.3ijl | 1RM: 1±85 poundsi | 3.1±1.4il | FSQ: 1.3±1.5il | 8.4±10.1 (↑ 14%)imn | |
| Exercise | 99.2±17.4 | NR | −0.5±3.6i (−0.5)t | .71k | VO2peak: 1.4±1.0ijn | 1RM: 174±166 poundsi | 4.0±2.5in | FSQ: 1.8±2.7in | 5.7±8.0 (↑ 10%)imn | |
| Diet and exercise | 99.1±16.8 | NR | −8.6±3.8i (−8.7)t | .67, <.001o | VO2peak: 3.1±2.4ijpq | 1RM: 164±124 poundsi | 5.4±2.4ipt | FSQ: 2.7±2.6ip | 8.6±9.3 (↑ 15%)i | |
L/min.
Reported functional status was calculated using a composite score of four independent measures (number of chair stands, number of arm curls, 2-minute step test/number of steps, 8-ft up-and-go). Because the unit measures were different for each test, a composite score was calculated. (The change for each test was normalized using z scores, and then those scores were averaged.)
Significant (p<.001) strength improvements from Week 4 to 24 as determined using the paired t-test.
Significant pre- vs postintervention treatment differences compared with control group (p<.05).
Significant pre- vs postintervention treatment differences compared with resistance group (p<.05).
Pairwise group comparisons using analysis of variance (ANOVA) with Tukey studentized range tests adjusted for multiple comparisons with statistically significant differences (p<.05) compared with fcontrol group and gresistance exercise group.
Significant pre- vs postintervention treatment differences compared with aerobic exercise group (p<.05).
Reported at 1 year.
mL/kg per minute.
P-values represent comparisons between diet and control and between diet and exercise groups.
Diet vs control p<.05
Quality of life as represented by the Medical Outcomes Study 36-item Short-Form Survey Physical Component Subscale.
Exercise vs control p<.05
P-value represents comparison between diet and exercise vs diet and between diet and exercise vs exercise.
Diet and exercise vs diet p<.05.
Diet and exercise vs exercise p<.05.
Absolute change.
Results for physical function.
Baseline minus follow-up divided by baseline.
VO2peak=peak oxygen consumption; 6MWT=6-minute walk test; WOMAC=Western Ontario McMaster University Arthritic Index (range 1–100; higher scores indicate greater impairment); FSQ=Functional Status Questionnaire (range 0–36, higher scores indicating better functional status); 1-RM=one repetition maximum (sum of maximum weights lifted in the bicep curl, bench press, seated row, knee extension, knee flexion, leg press).
Assessment of Methodological Risk of Bias
Studies generally had negative or unclear risk of bias. The main methodological problems were lack of blinding of participants and healthcare providers and allocation concealment. All included studies except one24 reported eligibility criteria and prespecified measures for primary outcomes (selective outcome reporting). The overall percentage of included trials (range 0–100%) in which the author’s judgment of a summary assessment outcome was met according to the Cochrane Collaboration tool for assessing risk of bias (categories: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting or other sources of bias) is indicated in Table 1. Overall methodological quality was considered low to moderate.
Study Characteristics
All six of the parent studies included were based in North America. All were performed at single centers. All were performed in a research center—none in primary care settings. All studies ranged from 6 to 18 months (median 26 weeks) and were RCTs.
Participant Characteristics
There were 405 participants in the parent studies. The number of participants varied from 9 to 44 per intervention arm. All studies but one (n=44)27 had an overall sample size of less than 30 subjects in each intervention arm. Recruitment methods were specified in each included randomized trial, and exclusion criteria were explicitly stated in each study. Mean age ranged from 66.7 to 71.1 in each intervention arm. All participants in intervention arms had obesity (mean BMI 29.2–39.0 kg/m2). One study25 had participants with a BMI less than 30.0 kg/m2, but subjects were classified as being obese based on WC. One study24 did not present mean BMI data but included subjects with a BMI of 30 kg/m2 or greater. All recruited subjects were community living. Loss to follow-up ranged from 0% to 13%. Participant baseline characteristics varied. Subjects were sedentary in one study24, frail or functionally impaired in four studies9, 26–28, and lacked significant comorbidity in one study25.
Study Intervention
A wide range of designs and interventions were used in the included studies. Four studies had two arms, one had three arms, and one had four arms. Control groups included routine physician care, a technology device, no exercise, or usual care (no treatment). Caloric reduction ranged from 500- to 1000-kcal/d deficits. Exercise arms varied in duration of aerobic and resistance exercises. Multidisiplinary staff were used in the included studies. Participants were provided with protein, calcium, and vitamin D supplements in only two studies9, 28. The review did not demonstrate consistency in the interventions provided to participants.
Effect on Outcome Measures: Weight Loss, Physical Function, Quality of Life
Weight loss was measured in each included study and ranged from 0.5 to 10.7 kg (0.1% to 9.3%). Markedly greater weight loss was observed in groups with a dietary component than in those with exercise alone. Five studies used structured resistance programs to preserve lean mass. Dietary interventions were consistently associated with weight loss and improvement in function, whereas exercise-alone interventions led to better function but no significant weight loss. Body composition was measured in five studies using dual-energy X-ray absorptiometry and one study using magnetic resonance imaging. Only one study27 reported participants with clinically significant weight loss of more than 5% (84% of subjects).
Physical function was measured using physical performance testing, the 6MWT, the Western Ontario McMaster Arthritis Index, and the Functional Status Questionnaire. A combined dietary and exercise intervention led to weight loss and less loss of muscle mass, with concomitant improvement in physical function. All studies other than two26,27 assessed VO2peak. One study26 did not report physical function outcomes. Two studies9, 29 used the Medical Outcomes Study 36-item Short-Form Survey to assess self-reported health or quality of life. In both studies, the combined diet and exercise groups had marked improvement in their self-reported health scores.
Other Findings
Exercise alone led to greater fat-free mass, and diet alone led to lower fat mass and greater loss of fat-free mass. A combination of diet and exercise resulted in a relative preservation of fat-free mass. Diet alone led to reductions in bone mineral density, which exercise partially mitigated. Diet and exercise led to greater improvements than control in glucose homeostasis, bone mineral density, cognition, and inflammatory markers. Adverse events were minimal (a fall, dizziness, musculoskeletal complaints) and were reported in only three studies.
DISCUSSION
This review provides an evaluation of the literature on geriatric obesity interventions since 2005 using a two-tiered screening approach. Despite the importance of this public health concern, the number of published randomized trials is limited, highlighting a critical need to develop interventions to assess outcomes in this high-risk population. The geriatric obesity interventions assessed generally led to weight loss and improved quality of life and physical function, as measured using VO2peak and muscle strength.
This review was deliberately focused on quality of life and physical function in addition to weight loss as important health indicators in older adults30–32. The interventions generally emphasized weight loss as a common approach to obesity management. Only one study reported the proportion of subjects with clinically significant weight loss (≥5% of body weight)8, used as a surrogate for success in adult guidelines. Whether this threshold should be considered in older adults is unclear. Objective and subjective improvements in these domains were observed in the majority of the studies. The data demonstrate the general trends in achieving these outcomes. The current findings provide additional methodological data suggesting the importance of focusing on variables beyond weight loss in this population. Outcomes in older adults, such as functional status and self-reported health, may be useful to enhance geriatric obesity strategies and should be incorporated into daily practice.
The effect on physical function independent of weight loss should not be understated. Evidence of this phenomenon was observed particularly in subjects engaged in combined diet and exercise or exercise-only (aerobic or resistance) interventions. Although weight loss leads to improvements in function, the results suggest that functional improvements can be achieved with exercise alone. Even in the study consisting of less than 5% weight loss25, improvements in function were observed, yet this was predominantly based on a healthy diet and an exercise program. Improvement of function promotes healthy aging and prevents ensuing disability, all of which can lead to better quality of life. Clinicians should be reluctant to consider weight loss with dietary measures alone if the desired outcome is improvement in physical performance, although combining weight loss and exercise results in maximum improvement in physical function and could mitigate the concern of potential sarcopenia and bone loss in older adults. In the studies with a diet-only or control arm that did not have any resistance exercise program, findings highlight the emergence of sarcopenia and bone loss, an important yet overlooked phenomenon of geriatric obesity interventions. Dietary weight loss leads to loss of fat mass and fat-free mass. These trials demonstrate the importance of unopposed weight loss in this population. Sarcopenia progresses with age, and older adults have lower compensatory capacity to offset the loss of muscle mass and strength that may hasten functional impairment and incident disability11, 33, 34. Clinicians should evaluate each person individually and focus on wellness and prevention of sarcopenia and bone loss when recommending weight-loss therapy. The benefits of intentional weight loss observed might not apply to those whose weight loss is unintentional and should be monitored in the course of practice.
This review highlights critical concerns in examining and addressing obesity in older adults. First, high-quality RCTs are needed. Second, longer follow-up and effectiveness trials will clarify sustainability and outcomes of these interventions, which are related to geriatric life expectancy. Shared decision-making should be integrated into patient encounters. Third, pragmatic approaches are critically needed within a primary care infrastructure to manage this disorder. None of the studies tested interventions in primary care, arguably the most common setting for individuals to receive chronic disease management, although each study intervention was labor intensive, and participants engaged in behavioral change through nutritional and physical activity approaches. Hence, their implementation within a primary care or specialty setting may be challenging and face obstacles. Fourth, consensus is needed to standardize the structure of geriatric obesity interventions. Combined diet and exercise strategies, consisting of caloric reduction of at least 500 kcal/d, with appropriate protein and dietary supplementation and resistance exercise, may prevent sarcopenia and bone loss, which are associated with worse function9.
Pharmacological and surgical therapy were deliberately not assessed. Newer medications should be used with caution in older adults because they have considerable neuropsychiatric side effects, including memory impairment. These side effects may exacerbate underlying and compensated cognitive function in an age group already at risk of this condition. Bariatric surgery is an approved therapy for obesity, but the literature remains unclear as to its general benefits in adults aged 65 and older35, although emerging long-term mortality benefits have been reported36. Careful selection of older eligible adults undergoing an evaluation has been recommended37. The definition of obesity in older adults is debated extensively38. Measures that could be performed practically and economically in a clinical care setting such as BMI and WC were intentionally chosen. The specificity and sensitivity of these measures differ from those of body composition measures assessed using computed tomography, dual-energy X-ray absorptiometry, or magnetic resonance imaging, which cannot be reasonably performed on a population level. A BMI cutoff of 30.0 kg/m2 is a well-established cutoff in defining obesity and is used to identify older adults eligible for the Medicare Intensive Behavioral Therapy benefit13. It is also associated with greater risk of death3, 15. A number of subjects classified as overweight were eliminated from this review who would not only be eligible for treatment if they were younger8, but otherwise might have adiposity based on other assessment measures38. Using WC may be reasonable and helpful in recognizing persons with normal central obesity who may have different underlying treatment and weight-loss strategies and provides a rationale for including such subjects in further study. There is also strong epidemiological evidence suggesting that a BMI of 25.0 to 29.9 kg/m2 is associated with low mortality and functional impairment in older adults who otherwise would not be at high risk of death after weight-loss therapy39, 40. BMI also incorporates fat and muscle mass, and relying solely on this measure ignores sarcopenia, sarcopenic obesity, and normal-weight obesity33, 41.
Interventions focusing on obesity in the general population are often short in duration, and the current results suggest that this is not an exception in older adults. Most weight-loss studies, such as the Diabetes Prevention Program42 or Action for Health in Diabetes43, have demonstrated initial weight loss within the first few months; the physiology and management actually differ in the weight maintenance phase. With the exception of one study identified in this review,9 all were of short duration. The shorter study duration raises considerable interest because it is likely that short-term outcomes improved, but whether they were sustained is unclear. Studies of 6 months or longer that concentrated specifically on sustained efficacy treatment trials, in accordance with the recommended weight loss guidelines,8 were deliberately focused on. Future studies need to examine long-term follow-up in this population.
The strengths of this review include the use of the PRISMA criteria, which reduces bias and error and improves the reproducibility and transparency of the process. The review emphasizes the importance of empirical evidence over preconceived knowledge by identifying knowledge gaps and highlighting methodological inconsistencies and weaknesses. A validated and systematic approach using validated PRISMA criteria with the assistance of an interdisciplinary team that includes experienced librarians increases the validity of the process. Screening was piloted to ensure consistency. The results were useful in identifying future research priorities. Availability of data and quality of the original reports inherently limit literature reviews. Incomplete reporting and negative trials are likely to be subject to reporting bias and may not be published. A priori, the authors were aware of the clinical heterogeneity observed in the known randomized trials and systematic reviews. The current results confirmed considerable methodological heterogeneity as well, so it was decided not to perform a metaanalysis. Although the data were diverse, in addition to weight loss, outcomes that were person-specific and meaningful in an aging population were focused on. Observational studies were deliberately not included to preserve validity. Considerable information can be concluded from well-conducted observational studies, although selection bias is unavoidable in this type of design. Therefore, results cannot be used to definitively support conclusions about obesity interventions based on the outcomes observed in this review. Individuals whose group mean age was 65 and older were included, and studies with subjects younger than 60, which have been included in previous systematic reviews, were omitted2, 17–20, 23. Middle-aged individuals have different physiology and homeostasis and should be considered differently. Lastly, publication bias may affect the number of studies included in this study. Including studies with participants aged 60 to 64 also may be perceived as a limitation of this analysis.
CONCLUSION
Although there were a limited number of high-quality studies to support geriatric obesity interventions, current RCTs suggest that a reduction in weight can lead to improvements in physical function and quality of life. Body composition changes such as loss of fat mass and preservation of fat-free mass are favorable, particularly when resistance exercise programs are integrated into a program of caloric restriction. Well-designed RCTs are needed in this high-risk population to provide definitive guidance in a clinical care setting.
Supplementary Material
Acknowledgments
We would like to thank Patricia Erwin at Mayo Clinic Rochester for her assistance in the literature review.
Financial Disclosure
Dr. Batsis receives funding from the Health Resources Services Administration (U1QHP28718) for medical geriatric teaching; the Junior Faculty Career Development Award, Department of Medicine, Dartmouth-Hitchcock Medical Center; and the Dartmouth Centers for Health and Aging. Dr. Bartels receives the following funding: National Institute of Mental Health (NIMH) K12 HS021695; National Institutes of Health, National Center for Advancing Translational Sciences 1KL2TR001088; Centers for Disease Control and Prevention U4DP005018; Health Resources and Services Administration U1QHP28718; NIMH: R01 MH102325, R01 MH104555, T32 MH073553. Lydia Gill is a premedical student funded by The Dartmouth Centers for Health and Aging. Rebecca Masutani is a third-year medical student funded the Medical Student Research Fellowship at the Geisel School of Medicine. Support was provided by the Dartmouth Health Promotion and Disease Prevention Research Center, supported by Cooperative Agreement U48DP005018 from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Contributions
Batsis: conception and design, acquisition of data, analysis, interpretation of data; drafting the article, critical revision for important intellectual content; final approval of the version to be published. Gill: conception and design, acquisition of data, analysis, interpretation of data; critical revision for important intellectual content; final approval of the version to be published. Masutani: acquisition of data, analysis, interpretation of data; critical revision for important intellectual content; final approval of the version to be published. Adachi-Mejia: conception and design, acquisition of data, interpretation of data; critical revision for important intellectual content; final approval of the version to be published. Blunt, Bagley: conception and design, acquisition of data, critical revision for important intellectual content; final approval of the published version. Lopez-Jimenez, Bartels: conception and design, interpretation of data; critical revision for important intellectual content; final approval of the version to be published.
Sponsor’s Role
The funders played no role in the study design, collection, analysis, interpretation of data, writing of the report, or in the decision to submit the paper for publication. They accept no responsibility for the contents.
Footnotes
Presented at the American Geriatrics Society Annual Meeting, Long Beach, California, May 19 to 21, 2016.
Conflict of Interest: None.
References
- 1.Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern Med. 2015;175:1412. doi: 10.1001/jamainternmed.2015.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McTigue KM, Hess R, Ziouras J. Obesity in older adults: A systematic review of the evidence for diagnosis and treatment. Obesity (Silver Spring) 2006;14:1485–1497. doi: 10.1038/oby.2006.171. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am J Clin Nutr. 2009;89:1213–1219. doi: 10.3945/ajcn.2008.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowd JB, Zajacova A. Long-term obesity and physical functioning in older Americans. Int J Obes (Lond) 2015;39:502–507. doi: 10.1038/ijo.2014.150. [DOI] [PubMed] [Google Scholar]
- 5.Batsis J, Zbehlik A, Barre L, et al. The impact of waist circumference on function and physical activity in older adults: Longitudinal observational data from the osteoarthritis initiative. 2014;13:81. doi: 10.1186/1475-2891-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 7.Villareal DT, Apovian CM, Kushner RF, et al. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–S238. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batsis JA, Bynum J. Uptake of the Centers for Medicare and Medicaid Obesity Benefit: 2012–2013. Obesity. 2016 doi: 10.1002/oby.21578. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batsis JA, Huyck KL, Bartels SJ. Challenges with the Medicare obesity benefit: Practical concerns and proposed solutions. J Gen Intern Med. 2015;30:118–122. doi: 10.1007/s11606-014-3031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie CS, Locher JL, Roth DL, et al. Unintentional weight loss predicts decline in activities of daily living function and life-space mobility over 4 years among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63A:67–75. doi: 10.1093/gerona/63.1.67. [DOI] [PubMed] [Google Scholar]
- 15.Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: A meta-analysis of randomized clinical trials. PLoS One. 2015;10:e0121993. doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: From global epidemiology to individualized medicine. Clin Pharmacol Ther. 2007;82:509–524. doi: 10.1038/sj.clpt.6100355. [DOI] [PubMed] [Google Scholar]
- 17.Bales CW, Buhr G. Is obesity bad for older persons? A systematic review of the pros and cons of weight reduction in later life. J Am Med Dir Assoc. 2008;9:302–312. doi: 10.1016/j.jamda.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Felix HC, West DS. Effectiveness of weight loss interventions for obese older adults. Am J Health Promot. 2013;27:191–199. doi: 10.4278/ajhp.110617-LIT-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poggiogalle E, Migliaccio S, Lenzi A, et al. Treatment of body composition changes in obese and overweight older adults: Insight into the phenotype of sarcopenic obesity. Endocrine. 2014;47:699–716. doi: 10.1007/s12020-014-0315-x. [DOI] [PubMed] [Google Scholar]
- 20.Porter Starr KN, McDonald SR, Bales CW. Obesity and physical frailty in older adults: A scoping review of lifestyle intervention trials. J Am Med Dir Assoc. 2014;15:240–250. doi: 10.1016/j.jamda.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amaral S, Liberali R, Navarro F, et al. The influence of resistance training variables in reducing body weight in obese and overweight: A systematic review. Revista Brasileira De Obesidade Nutricao E Emagrecimento. 2015;9:41–48. [Google Scholar]
- 22.Maderuelo-Fernandez JA, Recio-Rodriguez JI, Patino-Alonso MC, et al. Effectiveness of interventions applicable to primary health care settings to promote Mediterranean diet or healthy eating adherence in adults: A systematic review. Prev Med. 2015;76(Suppl):S39–S55. doi: 10.1016/j.ypmed.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr Rev. 2010;68:375–388. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 24.Shah K, Stufflebam A, Hilton TN, et al. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–2168. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: A randomized controlled trial. Arch Intern Med. 2009;169:122–131. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 26.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GD, Nicklas BJ, Davis C, et al. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14:1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 28.Villareal DT, Banks M, Sinacore DR, et al. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med. 2006;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 29.Villareal DT, Miller BV, III, Banks M, et al. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84:1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 30.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rejeski WJ, King AC, Katula JA, et al. Physical activity in prefrail older adults: Confidence and satisfaction related to physical function. J Gerontol B Psychol Sci Soc Sci. 2008;63B:P19–P26. doi: 10.1093/geronb/63.1.p19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rejeski WJ, Mihalko SL. Physical activity and quality of life in older adults. J Gerontol A Biol Sci Med Sci. 2001;56A(Spec No 2):23–35. doi: 10.1093/gerona/56.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- 33.Batsis JA, Barre LK, Mackenzie TA, et al. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: Dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61:974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 34.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69A:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flum DR, Salem L, Elrod JA, et al. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. JAMA. 2005;294:1903–1908. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 36.Giordano S, Victorzon M. Bariatric surgery in elderly patients: A systematic review. Clin Interv Aging. 2015;10:1627–1635. doi: 10.2147/CIA.S70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batsis JA, Dolkart KM. Evaluation of older adults with obesity for bariatric surgery: Geriatricians’ perspective. J Clin Gerontol Geriatr. 2015;6:45–53. [Google Scholar]
- 38.Batsis JA, Mackenzie TA, Bartels SJ, et al. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond) 2016;40:761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuk JL, Ardern CI. Influence of age on the association between various measures of obesity and all-cause mortality. J Am Geriatr Soc. 2009;57:2077–2084. doi: 10.1111/j.1532-5415.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 40.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi: 10.1093/epirev/mxs006. [DOI] [PubMed] [Google Scholar]
- 41.Batsis JA, Zbehlik AJ, Scherer EA, et al. Normal weight with central obesity, physical activity, and functional decline: Data from the Osteoarthritis Initiative. J Am Geriatr Soc. 2015;63:1552–1560. doi: 10.1111/jgs.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: The Look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villareal DT, Shah K, Banks MR, et al. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: A one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181–2187. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villareal DT, Banks MR, Patterson BW, et al. Weight loss therapy improves pancreatic endocrine function in obese older adults. Obesity (Silver Spring) 2008;16:1349–1354. doi: 10.1038/oby.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Miller GD, Messier SP, et al. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62A:866–871. doi: 10.1093/gerona/62.8.866. [DOI] [PubMed] [Google Scholar]
- 47.Miller GD, Nicklas BJ, Loeser RF. Inflammatory biomarkers and physical function in older, obese adults with knee pain and self-reported osteoarthritis after intensive weight-loss therapy. J Am Geriatr Soc. 2008;56:644–651. doi: 10.1111/j.1532-5415.2007.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller GD. Improved nutrient intake in older obese adults undergoing a structured diet and exercise intentional weight loss program. J Nutr Health Aging. 2010;14:461–466. doi: 10.1007/s12603-010-0100-3. [DOI] [PubMed] [Google Scholar]
- 49.Miller GD, Jenks MZ, Vendela M, et al. Influence of weight loss, body composition, and lifestyle behaviors on plasma adipokines: A randomized weight loss trial in older men and women with symptomatic knee osteoarthritis. J Obes. 2012;2012:708505. doi: 10.1155/2012/708505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armamento-Villareal R, Sadler C, Napoli N, et al. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27:1215–1221. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Napoli N, Shah K, Waters DL, et al. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am J Clin Nutr. 2014;100:189–198. doi: 10.3945/ajcn.113.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Armamento-Villareal R, Aguirre L, Napoli N, et al. Changes in thigh muscle volume predict bone mineral density response to lifestyle therapy in frail, obese older adults. Osteoporos Int. 2014;25:551–558. doi: 10.1007/s00198-013-2450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: Results of a randomized controlled trial. Int J Obes (Lond) 2014;38:423–431. doi: 10.1038/ijo.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armamento-Villareal R, Aguirre LE, Qualls C, et al. Effect of lifestyle intervention on the hormonal profile of frail, obese older men. J Nutr Health Aging. 2016;20:334–340. doi: 10.1007/s12603-016-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.