Abstract
Background:
There is no much information about how tuberculous lesions of the spine progress/heal; what clinical and radiological features suggest progression/healing; what is the optimal duration of antitubercular treatment; and what clinical, laboratory, and radiological investigations and their frequency should be done to monitor the disease course.
Aims:
The present study aimed to evaluate what specific clinicoradiologic features suggest involvement and healing in tuberculosis of the spine.
Subjects and Methods:
Fifty spinal tuberculosis patients (30 males and 20 females) diagnosed clinicoradiologically were enrolled in the study. Patients were evaluated clinically, radiographically, and by magnetic resonance imaging (MRI) at regular intervals to monitor the disease course till 24 months of the initial presentation.
Results:
Wedge/collapse (23/50 cases), soft tissue mass (29/50 cases), disc narrowing (45/54 discs), and endplate erosions (89/107 endplates) were the plain radiological findings of tubercular spinal involvement. Earliest sign of healing on plain radiography was decrease in fuzziness of endplate, ultimately leading to either sclerosis of endplate or fusion of adjacent vertebrae. Initial MRI findings included bone marrow edema (50/50 cases), discitis (53/62 discs), endplate erosions (105/123 endplates), pre- and para-vertebral collections (45/50 cases), epidural involvement (26/50 cases), epidural spread (77/109 vertebrae), and subligamentous spread (42/50 cases). Earliest feature of healing on magnetic resonance (MR) examination was decrease in inflammatory soft-tissue masses and reduction in marrow edema.
Conclusions:
Salient features of spinal involvement in tuberculosis on plain radiograph were paradiscal involvement, endplate destruction, and soft tissue masses. Marrow edema, paravertebral collections, subligamentous spread, extradural component, endplate erosion, and discitis suggested tubercular involvement of the spine on MRI. A decrease in these was observed to have prognostic value both in monitoring disease course and response to chemotherapy. Based on the clinicoradiologic findings of the present study, we propose decision-making algorithm, follow-up algorithm, and MR examination protocol for spinal tuberculosis.
Level of evidence:
This was a Level II study.
Keywords: Clinicoradiologic features, Magnetic resonance imaging, Spine, Tuberculosis, X-rays
Introduction
The morphologic and pathophysiologic changes encountered in spinal tuberculosis are most severe and most varied among infective spondylitis.[1,2] Early diagnosis and prompt treatment are essential to prevent permanent neurological deficit and/or spinal deformity. However, it can only be made if one detects early clinical and radiological features suggestive of spinal tuberculosis.[1,2] Imaging plays a major role in the overall evaluation of these lesions. An ideal modality of investigation is expected to provide information that will help identify the nature of disease, show the location and extent of involvement, suggest the type of infection, guide biopsy and/or drainage procedure, indicate method of therapy, and help assess response to therapy.[3]
Radiographs are the first line of investigations to substantiate or refute a clinical diagnosis of tuberculosis of the spine. Early lesions are usually missed on plain radiography because at least 30%–40% mineral density should be lost before changes appear on radiographs.[4] Computed tomography (CT) scanning can determine posterior extension and encroachment of inflammatory tissue, bone, or disc material and diagnose posterior spinal disease and involvement of sacroiliac joints and sacrum. It helps in guiding biopsies and planning operative intervention.[1,2,5,6] Magnetic resonance imaging (MRI) is considered an ideal modality for making the diagnosis, demonstrating the extent of disease, identifying complications, and assessing response to treatment.[7,8] It has an excellent contrast resolution and multiplanar capabilities with equally good definition of bone and soft tissues.[3,9] A major advantage of MRI, compared with CT scan and plain radiography, is the higher sensitivity for detection of early inflammatory bone marrow changes in the vertebra. MRI is mostly useful in delineating paravertebral, epidural, and intraosseous abscesses and in evaluating the extent of cord compression and presence of intramedullary lesions.[10]
Eradication of infection is the primary aim of treatment in spinal tuberculosis. In addition, correction or prevention of increase in angular deformity together with recovery from any neurological deficit is the further aim.[1,2,9] The present study aimed to evaluate what specific clinicoradiologic features suggest involvement and healing in tuberculosis of the spine. It was hypothesized that it would help in better understanding of characteristic clinicoradiologic features of involvement and healing and defining imaging and treatment protocols in tuberculosis of spine.
Subjects and Methods
This prospective study was carried in fifty patients aging more than 15 years and suffering from tuberculosis of the spine. The study started in November 2007 and completed in November 2012. After taking informed consent and ethical clearance, a total of 68 consecutive patients were enrolled during this period, but only fifty patients completed full 2 years of regular follow-up with all relevant investigations as per the study protocol. The diagnosis was confirmed by clinicoradiologic evaluation and observing response to antitubercular treatment. Patients with severely immunocompromising disorders likely to affect healing and involvement pattern, for example, HIV-positive patients, and patients with tumorous conditions were excluded from the study.
Clinical evaluation
Detailed informative history of the patient was taken. Thorough clinical examination, both physical and neurological, was performed. Neurological classification was done according to Tuli's classification of stages of paraplegia.[11] Baseline hematological investigations such as complete blood count, erythrocyte sedimentation rate (ESR), liver function tests, renal function tests, serum proteins, albumin-globulin ratio, human immunodeficiency virus, and hepatitis-B antigen were carried out.
Radiographic evaluation
Plain X-rays of the chest, affected vertebral column, and any other affected part of the body were carried out. Ultrasonography of the cold abscess (paraspinal, psoas) was done to know about initial size of the abscesses.
Magnetic resonance imaging evaluation
MRI to localize the site and amount of destruction, vertebral body part affected, skip lesion, cord condition, epidural abscess, and pre- and para-vertebral abscesses was done. Sagittal, axial, and coronal T1-weighted images and T2-weighted fast spin-echo images were taken on different MRI systems with magnetic field strengths ranging from 1 to 1.5 Tesla.
Miscellaneous investigations
Other investigations such as fine needle aspiration cytology (if diagnosis was doubtful), and in case of therapeutic drainage of pus, it was sent for Ziehl–Neelsen staining and culture sensitivity. In eleven patients who were operated for neurological deteriorations, removed tissue was sent for histopathological examination and pus and granulation tissue were sent for Ziehl–Neelsen staining and culture sensitivity. Three sputum samples were tested for acid-fast bacilli. Tuberculin skin test (Mantoux test) was done.
After initial clinical examination and requisite investigations, protocol of treatment was decided. Patients requiring conservative treatment were treated with antitubercular treatment for 12 months. Braces (thoraco-lumbo-sacral orthosis [Taylor brace for lesion from fourth dorsal to second lumbar vertebrae; lumbosacral orthosis (Goldthwait brace for second lumbar to lumbosacral region)]) were applied to prevent deformity of the spine. Requisite surgical procedures along with antitubercular treatment were followed in patients requiring surgery both for neurological involvements and drainage of pus. Follow-up was done monthly for the first 3 months, three monthly for 1 year, and six monthly for 2 years. At any time during follow-up, if patient needed alteration in antitubercular treatment or surgical intervention, it was done. Patients showing healing of tuberculous lesions on evaluation both clinical and investigations (hematologic and radiologic) after 6 months of antitubercular treatment were the candidates for stopping further therapy. Patients not showing healing even after 12 months of treatment were considered for appropriate extended antitubercular treatment. These cases were discussed with the chest and tuberculosis specialists from the institute. Usually, rifampicin and isoniazid were given for the extended period. Complete physical and neurological examinations were done at each follow-up. Complete blood count and ESR were done to observe the healing response. Plain X-ray of the part was done at each follow-up. MRI scans were done at 6, 12, and 24 months.
Statistical analysis
All the radiological measurements were performed by the first author three times at different occasions, and mean of all the three measurements was taken for the research purpose. At the end of the study, the data were collected and analyzed using Student's t-test, Chi-square test, and repeated measures ANOVA.
Institutional Review Board permission
Institutional Review Board clearance was taken for the study and it was also ethically cleared.
Results
Clinical evaluation
The mean age of the fifty subjects was 42.76 (15.74) years (ranged: 17–70 years). There were 30/50 (60%) males and 20/50 (40%) females. Patients were suffering from the symptomatology (pain in the back, constitutional symptoms, deformity, and neurological involvement either alone or in combination) of tuberculosis with a mean duration of 8.3 (8.89) months (range: 1–36 months). Table 1 shows the symptomatology of the patients. Backache was the most common symptom in this series of patients. Majority of the patients were seen and treated by the health practitioners with nonsteroidal anti-inflammatory drugs with a presumptive diagnosis of other mechanical problems of the back. The cervical spine (n = 2), dorsal spine (n = 12), thoracolumbar junction (n = 7), lumbar spine (n = 20), and lumbosacral junctions were affected in nine patients. Erythrocytic sedimentation rate was raised in all the patients at the time of presentation, with a mean value of 53.54 (19.9) mm first hour rate (FHR). Mean ESR value normalized around 20 mm FHR at the 12th-month follow-up. Graph 1 shows trends of the ESR during 24 months of follow-up.
Table 1.
Signs and symptoms of patients

Graph 1.
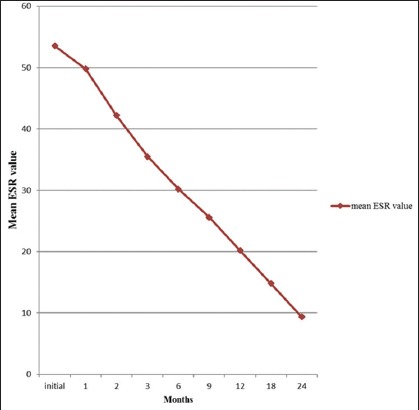
Mean erythrocyte sedimentation rate values and its declining trends during disease course
Radiographic evaluation
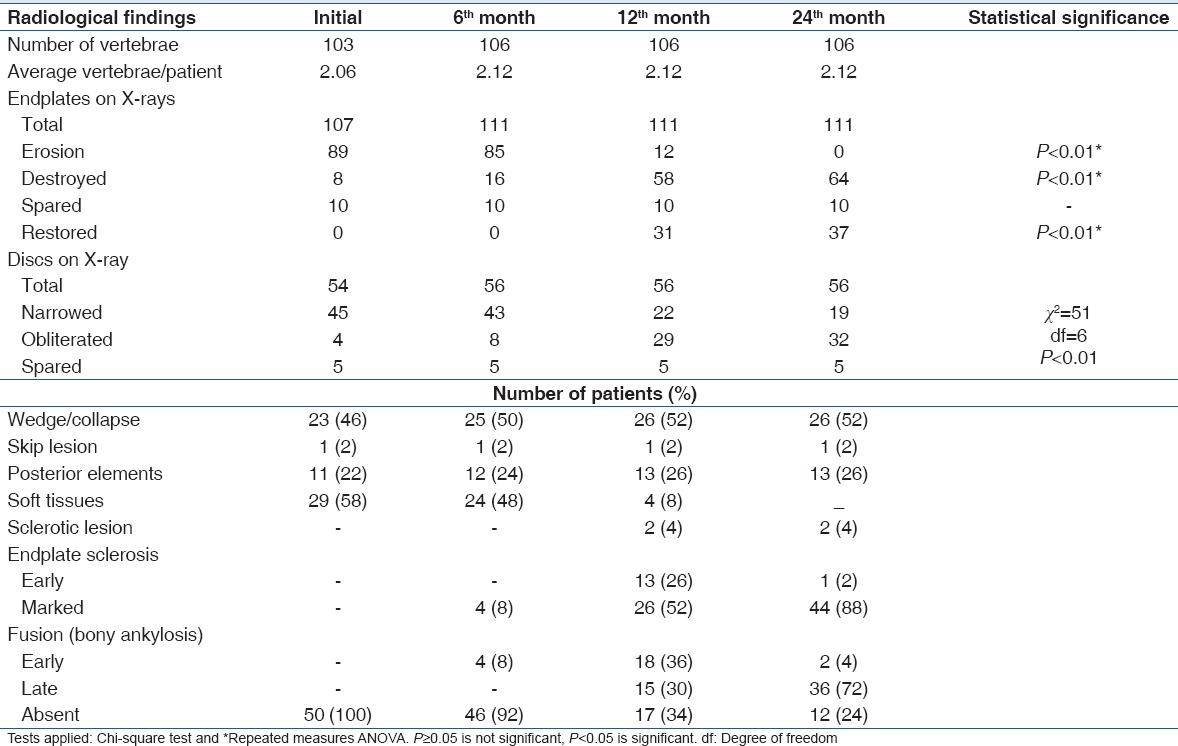
Chest radiographs revealed associated pulmonary tuberculosis in 16 (32%) patients. Plain radiography of the spine showed 51 lesions in fifty patients. There was paradiscal type of lesion in 47 patients; one patient each showed anterior, central, and posterior element involvement. Other radiological findings are shown in Table 2.
Table 2.
Other radiological findings
Magnetic resonance imaging evaluation
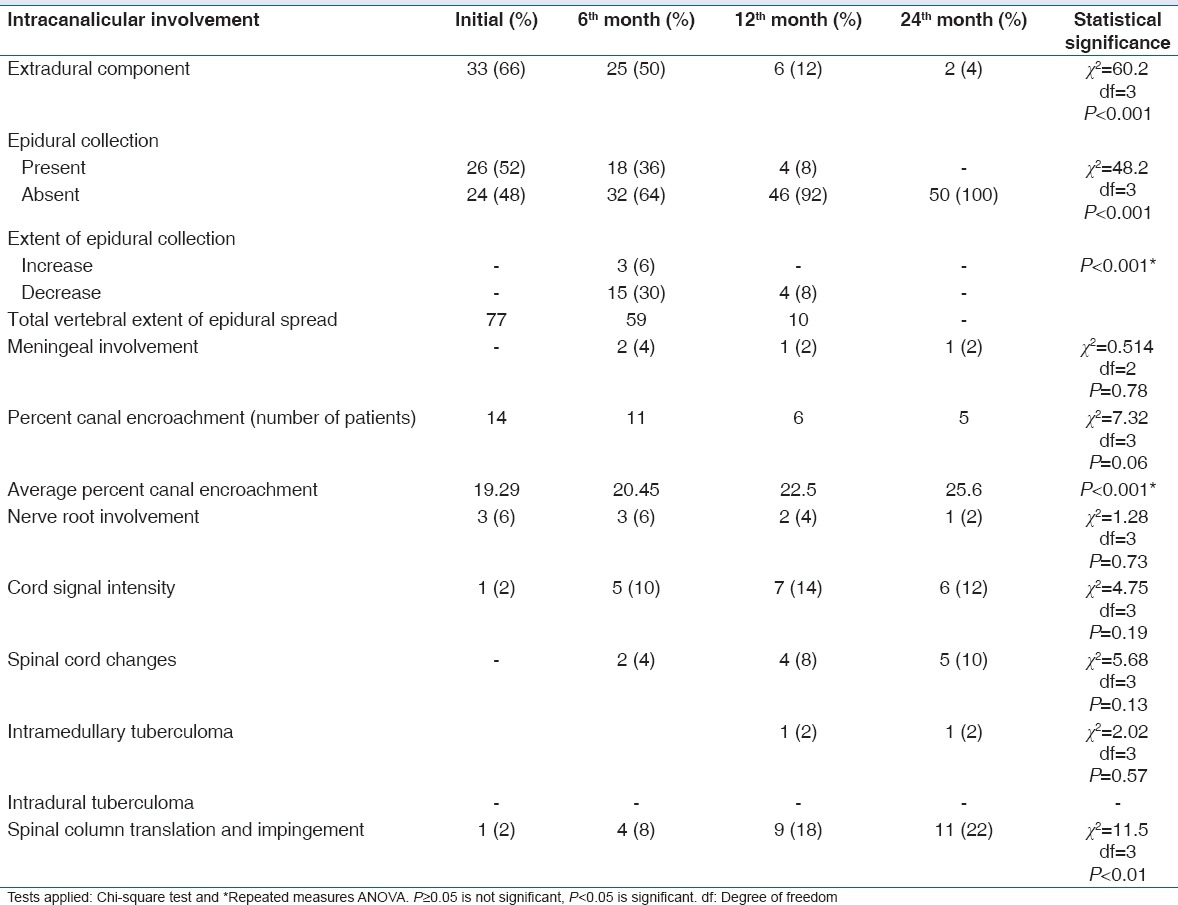
Table 3 shows signal intensity changes, involvement of number of vertebrae, endplates, discs, and bone marrow edema at various stages of follow-up. On MRI, 47 (94%) patients showed paradiscal type of lesion. Anterior, central, and posterior types of involvement were present in 1 (2%), 1 (2%), and 1 (2%) patients, respectively. Table 4 shows extent of pre- and para-vertebral collection at various stages of the disease. Psoas abscess was seen in 9 (18%) patients initially. Intracanalicular involvements are shown in Table 5.
Table 3.
Magnetic resonance imaging findings

Table 4.
Pre- and para-vertebral collections on magnetic resonance imaging

Table 5.
Intracanalicular involvement observed on magnetic resonance imaging
Various combinations of the initial MRI features observed were as follows: Ninety-two percent of cases had a combination of marrow edema and paravertebral collections. Eighty-eight percent of cases had a combination of marrow edema and subligamentous spread of disease. Eighty-two percent of cases had a combination of marrow edema, paravertebral collections, and endplate erosion. Eighty percent cases had a combination of marrow edema, paravertebral collections, endplate erosion, and subligamentous spread. Fifty-two percent of cases had a combination of subligamentous spread and epidural abscess. Sixty-six percent of cases had a combination of subligamentous spread and extradural component (caseous/granulation tissue) suggestive of spread anterior and posterior to vertebral body. Sixty-four percent of cases had a combination of marrow edema, paravertebral collections, subligamentous spread, extradural component, endplate erosion, and discitis.
Mean duration of antitubercular treatment given to the patients was 13.12 (1.8) months (range: 12–18 months). All the patients were declared healed clinicoradiologically although some residual MRI abnormalities were observed even at 24-month follow-up in few patients.
Discussion
The present study was undertaken to document various clinicoradiologic features at presentation, during healing phase, and 24 months after initial presentation and to define treatment and investigation protocols in spinal tuberculosis. Relevant literature was also reviewed.[3,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]
Clinical evaluation
The opportunity of early diagnosis was missed in majority of the patients as the mean duration of symptoms in the present study was 8.3 (8.89) months (range: 1–36 months). Backache was the most common presenting symptom in our study. Deformity kept on increasing as the disease progressed and 32 patients developed deformity by the 12th month and it persisted until the end of the study. Neurological deficit improved in 9 (18%) patients but persisted in 2 (4%) patients. The clinical features of tuberculosis spine in the present series were fairly similar to that described in the previous studies.[16,17,18] The frequency of neurological involvement has been found to vary across studies from 23% to 76% of patients.[19,20]
An increased ESR is not thought to be significant in diagnosis of tuberculosis but is considered to be a useful measure for assessing the response to chemotherapy.[19] The data reported in the literature suggest that laboratory inflammatory syndrome should disappear after three months of treatment and patient should recover their previous clinical status after 6 months of treatment. In the present study, ESR was found to be raised in all the patients in the initial visit, with a mean value of 53.54. Sustained increase in mean ESR value (>20 mm/h) was observed till 12th-month follow-up.
Radiographic evaluation
Spinal tuberculosis most commonly involves the thoracic spine and the lumbar spine; involvement of the cervical region and sacrum is less common.[16,17,21,22,23,24] In our study also, these two regions were predominantly involved. Most common type of involvement seen in tuberculous spondylitis is paradiscal type.[7] In the present study, 94% of patients had this type of involvement [Figures 1, 2a, and 3a]. Posterior type of involvement was seen only in 2% of the patients. These findings are comparable to those reported in the literature.[7,13]
Figure 1 (A).

Radiological and magnetic resonance imaging pictures of a 27-year-old male with tubercular spondylitis D10–12 with neurological deficit. Patient treated with anterolateral decompression and antituberculous treatment and he had partial recovery. (a-d): Plain X-rays at presentation, 6 months, 12 months, and 24 months. (a) Initial X-rays both anteroposterior and lateral show paradiscal lesion D10–D12 with endplate destruction, vertebral body destruction and soft tissue shadows. Lesions showed healing with reduction in soft tissue shadows (b) with endplate sclerosis and fusion (c and d). (e-h) Short tau inversion recovery images of magnetic resonance imaging. (e) Initial short tau inversion recovery images show hyperintense signal intensity which decreased with time (f-h)
Figure 2 (A).
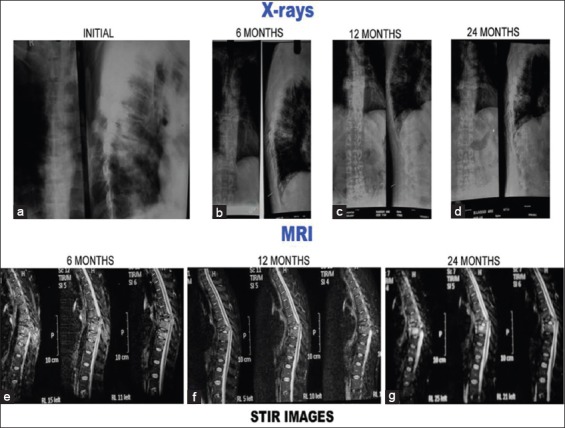
Plain X-rays and magnetic resonance imaging pictures of a 40-year-old male with tubercular spondylitis D7–8 with neurological deficit. Patient treated with anterolateral decompression and antituberculous treatment. Patient had full neurological recovery. (a-d): Plain X-rays at presentation, 6 months, 12 months, and 24 months. (a) Initial X-rays both anteroposterior and lateral show paradiscal lesion D7–D8 with endplate destruction, vertebral body destruction and soft tissue shadows. Lesions healed with endplate sclerosis, fusion, and kyphosis (b-d). (b, e-h) Short tau inversion recovery images of magnetic resonance imaging. (e) Initial short tau inversion recovery images show hyperintense signal intensity which decreased with time (f-g)
Figure 3 (A).
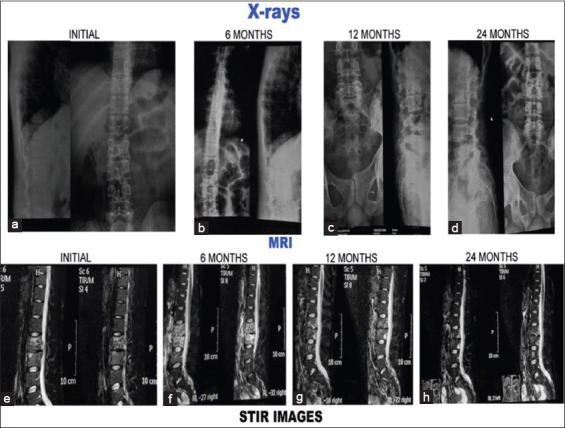
Plain X-rays and magnetic resonance imaging pictures of 20-year-old male with tubercular spondylitis L2–L3. Patient treated conservatively. Patient had associated polycystic kidney disease.(a-d): Plain X-rays at presentation, 6 months, 12 months, and 24 months. (a) Initial X-rays both anteroposterior and lateral show paradiscal lesion L2–L3 with endplate destruction, vertebral body destruction and narrow disc space. Endplate sclerosis occurred and lesion healed with narrow disc space (b-d). (e-h) short tau inversion recovery images of magnetic resonance imaging. (e) Initial short tau inversion recovery images show hyperintense signal intensity which subsequently decreased (f-g) to become isointense at 24 months (h)

Radiographic changes associated with tuberculosis spine include rarefaction of the vertebral endplates, disc space narrowing, anterior wedging, and bone destruction, but these findings may not be visible on plain radiograph up to 8 weeks.[14] Various studies report that plain radiograph demonstrated changes consistent with spinal tuberculosis in 84%–99% cases.[16,22] In the present study, 88% of the patients showed radiographic changes consistent with spinal tuberculosis. In the present study, at the initial presentation, 2.06 average numbers of vertebrae were affected and these increased to 2.12 at subsequent follow-up; these findings are consistent with other studies.[14,15,25] Wedging was observed in 46% of the patients on X-rays. Reported incidence of wedging by various authors ranges from 15.5% to 69.3%.[13,14,15] Forty-four cases (88%, 97/107 endplates) on the X-rays showed endplate erosions/destructions at initial presentation. At 24 months, 64 endplates (57.6%) were destroyed (statistically significant, P < 0.01) and 37 endplates (33.3%) showed healing either in the form of sclerosis or restoration of normal contour. All the sclerosed and healed endplates were not of completely normal contour. Danchaivijitr et al.[13] and Jain et al.[15] reported endplate erosion and fuzzy paradiscal margins in 43.8% and 98% of the cases, respectively. On X-rays, 45 discs were narrowed, 4 obliterated, and 5 spared at initial presentation. At 24 months, a significant number of discs were obliterated (statistically significant, P < 0.01). Jain et al. reported uniformly decreased disc space in all the 49 patients on X-rays.[15] Decrease in the initial blurring/ill definition of the endplate and decrease in soft tissues (abscesses) were the first signs of healing observed on the plain radiographs of the affected spine in the present study.
Magnetic resonance imaging evaluation
On T1-weighted images, all the patients (100%) showed hypointense signal intensity [Figures 1b, 2b and 3b]. On T2-weighted images, 49 patients (98%) showed hyperintense signal intensity and only one patient (2%) showed mixed signal intensity. On short tau inversion recovery (STIR) images, 48 patients (96%) showed hyperintensity and only two patients (4%) showed isointense signal intensity. Similar findings had also been reported in the literature.[14]
Figure 1 (B).
T1-weighted images (sagittal and axial) of the same patient. (a-d) Sagittal sections at presentation, 6 months, 12 months, and 24 months. (a) Initial sagittal sections show paradiscal lesion with endplate erosions, intraosseous caseation, marrow edema, epidural collection compressing spinal cord. Signal intensity change with decrease in findings of the initial magnetic resonance imaging is seen in subsequent magnetic resonance imaging sections. (e-h) Axial sections at presentation, 6 months, 12 months, and 24 months. (e) Initial axial sections show paravertebral collection, intraosseous caseation with epidural collection. All these resolved subsequently (f-h)
Figure 2 (B).

T1-weighted images (sagittal and axial) of the same patient. (a-d) Sagittal sections at presentation, 6 months, 12 months, and 24 months. (a) Initial sagittal sections show hypointense signal intensity with paradiscal lesion, endplate erosions, intraosseous caseation, marrow edema, epidural collection compressing the spinal cord. All these features resolved on subsequent magnetic resonance imaging sections. (e-h) Axial sections at presentation, 6 months, 12 months, and 24 months. (e) Initial axial sections show paravertebral collection, intraosseous caseation with epidural collection. All these resolved subsequently (f-h)
Figure 3 (B).
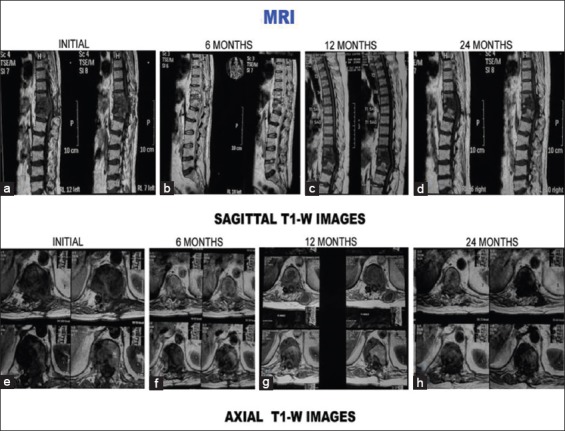
T1-weighted images (sagittal and axial) of the same patient. (a-d) Sagittal sections at presentation, 6 months, 12 months, and 24 months. (a) Initial sagittal sections show hypointense signal intensity with paradiscal lesion, discitis, endplate erosions, intraosseous caseation, and marrow edema. Six-month magnetic resonance imaging sections show increase in number of vertebrae involvement and increased edema (b). Healing occurred with increase in signal intensity on T1-weighted images (e-h) axial sections at presentation, 6 months, 12 months, and 24 months. (e) Initial axial sections show paravertebral collection, intraosseous caseation with mild epidural collection. All these resolved subsequently (f-h)
Paradiscal lesions are the most common pattern of spinal tuberculosis, and this was also observed in the present series.[12,14,15] Tuberculosis of the posterior element is considered rare. Associated posterior element involvement had been reported between 8% and 68%.[14,22,23,27,28] On MR imaging, 109 vertebrae were involved in the study population comprising fifty patients. The average number of vertebrae affected per patients was 2.18. Kim et al.[25] and al-Mulhim et al.[14] reported an average number of affected vertebrae per patient as 2.8 and 2.3, respectively. With the advent of newer imaging techniques that enable the early detection of tuberculosis, the average number of affected vertebrae is on declining trend.
Destruction of endplate is considered typical for disc infection.[15,23] Out of the total 123 endplates on MRI, 105 showed erosion, 8 were destroyed, and 10 spared at initial presentation. There was significant increase in destroyed discs at subsequent follow-up (statistically significant, P < 0.01). Forty-nine out of 117 involved endplates showed preservation of the endplates on MRI at 24 months and 12 endplates were spared. Jain et al. reported that out of total 220 endplates, 192 showed erosions, 26 were destroyed, and two were spared.[15] Various authors have reported incidence of endplate erosions between 25% to 100%.[4,6,13,15,27,28] Out of the total 62 discs at initial presentation; 53 showed discitis, 5 were spared, and 4 were destroyed. The reported incidence of disc involvement varies from 33% to 100%.[14,16,17,23,26,29]
Edema on vertebral bodies was observed on initial MRI in all the patients. In the literature also, it has been reported that almost 100% patients with spinal tuberculosis have marrow edema.[12,13,23,25,26,27]
Paravertebral abscesses were present in 90% of the cases with total spread of 138 vertebral levels. Average vertebral extent of soft tissue collection was three vertebrae. Enhancement was seen in all the cases in whom gadolinium was administered, but we were not able to demonstrate any calcification in these abscesses on MRI [Figure 3d]. Reported incidence of paravertebral abscess is between 58% and 100%.[12,14,16,29,30] In the present study, subligamentous spread was seen in 42 (84%) of the patients with total spread over 108 vertebral levels. Average vertebral extent of subligamentous spread per patient was 2.57. The reported incidence of subligamentous spread in various studies is between 58% and 93%.[23,26,29,30] Various studies have reported incidence of epidural abscess between 53.3% and 85.7%.[14,15,16,27] Epidural collections in the present study were observed in 26 (52%) patients with total vertical extent of 77 vertebral bodies. Following intracanalicular findings were observed: extradural component (caseous/granulation tissue) in 33 (66%), canal encroachment in 14 (28%), nerve root involvement in 3 (6%), and cord signal intensity change in 1 (2%) case. Le Page et al. reported spinal cord compression in 64% and radicular compression in 36% of the cases.[16] Extradural component was present in 33 patients (66%), but only 11 patients (22%) developed neurological deficit in the present study. This is because not all cord compressions lead to neurological deficit. Compression of 60% or more above the level of conus medullaris resulted in neurological deficit, while compression below the conus level resulted in no neurological sign.[23] Cormican et al. reported MR evidence of spinal cord compression, of cauda equina compression, and of radicular disease in 13 (65%), 2 (10%), and 2 (10%) patients, respectively.[17] Andronikou et al. reported that cord was abutted on in 85% and compressed in 79% of their patients by soft tissue mass projecting into the spinal canal. In 67% of patients with axial imaging, the thecal sac was completely obliterated.[12] Focal myelopathy presenting as an increased signal of the cord on T2-weighted-images had been noted in adults and was thought to indicate a poor prognosis.[23] Similar observations were also made in the present study [Figures 1A–C].
Figure 3 (D).
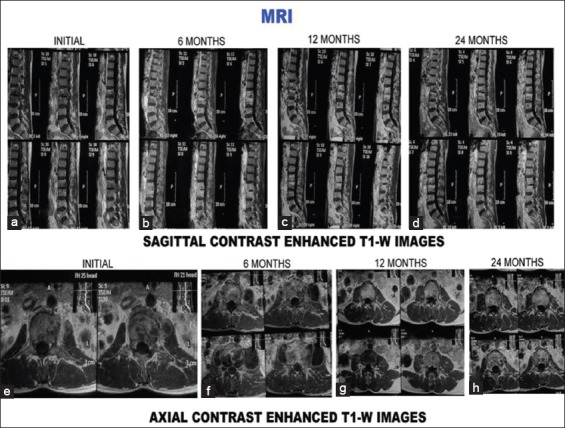
Contrast enhanced T1-weighted images (sagittal and axial) of the same patient. Contrast enhancement is seen in initial and 6 months scans both sagittal and axial magnetic resonance imaging scans (a, b, e, f). It is absent at 12 months and 24 months (c, d, g, h)
Figure 1 (C).
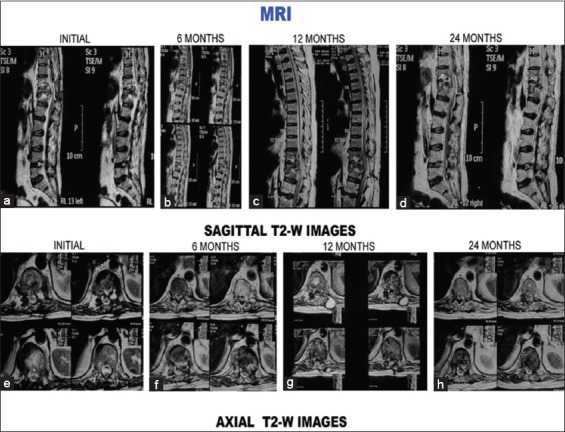
T2-weighted images (sagittal and axial) of the same patient. (a-d) Sagittal sections at presentation, 6 months, 12 months, and 24 months. (a) Initial sagittal sections show paradiscal lesion with endplate erosions, intraosseous caseation, marrow edema, epidural collection compressing spinal cord. All these features subsequently decreased (b-d) decrease in findings of the initial magnetic resonance imaging is seen in subsequent magnetic resonance imaging sections. Twenty-four months magnetic resonance imaging sections show cord signal intensity change and cord changes (d). (e) Initial axial sections show paravertebral collection, psoas abscess. All these resolved subsequently (f-h)
Various classical features of the tuberculosis of the spine in combination on MRI can be used with confidence to diagnose it. Ninety-two percent cases had a combination of marrow edema and paravertebral collections. Eighty-eight percent cases had a combination of marrow edema and subligamentous spread of disease. Eighty-two percent cases had a combination of marrow edema, paravertebral collections, and endplate erosion. Eighty percent cases had a combination of marrow edema, paravertebral collections, endplate erosion, and subligamentous spread. Fifty-two percent cases had a combination of subligamentous spread and epidural abscess. Sixty-six percent cases had a combination of subligamentous spread and extradural component (caseous/granulation tissue), suggestive of spread anterior and posterior to vertebral body. Sixty-four percent cases had a combination of marrow edema, paravertebral collections, subligamentous spread, extradural component, endplate erosion, and discitis. A combination of marrow edema, paravertebral collections, endplate erosion, and subligamentous spread on MRI present in majority of the cases (88% of cases) is the classical feature of tuberculosis spine. Only one recent study in 2012 by Jain et al. reported all cases (100%) had a combination of marrow edema and paravertebral collections; 92% had marrow edema and subligamentous spread; 98% marrow edema, paravertebral collections, and endplate erosion; and 91.8% had marrow edema, paravertebral collections, endplate erosion, and subligamentous spread. A total of 83% had subligamentous spread and epidural components and 83% had marrow edema, paravertebral collections, subligamentous spread, extradural component, endplate erosion, and discitis.[15]
Magnetic resonance imaging features during and after healing
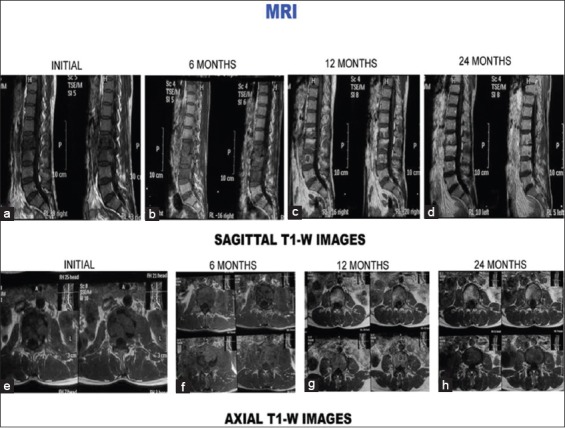
Reversal of signal intensity changes was observed in follow-up magnetic resonance (MR) examination. There was statistically significant increase in the hyperintense signal intensity on T1-weighted images (P < 0.01). Forty-six percent patients at 12 months and 74% at 24 months showed suppression of signal intensity on STIR images [Figures 1A, 2A, and 3A]. Increased signal intensity on T1-weighted images from previously affected vertebrae indicates healing and has been found to correlate well with clinical signs and symptoms.[3] Figures 1B, 2B, and 3B show signal change in T1-weighted images consistent with healing. Sharif et al. reported reversal of signal intensity on T1-weighted images of previously infected vertebrae in 12 of 25 patients who underwent follow-up examination 1–12 months after the initial examination.[3] They also reported a gradual decrease in signal intensity of the affected spinal region on T2-weighted images in all the patients. They reported that the longer the follow-up, the less abnormal is the signal intensity.[3] Similar observation was also made in the present study [Figures 1C, 2C, and 3C].
Figure 2 (C).
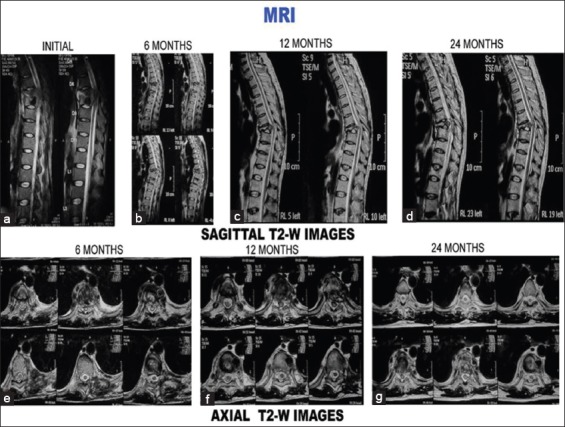
T2-weighted images (sagittal and axial) of the same patient. (a-d) Sagittal sections at presentation, 6 months, 12 months, and 24 months. (a) Initial sagittal sections show paradiscal lesion with endplate erosions, intraosseous caseation, marrow edema, epidural collection compressing spinal cord. All these features resolved subsequently. Disc is preserved although narrowed (b-d). (e) Axial sections at 6 months show septate paravertebral collection and intraosseous caseation. All these resolved subsequently (f-g)
Figure 3 (C).
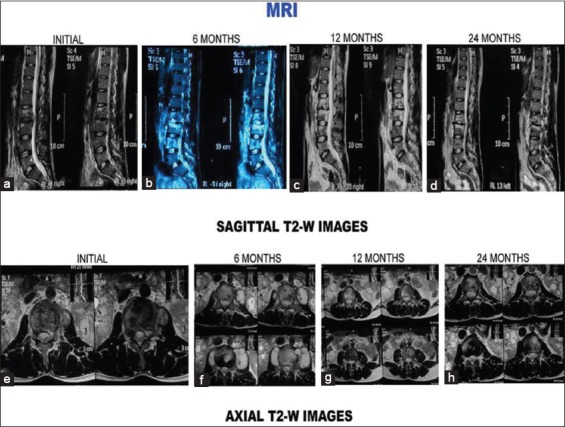
T2-weighted images (sagittal and axial) of the same patient. (a-d) Sagittal sections at presentation, 6 months, 12 months, and 24 months. (a) Initial sagittal sections show paradiscal lesion with hyperintense signal intensity, endplate erosions, intraosseous caseation, marrow edema, mild epidural collection. (b) Six-month magnetic resonance imaging sections show increase in number of vertebrae involvement and increased edema. Healing observed on the subsequent magnetic resonance imaging scans with disc space narrowing (b-d). (e) Initial axial sections show septate paravertebral collection and intraosseous caseation. Paravertebral collections and intraosseous caseation increased during first 6 months (f). Healing was observed with resolution of caseation and paravertebral collections (g-h)
Bone marrow edema decreased with passage of time. At 1 year MR images, it was decreased in 50% and absent in other 50% of the cases (statistically significant, P < 0.001). Le Page et al. reported conversion of initial edematous of the vertebral body gradually to fatty signals in 40% cases at 6 months and 75% at 12 months.[16] This change (increase in T1-weighted signal intensity) is a sign of cure.[32,33] Sharif et al. reported conversion to a fatty signal in 48% of cases at 1 year.[32]
There was significant increase in the vertebral involvement in the first 6 months (P = 0.01) although incidence of disc involvement remained fairly constant. Paravertebral abscesses showed statistically significant steady decline in incidence and size (P < 0.01). Decrease in the size of paravertebral abscesses was the first sign of healing observed on MRI in the present study. However, 13 (26%) and 1 (2%) of the patients showed small paravertebral abscesses at the end of 12 and 24 months, respectively. Similar observations were also made in regard to subligamentous spread and epidural abscesses. Only 10 (20%) and 4 (8%) patients showed subligamentous spread and epidural abscesses, respectively, at 12 months. None of the patients had these involvements at the 24-month follow-up MR images. Studies in the literature had also reported similar changes during 1st year of disease activity with varying incidence.[3,12,15,16,17]
Healing of spondylitis is associated with longstanding signal alterations on MR imaging even if there is no clinical evidence of persistent infection. The earliest sign of healing is a reduction of the thickness of the inflammatory tissue mass.[15,32] Decrease in bone marrow edema and soft-tissue mass was the earliest sign of healing observed on the MRI in the present study. It was also observed that the marrow edema increased in 20% of the patients and average vertebral involvement per person increased to 2.38 during first 6 months of the disease. It has been reported that during healing phase, imaging evidence of bone destruction can progress up to 14 months, and recovery of vertebral height may not be seen earlier than 15 months after starting treatment. Thus, progression of bone destruction while on therapy should not necessarily be considered a sign of treatment failure if there is a clinical improvement. In longstanding compression, some permanent changes in the cord may be responsible for nonrecovery or poor recovery.[31] This was also observed in one case in the present study [Figure 1–C].
In the present study, treatment lasted for a mean duration of 13.1 months (range 12–18 months). Medical Research Council (MRC) advocates short-course chemotherapy (6 months) for uncomplicated spinal tuberculosis.[34] In the study by Cormican et al., mean duration of treatment was 13 months (range: 9–24 months).[17] The total duration of treatment and number of drugs required are still controversial.[35] A critical analysis of the literature shows that proof is still lacking the equivalence between 6 and 9 months and 12 months of the treatment.[35]
We agree with Cormican et al. that there is no guidance on soft tissue or vertebral changes noted on MR spine examination, during, or after antitubercular therapy.[17] These days more emphasis is on short-course chemotherapy (6 months) for adult uncomplicated fully sensitive tuberculosis.[17,18] Moreover, similar to the study by Cormican et al.,[17] we are also of the opinion that in significant proportion of the patients, longer duration of treatment may be required as their MR scans and markers of the inflammation were abnormal even though there was apparent disease resolution clinically.
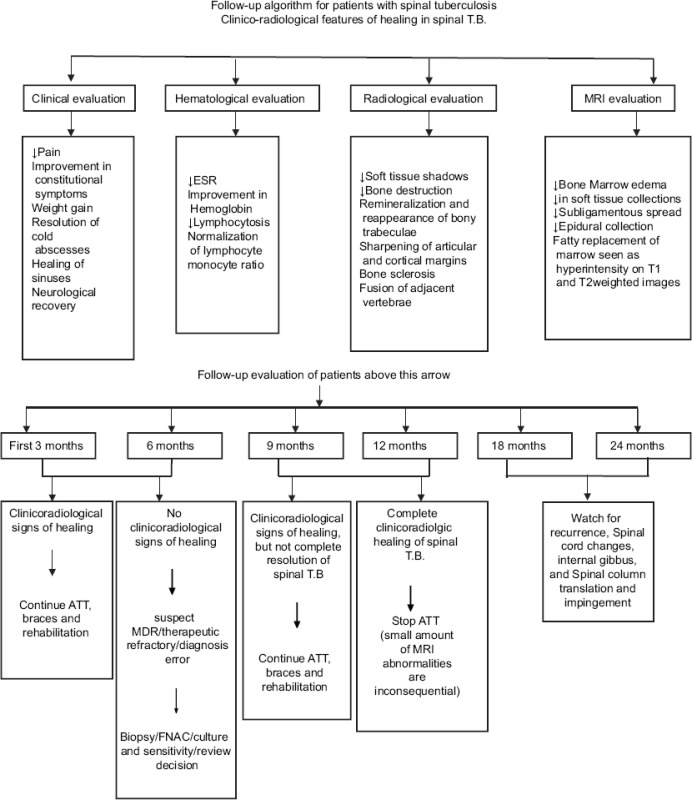
There is no guidance on appropriate imaging modality and frequency of use in the follow-up of spinal tuberculosis. Although the MRC trials have shown persistent changes on plain radiological appearances of vertebral column up to 3 years after completion of treatment, these changes were attributed to new bone formation and ankylosis.[18] In the present study also, plain film radiology has shown its utility in diagnosis, treatment, and subsequent follow-ups.
Strengths and limitations of the study
The present study had a limitation that a relatively small number of patients were enrolled. However, the strength of the study is the regular follow-up for a longer period of 2 years to observe the clinicoradiologic features in these patients.
Future research directions
Multicentric studies with more number of patients should be conducted to further validate the findings of the present study.
Conclusions
Salient features of spinal involvement in tuberculosis on plain radiograph are paradiscal involvement, endplate destruction, and soft tissue masses. Earliest sign of healing on plain radiography is decrease in fuzziness of endplate, ultimately leading to either sclerosis of endplate or fusion of adjacent vertebrae. MRI serves as an important diagnostic tool in early diagnosis of the disease, to see the response to the chemotherapy and to decide the need for the surgery and approach to be used. Decrease in inflammatory soft tissue masses and reduction in vertebral body marrow edema are the earliest feature of healing observed on MR examination. Increase or decrease in marrow edema, paravertebral collections, subligamentous spread, extradural component, endplate erosion, and discitis are the prognostic indicators of response to the chemotherapy. Short-course chemotherapy (6 months) may not be effective in completely curing spinal tuberculosis as evidenced by the clinical, laboratory, plain radiography, and MRI parameters evaluated in the present study. In the light of the findings of the present study, we propose decision-making algorithm, follow-up algorithm, and MR examination protocol (at presentation and at 6, 12, and 24 months) for spinal tuberculosis. These will help in diagnosis, monitoring the response to chemotherapy and disease course, and detecting any early recurrence.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hoffman EB, Crosier JH, Cremin BJ. Imaging in children with spinal tuberculosis. A comparison of radiography, computed tomography and magnetic resonance imaging. J Bone Joint Surg Br. 1993;75:233–9. doi: 10.1302/0301-620X.75B2.8444943. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S, Jain UK. Management of spinal tuberculosis – Current concepts. J Indian Med Assoc. 2004;102:164–7, 169. [PubMed] [Google Scholar]
- 3.Sharif HS, Clark DC, Aabed MY, Haddad MC, al Deeb SM, Yaqub B, et al. Granulomatous spinal infections: MR imaging. Radiology. 1990;177:101–7. doi: 10.1148/radiology.177.1.2399306. [DOI] [PubMed] [Google Scholar]
- 4.Teo EL, Peh WC. Imaging of tuberculosis of the spine. Singapore Med J. 2004;45:439–44. [PubMed] [Google Scholar]
- 5.Moore SL, Rafii M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am. 2001;39:329–42. doi: 10.1016/s0033-8389(05)70280-3. [DOI] [PubMed] [Google Scholar]
- 6.Joseffer SS, Cooper PR. Modern imaging of spinal tuberculosis. J Neurosurg Spine. 2005;2:145–50. doi: 10.3171/spi.2005.2.2.0145. [DOI] [PubMed] [Google Scholar]
- 7.Tuli SM, editor. Tuberculosis of the Skeletal System. 3rd ed. New Delhi: Jaypee Brothers Medical Publishers Ltd.; 2004. [Google Scholar]
- 8.Moon MS, Ha KY, Sun DH, Moon JL, Moon YW, Chung JH. Pott's Paraplegia – 67 cases. Clin Orthop Relat Res. 1996;323:122–8. [PubMed] [Google Scholar]
- 9.Jain AK. Tuberculosis of the spine: A fresh look at an old disease. J Bone Joint Surg Br. 2010;92:905–13. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 10.De Backer AI, Mortelé KJ, Vanschoubroeck IJ, Deeren D, Vanhoenacker FM, De Keulenaer BL, et al. Tuberculosis of the spine: CT and MR imaging features. JBR-BTR. 2005;88:92–7. [PubMed] [Google Scholar]
- 11.Tuli SM. Judicial management of tuberculosis of bones, joints and spine. Indian J Orthop. 1985;19:147–66. [Google Scholar]
- 12.Andronikou S, Jadwat S, Douis H. Patterns of disease on MRI in 53 children with tuberculous spondylitis and the role of gadolinium. Pediatr Radiol. 2002;32:798–805. doi: 10.1007/s00247-002-0766-8. [DOI] [PubMed] [Google Scholar]
- 13.Danchaivijitr N, Temram S, Thepmongkhol K, Chiewvit P. Diagnostic accuracy of MR imaging in tuberculous spondylitis. J Med Assoc Thai. 2007;90:1581–9. [PubMed] [Google Scholar]
- 14.al-Mulhim FA, Ibrahim EM, el-Hassan AY, Moharram HM. Magnetic resonance imaging of tuberculous spondylitis. Spine (Phila Pa 1976) 1995;20:2287–92. doi: 10.1097/00007632-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Jain AK, Sreenivasan R, Saini NS, Kumar S, Jain S, Dhammi IK. Magnetic resonance evaluation of tubercular lesion in spine. Int Orthop. 2012;36:261–9. doi: 10.1007/s00264-011-1380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Page L, Feydy A, Rillardon L, Dufour V, Le Hénanff A, Tubach F, et al. Spinal tuberculosis: A longitudinal study with clinical, laboratory, and imaging outcomes. Semin Arthritis Rheum. 2006;36:124–9. doi: 10.1016/j.semarthrit.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Cormican L, Hammal R, Messenger J, Milburn HJ. Current difficulties in the diagnosis and management of spinal tuberculosis. Postgrad Med J. 2006;82:46–51. doi: 10.1136/pgmj.2005.032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A 15-year assessment of controlled trials of the management of tuberculosis of the spine in Korea and Hong Kong. Thirteenth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br. 1998;80:456–62. doi: 10.1302/0301-620x.80b3.8544. [DOI] [PubMed] [Google Scholar]
- 19.Moon MS. Tuberculosis of the spine. Controversies and a new challenge. Spine (Phila Pa 1976) 1997;22:1791–7. doi: 10.1097/00007632-199708010-00022. [DOI] [PubMed] [Google Scholar]
- 20.Jain R, Sawhney S, Berry M. Computed tomography of vertebral tuberculosis: Patterns of bone destruction. Clin Radiol. 1993;47:196–9. doi: 10.1016/s0009-9260(05)81162-6. [DOI] [PubMed] [Google Scholar]
- 21.Bakhsh A. Medical management of spinal tuberculosis: An experience from Pakistan. Spine (Phila Pa 1976) 2010;35:E787–91. doi: 10.1097/BRS.0b013e3181d58c3c. [DOI] [PubMed] [Google Scholar]
- 22.Alothman A, Memish ZA, Awada A, Al-Mahmood S, Al-Sadoon S, Rahman MM, et al. Tuberculous spondylitis: Analysis of 69 cases from Saudi Arabia. Spine (Phila Pa 1976) 2001;26:E565–70. doi: 10.1097/00007632-200112150-00020. [DOI] [PubMed] [Google Scholar]
- 23.Desai SS. Early diagnosis of spinal tuberculosis by MRI. J Bone Joint Surg Br. 1994;76:863–9. [PubMed] [Google Scholar]
- 24.Shanley DJ. Tuberculosis of the spine: Imaging features. AJR Am J Roentgenol. 1995;164:659–64. doi: 10.2214/ajr.164.3.7863889. [DOI] [PubMed] [Google Scholar]
- 25.Kim NH, Lee HM, Suh JS. Magnetic resonance imaging for the diagnosis of tuberculous spondylitis. Spine (Phila Pa 1976) 1994;19:2451–5. doi: 10.1097/00007632-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Liu GC, Chou MS, Tsai TC, Lin SY, Shen YS. MR evaluation of tuberculous spondylitis. Acta Radiol. 1993;34:554–8. [PubMed] [Google Scholar]
- 27.Loke TK, Ma HT, Chan CS. Magnetic resonance imaging of tuberculous spinal infection. Australas Radiol. 1997;41:7–12. doi: 10.1111/j.1440-1673.1997.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 28.Narlawar RS, Shah JR, Pimple MK, Patkar DP, Patankar T, Castillo M. Isolated tuberculosis of posterior elements of spine: Magnetic resonance imaging findings in 33 patients. Spine (Phila Pa 1976) 2002;27:275–81. doi: 10.1097/00007632-200202010-00015. [DOI] [PubMed] [Google Scholar]
- 29.Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol. 2004;182:1405–10. doi: 10.2214/ajr.182.6.1821405. [DOI] [PubMed] [Google Scholar]
- 30.Yusof MI, Hassan E, Rahmat N, Yunus R. Spinal tuberculosis: The association between pedicle involvement and anterior column damage and kyphotic deformity. Spine (Phila Pa 1976) 2009;34:713–7. doi: 10.1097/BRS.0b013e31819b2159. [DOI] [PubMed] [Google Scholar]
- 31.Jain AK, Jena A, Dhammi IK. Correlation of clinical course with magnetic resonance imaging in tuberculous myelopathy. Neurol India. 2000;48:132–9. [PubMed] [Google Scholar]
- 32.Sharif HS, Morgan JL, al Shahed MS, al Thagafi MY. Role of CT and MR imaging in the management of tuberculous spondylitis. Radiol Clin North Am. 1995;33:787–804. [PubMed] [Google Scholar]
- 33.Gouliamos AD, Kehagias DT, Lahanis S, Athanassopoulou AA, Moulopoulou ES, Kalovidouris AA, et al. MR imaging of tuberculous vertebral osteomyelitis: Pictorial review. Eur Radiol. 2001;11:575–9. doi: 10.1007/s003300000631. [DOI] [PubMed] [Google Scholar]
- 34.Five-year assessment of controlled trials of short-course chemotherapy regimens of 6, 9 or 18 months’ duration for spinal tuberculosis in patients ambulatory from the start or undergoing radical surgery. Fourteenth report of the Medical Research Council Working Party on Tuberculosis of the Spine. Int Orthop. 1999;23:73–81. doi: 10.1007/s002640050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazra A, Laha B. Chemotherapy of osteoarticular tuberculosis. Indian J Pharmacol. 2005;37:5–12. [Google Scholar]


