Abstract
The renin-angiotensin-aldosterone system is a signaling pathway which responsible in the blood pressure regulation. Angiotensin-converting enzyme (ACE) is one of the key elements responsible for the hypertensive mechanism. It converts angiotensin-I to angiotensin-II. The discovery history of the ACE inhibitory activity assay method has been through a long stage for decades and development continues until today. The ACE inhibitory activity has become an effective screening method in the search for new antihypertensive agents from herbal plants. Some of in vitro assay methods were used to examine the activity of ACE inhibitors based on the substrate usage, such as; Cushman and Cheung Method using a substrate hippuryl-histidyl-leucine (HHL), Holmquist method using a substrate furanacryloyl-tripeptide, Elbl and Wagner method using a substrate benzoil-[l-14C] glicyl-L-histidine-L-leucine, Carmel and Yaron method using a substrate o-aminobenzoylglycyl-p-nitrophenylalanilproline, and Lam method using 3-hydroxybutyrylglycyl-glycyl-glycine as substrate. Several different methods to measure the results of enzymatic reactions or separating substrate with products, including spectrophotometric, fluorometric, high-performance liquid chromatography, electrophoresis, and radiochemistry. Application of the test method for screening the ACE inhibitors activity and investigation of active compounds from natural products can be done easily with this method, it is very helpful in research because the results obtained are simple, accurate, and rapid.
Keywords: Angiotensin-converting enzyme inhibitory activity, angiotensin-converting enzyme, herbal plants, in vitro assay method, Renin-angiotensin-aldosterone system
INTRODUCTION
Initially, the screening for antihypertensive effect in the drugs discovery from natural products mainly used empirically been done over the years and have used several experiments on animal models.[1,2,3] Studies in the drug discovery, especially as an antihypertensive has developed rapidly since the discovery of the angiotensin-converting enzyme (ACE). The ACE converts angiotensin decapeptide inactive into active octapeptide angiotensin II in the kidneys, especially in the renin-angiotensin-aldosterone system.[4,5,6,7,8] The activity of ACE inhibitory by in vitro has become an effective assay method in the drugs discovery as antihypertensive. This has been demonstrated by comparing the assay method of seven kinds medicines (captopril, enalapril, zofenopril, ramipril, fosinopril, lisinopril, and SQ 29852) as the ACE inhibitor.[9] However, in studies, the activity of ACE inhibitory for a positive control is more widely used a captopril because the drug is most widely used as antihypertensive and heart failure, and also have a free radical scavenger activity are highly relevant as an ACE inhibitor.[10]
In modern medicine, the drug discovery has become more specific and focus on particular target objectives. The identification of receptor or enzyme as a molecular target which has an important role in the disease regulation and then performs searches the ligand or substrate or inhibitor of a specific target is the reason behind this approach. The discovery of a new drugs mainly from natural materials are directly aimed at the molecular target (receptor or enzyme) is more effective and efficient than conventional methods using animal model experiments performed with the treatment and observation in general, and require treatment and observation are more complicated if performed on specific targets (e.g. receptors or enzymes), as well as the type of the test sample to be used.[2,9,11,12,13] Considering the potential of natural resources are abundant so that the necessary a special strategy conduct research one of which is an assay method of the ACE inhibitors activity in vitro. The present studies review aims to highlight the discover history, assay methods, and application in natural products drugs discovery of ACE inhibitory activity assay.
MATERIALS AND METHODS
This paper reviews about the ACE Inhibitory Activity Assay from comprehensive literature. The literature was searched between August and October 2016 from the electronic databases including PubMed, Scopus, ScienceDirect, and Google Scholar.
RESULTS AND DISCUSSION
The discover history of in vitro angiotensin-converting enzyme inhibitory assay methods
The assay method of ACE activity in vitro was begun in 1954–1957, when Skeggs et al. succeeded in isolating and purifying the enzyme which can hydrolyze decapeptide angiotensin I, then release vasopressive octapeptide angiotensin II and histidine-leucine dipeptide inactive or commonly referred to as “converting enzyme” from the horses plasma.[14] From the results of the discovery, the action mechanism of this enzyme can be determined [Figure 1].[15] However, at that time, there has been no progress on the development of in vitro assay methods.
Figure 1.
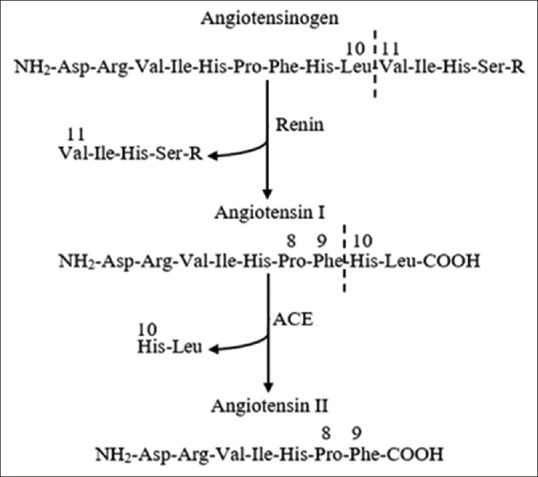
Scheme of angiotensin II formation by angiotensin-converting enzyme
About eleven years later (1968–1969) with the discovery of radiometric assay using the labeled angiotensin I substrate, wherein the release of radioactive histidine-leucine which serves as an enzymatic activity index[16] and further developed methods for chemical assay of the ACE, where the enzymatic reaction product based on the determination of histidine-leucine with fluorometric method on different substrates.[17] In 1970–1971, Cushman and Cheung managed to find a spectrophotometric assay method for measuring the amount of ACE to produce hippuric acid (HA) from hippuryl-histidyl-leucine (HHL) as substrate.[18]
Carmel and Yaron (1977–1978) developed a measurement method of the ACE inhibitory activity using an o-aminobenzoylglycine-p-nitrophenylalanylproline as a substrate and then hydrolyzed into o-aminobenzoylglicyl.[19] At the same time, Hayakari et al., developed assay methods of the ACE inhibitory activity in a spectrophotometry manner using HHL as substrate and a colorimetric reagent of HA namely 2,4,6-trichloro-s-triazine (TT).[20]
In 1979–1991, some research reported the usage of a substrate other than HHL for assay method of ACE inhibitory activity such as Holmquist et al. using tripeptide furanacryloyl (FA-PGG) as a substrate,[21] Baudin et al., using benzoyl-[l-14C] glicyl-L-histidyl-L-leucine as a substrate,[22] and the usage a substrate of chromophore- and fluorophore-labelled tripeptide dansyltriglycine[23] by Elbl and Wagner. Since 1993, Doig and Smiley have developed method of ACE inhibitory activity assay using a shielded hydrophobic phase (SHP) column for high-performance liquid chromatography (HPLC) instrument and HHL substrate.[24] Nakamura et al. have performed purification and characterization of ACE inhibitory using HPLC instrument.[25]
Initially, all methods are constantly being developed is only used against the pure compound and has not been in use on samples containing multi-compound as in plant extracts. However, Hansen et al. began to apply this method on the plant extracts.[26] In 2007, Lam le et al., managed to synthesize and use a substrate 3-hydroxybutyrylglycyl-glycyl-glycine (3HB-GGG) in the assay method of the ACE inhibitory activity,[27] and this method is also applicable to samples of plant extracts.
In vitro angiotensin converting enzyme assay methods
Several assay method of ACE inhibitory activity can be used to detect the activity of ACE inhibition from drugs or plant extract. Each method is distinguished by the use of substrates and measurement methods of enzymatic reaction products or separation of the substrate with the products. In addition, the author provides the names of each method based on the inventor names, ACE inhibitory activity test method is divided into several methods as follows:
Cushman and Cheung method
Cushman and Cheung (1970–1971) have developed the assay method of the activity of ACE inhibitors using a substrate hippuryl-histidyl-leucine (HHL), the ACE will hydrolyze HHL into HA [Figure 2].[28] The HA was measured at a wavelength of 228 nm to describe the ACE activity using a ultraviolet-visible (UV-Vis) spectrophotometer instrument. When there is an ACE inhibitor, the concentration of HA formed will be reduced.[18]
Figure 2.
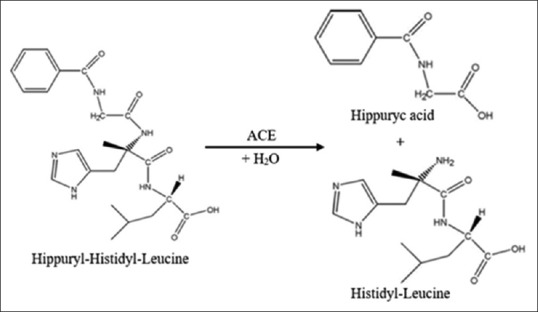
HHL hydrolysis by angiotensin-converting enzyme
The success of Cushman and Cheung’s method of assay the ACE inhibitors activity still depends on the ability to separate HA formed from the HHL substrate. Therefore, the another approach is required to facilitate the use of these methods optimally, among other: (a) The addition of TT in the sample mixture containing HA, a reaction between TT with HA formed should be measured at a wavelength of 382 nm.[20] (b) The usage of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) to stop the reaction and Cyanuric chloride in 1,4-dioxane as a color reagent measured in photometry at a wavelength of 504 nm.[29] (c) The addition of o-Phthaldialdehyde which then react with the substrate hydrolysis histidine-leucine is measured by a fluoro-colorimeter instrument at a wavelength of 495 nm emission and 365 nm excitation.[30] (d) The use of benzene-sulfonyl-chloride (BSC) as a color reagent in the presence of quinoline[31] (referred to as BSC-visible spectrophotometry method) and then modified using a microtiter plate reader.[32]
Besides using the UV-Vis spectrophotometer, can also use the high HPLC, however, require some modifications such as the use of SHP HPLC to separate HHL and HA, which can be injected directly for the measurement of the HA absorbance using a spectrophotometer wavelength at 254 nm with a 8.0 mL flow-cell.[24] Furthermore, Mehanna and Dowling, were able to develop a more simple HPLC method,[33] which further developed by using reverse-phase-HPLC (RP-HPLC) and HEPES as a buffer.[34]
Holmquist method
This method was first introduced by Holmquist et al. in 1979 using FA-PGG as a substrate. This method is based on the absorption spectrum blue shift that occurred on the substrate hydrolysis produces dipeptide and furanacryloyl-blocked amino acid.[21] This was confirmed by Lundberg et al., to perform measurements of the ACE inhibitory activity in serum using an FA-PGG substrate.[35] This method has been conducted optimization, validation, and modifications of the FA-PGG usage as a substrate for screening bioactive peptides.[36,37] The FA-PGG substrate is hydrolyzed into a dipeptide (glycylglycine) and furanacroyl-phenylalanine by ACE and measured at a wavelength of 328 nm and 352 nm,[37,38] the addition of ethylenediaminetetraacetic acid (EDTA) aimed to stopping the enzymatic reaction[37] and other modifications using a microplate reader.[38] Lahogue et al. has reported the application of HPLC-UV method for the determination of the activity of ACE inhibitors.[39]
Elbl and Wagner method
This method was developed by Elbl and Wagner in 1991 with the usage of the chromophore- and fluorophore-labeled tripeptide dansyl triglycine as substrate, which is cleaved by the ACE into dansylglycine and diglycine [Figure 3].[23] Furthermore, this method modified by using a microtitre plate which is injected directly into the UV detection.[26,40] Duncan et al. have been applying this method for screening the activity of ACE inhibitors from Zulu medicinal plants.[41]
Figure 3.
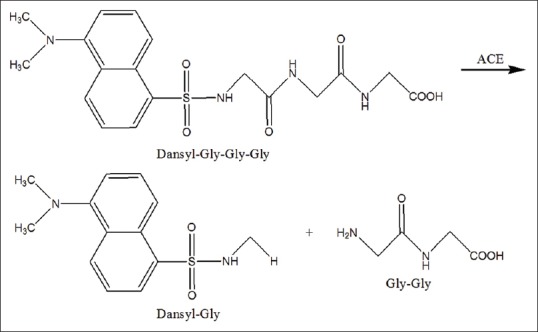
Dansyltriglycyne hydrolysis by angiotensin-converting enzyme
Lacaille-Dubois et al. modify Elbl and Wagner method in which the hippuric-glycine-glycine substrate is cleaved by ACE and react with trinitrobenzesulfonat acid to form 2,4,6-trinitrofenil-glycine-glycine were separated by RP-HPLC.[42,43] In addition, it also has been validated a colorimetric assay for screening ACE inhibitors from plant extracts in vitro by Serra et al.[44]
Baudin method
Baudin et al. have developed a method with radiometric technology to measure ACE activity in urine and using a substrate that has been labeled namely benzoyl-[l-14C] glycyl-L-histidyl-L-leucine. The substrate will be cleaved at the glycine-histidine bonding and form benzoyl-[l-14C] glycine. This test method can be used in the study of ACE in the urine as a marker if there is kidney damage.[22]
Carmel and Yaron method
The Carmel and Yaron methods began to be discovered and developed in 1978, an assay method of the ACE inhibitory activity using o-amino benzoyl glycyl-p-nitrophenilalanilproline as a substrate then hydrolyzed into o-amino benzoyl glycyl as a compound fluorescence, for discontinue enzymatic reaction can be used EDTA.[19] In 2006, Sentandreu and Toldrá using this method with slight modifications were measured using a multi-scanning microplate fluorometer. The advantage of this method is the usage capacity of large sample and in a short time.[45] However, this method is not widely used.
Lam method
Lam le et al., begun to study in 2007 managed to find a new substrate is 3HB-GGG for assay method of the activity of ACE. The 3HB-GGG is cleaved into Gly-Gly-Gly amino acid and 3-hydroxybutyric acid (3HB) by the ACE. Then 3HB measured using F-kit. This method is more sensitive, rapid, accurate, and suitable for conventional methods.[27] Then, Lam le et al. carried out development using a Water-Soluble Tetrazolium Salt (WST1) to detect 3-hydroxybutyrate formed.[46] The mechanism of the assay method of the ACE inhibitor activity using ACE kit-WST1 described in Figure 4 and this method using flow injection analysis to detect directly in a rapid, simple, and accurate.[47] This enzyme has been created in the form of a kit which has been patented with the name the ACE kit-WST1. The LAM method with the 3HB-GGG substrate using the ACE kit-WST can also be applied using microplate ELISA reader.[48,49,50]
Figure 4.
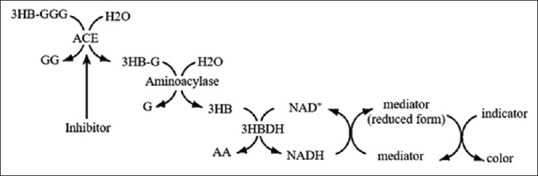
The principle of the assay method of angiotensin-converting enzyme inhibitors activity using angiotensin-converting enzyme kit-Water-Soluble Tetrazolium Salt 1
Applied of the angiotensin converting enzyme inhibitory assay method in drug discovery of natural products
The screening of antihypertensive activity on natural products, especially herbs used empirically as antihypertensive has been conducted over the years. The application of the assay method of the activity of ACE inhibitory for the activity screening in the natural product research is very helpful because the results obtained in a rapid, accurate, and simple. In general, the methods described above have been conducted optimization for the type and the materials concentration used so that the reaction mechanism of the enzyme occurs the same as in the actual state in the body,[29,36] this method also was performed standarization[30] and validation,[36] between each method as well as with conventional methods performed in vivo method. Selection of assay methods based on the substrate type depending on the availability of instruments that can be used to measure the inhibitory activity of ACE from the samples.[51] Some herbs that have been carried out the screening of activity using the in vitro assay method of ACE inhibitors activity as shown in Table 1. Based on the screening results of ACE inhibitors activity from various data sources [Table 1] show that the research of new drug discovery from natural materials is still very limited when compared with the availability of abundant natural resources around us.
Table 1.
The results data of angiotensin-converting enzyme inhibitors activity in vitro from plants
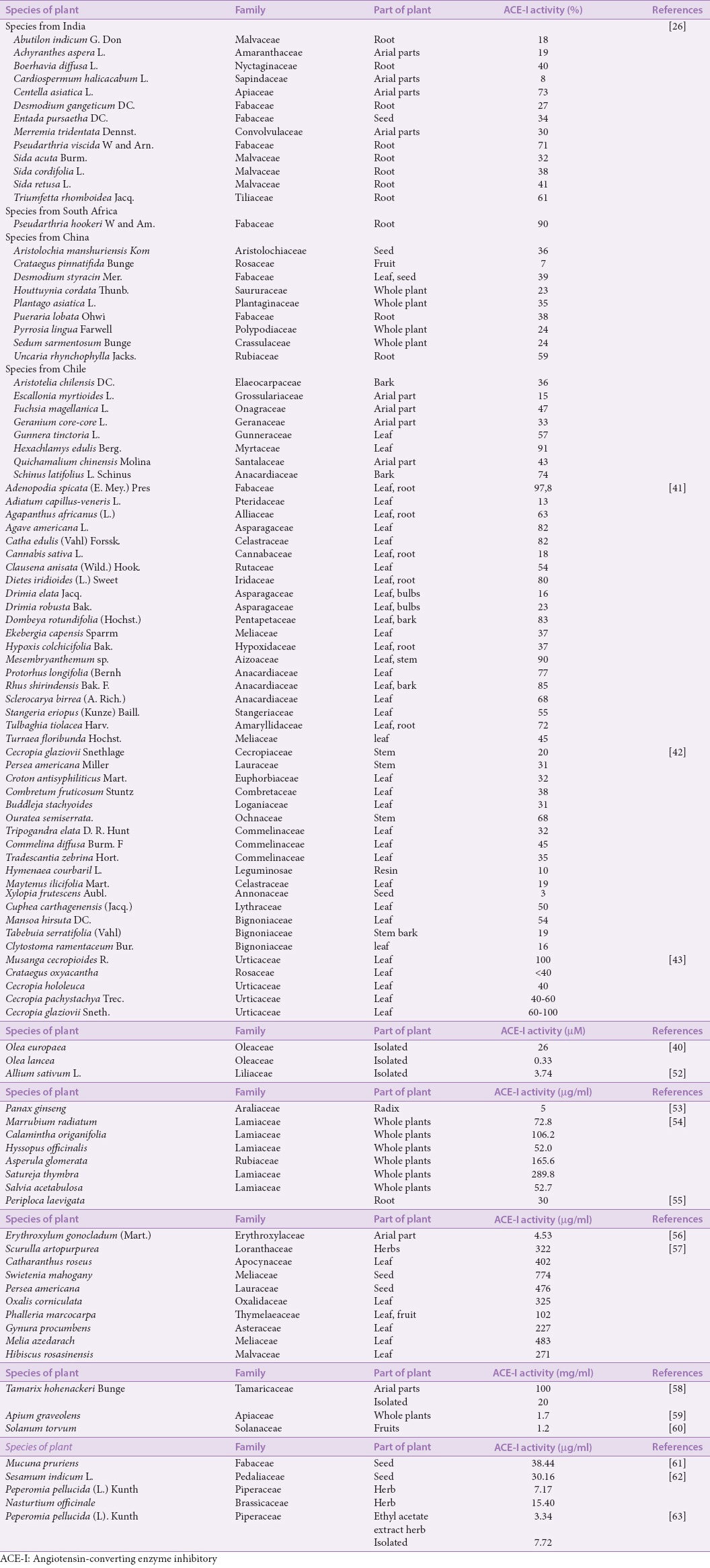
The new drug discovery from natural materials are not only limited to the screening phase the activity but till the determining phase of compounds is responsible as ACE inhibitors. The search of active compound can be performed easily with this method, in general, can be started from screening the activity of the extract, fractions, and isolates. Even for the isolates that have been characterized and determined the structure can be more optimal if combined with the in silico assay method[13,64] to obtain a prediction overview of the action mechanism and the group most responsible of the compounds studied. Several studies based on the empirical usage of natural products such as the celery plant (Apium graveolens) as antihypertensive and isolation of the junipediol A 8-OβD-glucoside (1-βD-glucosyloxy-2- (3-methoxy-4-hydroxyphenyl) -propane -1,3-diol) compound has the strongest activity as an ACE inhibitor compared to another known compound (such as, 11,21-dioxo-3 b, 15a, 24-trihydroxyurs-12-ene-24-O- b-D-glucopyranoside, chrysoeriol-7-O-b-D-apiosylglucoside, apiin, icariside D2, luteolin-7-O-b-D-glucoside, apigenin-7-O-b-D-glucoside, roseoside, and isofraxidin-b-D-glucoside).[59] Garlic (Allium sativum) has been known to dipeptide compounds that play a role as an ACE inhibitor, namely Gly-Phe, Ser-Phe, Gly-Tyr, Ser-Tyr, Asn-Phe, Asn-Tyr, and Phe-Tyr, with the IC50, were 277.9, 130.2, 72.1, 66.3, 46.3, 32.6, and 3.74 μM,[52] respectively. The (E)-2,3-dihydroxycyclopentyl-3-(3’,4’-dihydroxyphenyl) acrylate compound was isolated from wild Eggplant (Solanum torvum) (IC50 of 778 mg/mL).[60] Suaeda physophora Pall has isolated a quinoline alkaloids (Suaeda) and flavonoid compounds that also have a potential of ACE inhibitors activity.[65] The astilbin (with IC50 of 4.53 mg/mL) were isolated from Erythroxylum gonocladum plant.[56] The quercetin isolated from Peperomia pellucida L. Kunth herbs with IC50 of 7.72 mg/mL.[63] By knowing the active compounds that play a role as an ACE inhibitor, the researchers of natural products are more focused on enrichment, dereplication, and isolation the active compound that can be applied in the in vivo assay method and further development.
CONCLUSION
By the discovery of the various assay methods of ACE inhibitors activity. This method is very applicable for testing on natural products so that the researchers can perform quickly, accurately, and simple, so they can more focus on the development of extraction, fractionation, and isolation methods for the enrichment and dereplication of the active compounds. The selection of the assay methods for determining the inhibitory activity of ACE is only based on subject to availability of the substrate and the measurement instruments for example spectrophotometric, photo fluorometer, HPLC, and others.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors are thankful to Ministry of Research, Technology and Higher Education, Republic of Indonesia and Directorate of Research and Community Engagement (DRPM), Universitas Indonesia for financially supporting the study via a grant “Hibah PUPT 2015” and “PITTA 2016”.
REFERENCES
- 1.López-Fandiño R, Otte J, Camp JV. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int Dairy J. 2006;16:1277–93. [Google Scholar]
- 2.Fujita M, Ohnishi K, Takaoka S, Ogasawara K, Fukuyama R, Nakamuta H. Antihypertensive effects of continuous oral administration of nattokinase and its fragments in spontaneously hypertensive rats. Biol Pharm Bull. 2011;34:1696–701. doi: 10.1248/bpb.34.1696. [DOI] [PubMed] [Google Scholar]
- 3.Lingbeck JM, O’Bryan CA, Martin EM, Adams JP, Crandall PG. Sweetgum: An ancient source of beneficial compounds with modern benefits. Pharmacogn Rev. 2015;9:1–11. doi: 10.4103/0973-7847.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skeggs LT, Jr, Marsh WH, Kahn JR, Shumway NP. The existence of two forms of hypertensin. J Exp Med. 1954;99:275–82. doi: 10.1084/jem.99.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skeggs LT, Jr, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956;103:295–9. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gude D. How full is our antihypertensives pipeline? J Pharmacol Pharmacother. 2012;3:7–11. doi: 10.4103/0976-500X.92492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabassum N, Ahmad F. Role of natural herbs in the treatment of hypertension. Pharmacogn Rev. 2011;5:30–40. doi: 10.4103/0973-7847.79097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayari N, Saidi N, Sila A, Ellouz-Chaabouni S, Bougatef A. Chemical composition, angiotensin I-converting enzyme (ACE) inhibitory, antioxydant and antimicrobial activities of Ononis natrix leaves extracts. Free Radic Antioxid. 2016;6:23–33. [Google Scholar]
- 9.Cushman DW, Wang FL, Fung WC, Grover GJ, Harvey CM, Scalese RJ, et al. Comparisons in vitro, ex vivo, and in vivo of the actions of seven structurally diverse inhibitors of angiotensin converting enzyme (ACE) Br J Clin Pharmacol. 1989;28(Suppl 2):115S–30S. doi: 10.1111/j.1365-2125.1989.tb03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra M, Scott N, McMurray J, McLay J, Bridges A, Smith WE, et al. Captopril: A free radical scavenger. Br J Clin Pharmacol. 1989;27:396–9. doi: 10.1111/j.1365-2125.1989.tb05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuglsang A, Nilsson D, Nyborg NC. Characterization of new milk-derived inhibitors of angiotensin converting enzyme in vitro and in vivo. J Enzyme Inhib Med Chem. 2003;18:407–12. doi: 10.1080/1475636031000138723. [DOI] [PubMed] [Google Scholar]
- 12.Dost T, Kafkas S, Gokalp F, Karul A, Birincioglu M. Effects of angiotensin converting enzyme inhibition on adiponectin levels and lipid profile in the ovariectomized-aged rats. J Pharmacol Pharmacother. 2014;5:21–6. doi: 10.4103/0976-500X.124413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhammad SA, Fatima N. In silico analysis and molecular docking studies of potential angiotensin-converting enzyme inhibitor using quercetin glycosides. Pharmacogn Mag. 2015;11(Suppl 1):S123–6. doi: 10.4103/0973-1296.157712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skeggs LT, Jr, Kahn JR, Lentz K, Shumway NP. The preparation, purification, and amino acid sequence of a polypeptide renin substrate. J Exp Med. 1957;106:439–53. doi: 10.1084/jem.106.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guang C, Phillips RD, Jiang B, Milani F. Three key proteases – Angiotensin-I-converting enzyme (ACE), ACE2 and renin – Within and beyond the renin-angiotensin system. Arch Cardiovasc Dis. 2012;105:373–85. doi: 10.1016/j.acvd.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huggins CG, Thampi NS. A simple method for the determination of angiotensin I converting enzyme. Life Sci. 1968;7:633–9. doi: 10.1016/0024-3205(68)90086-6. [DOI] [PubMed] [Google Scholar]
- 17.Piquilloud Y, Reinharz A, Roth M. Studies on the angiotensin converting enzyme with different substrates. Biochim Biophys Acta. 1970;206:136–42. doi: 10.1016/0005-2744(70)90090-2. [DOI] [PubMed] [Google Scholar]
- 18.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–48. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 19.Carmel A, Yaron A. An intramolecularly quenched fluorescent tripeptide as a fluorogenic substrate of angiotensin-I-converting enzyme and of bacterial dipeptidyl carboxypeptidase. Eur J Biochem. 1978;87:265–73. doi: 10.1111/j.1432-1033.1978.tb12375.x. [DOI] [PubMed] [Google Scholar]
- 20.Hayakari M, Kondo Y, Izumi H. A rapid and simple spectrophotometric assay of angiotensin-converting enzyme. Anal Biochem. 1978;84:361–9. doi: 10.1016/0003-2697(78)90053-2. [DOI] [PubMed] [Google Scholar]
- 21.Holmquist B, Bünning P, Riordan JF. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal Biochem. 1979;95:540–8. doi: 10.1016/0003-2697(79)90769-3. [DOI] [PubMed] [Google Scholar]
- 22.Baudin B, Bénéteau-Burnat B, Baumann FC, Giboudeau J. A reliable radiometric assay for the determination of angiotensin I-converting enzyme activity in urine. J Clin Chem Clin Biochem. 1990;28:857–61. doi: 10.1515/cclm.1990.28.11.857. [DOI] [PubMed] [Google Scholar]
- 23.Elbl G, Wagner H. A new method for the in vitro screening of inhibitors of angiotensin-converting enzyme (ACE), using the chromophore- and fluorophore-labelled substrate, dansyltriglycine. Planta Med. 1991;57:137–41. doi: 10.1055/s-2006-960050. [DOI] [PubMed] [Google Scholar]
- 24.Doig MT, Smiley JW. Direct injection assay of angiotensin-converting enzyme by high-performance liquid chromatography using a shielded hydrophobic phase column. J Chromatogr B Biomed Sci Appl. 1993;613:145–9. doi: 10.1016/0378-4347(93)80208-l. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci. 1995;78:777–83. doi: 10.3168/jds.S0022-0302(95)76689-9. [DOI] [PubMed] [Google Scholar]
- 26.Hansen K, Nyman U, Smitt UW, Adsersen A, Gudiksen L, Rajasekharan S, et al. In vitro screening of traditional medicines for anti-hypertensive effect based on inhibition of the angiotensin converting enzyme (ACE) J Ethnopharmacol. 1995;48:43–51. doi: 10.1016/0378-8741(95)01286-m. [DOI] [PubMed] [Google Scholar]
- 27.Lam le H, Shimamura T, Sakaguchi K, Noguchi K, Ishiyama M, Fujimura Y, et al. Assay of angiotensin I-converting enzyme-inhibiting activity based on the detection of 3-hydroxybutyric acid. Anal Biochem. 2007;364:104–11. doi: 10.1016/j.ab.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Thongnopnua P, Poeaknapo C. High-performance liquid chromatographic determination of enalapril in human plasma by enzyme kinetic analytical method. J Pharm Biomed Anal. 2005;37:763–9. doi: 10.1016/j.jpba.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 29.Schnaith E, Beyrau R, Bückner B, Klein RM, Rick W. Optimized determination of angiotensin I-converting enzyme activity with hippuryl-L-histidyl-L-leucine as substrate. Clin Chim Acta. 1994;227:145–58. doi: 10.1016/0009-8981(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira EM, Santos RA, Krieger JE. Standardization of a fluorimetric assay for the determination of tissue angiotensin-converting enzyme activity in rats. Braz J Med Biol Res. 2000;33:755–64. doi: 10.1590/s0100-879x2000000700005. [DOI] [PubMed] [Google Scholar]
- 31.Li GH, Liu H, Shi YH, Le GW. Direct spectrophotometric measurement of angiotensin I-converting enzyme inhibitory activity for screening bioactive peptides. J Pharm Biomed Anal. 2005;37:219–24. doi: 10.1016/j.jpba.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Wang Y, Ye R, Wu Y, Xia W. Comparison of analytical methods to assay inhibitors of angiotensin I-converting enzyme. Food Chem. 2013;141:3329–34. doi: 10.1016/j.foodchem.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 33.Mehanna AS, Dowling M. Liquid chromatographic determination of hippuric acid for the evaluation of ethacrynic acid as angiotensin converting enzyme inhibitor. J Pharm Biomed Anal. 1999;19:967–73. doi: 10.1016/s0731-7085(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 34.Sharifi N, Souri E, Ziai SA, Amin G, Amini M, Amanlou M. Isolation, identification and molecular docking studies of a new isolated compound, from Onopordon acanthium: A novel angiotensin converting enzyme (ACE) inhibitor. J Ethnopharmacol. 2013;148:934–9. doi: 10.1016/j.jep.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Lundberg PA, Lindstedt G, Andersson T, Branegård B, Lundquister G, Nystron E. Single reagent assay for angiotensin converting enzyme in serum. J Clin Chem. 1984;30:163–8. [Google Scholar]
- 36.Vermeirssen V, Van Camp J, Verstraete W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J Biochem Biophys Methods. 2002;51:75–87. doi: 10.1016/s0165-022x(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 37.Murray BA, Walsh DJ, FitzGerald RJ. Modification of the furanacryloyl-L-phenylalanylglycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity. J Biochem Biophys Methods. 2004;59:127–37. doi: 10.1016/j.jbbm.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Zhou J, Huang K, Sun Y, Zeng X. Purification of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide with an antihypertensive effect from loach (Misgurnus anguillicaudatus) J Agric Food Chem. 2012;60:1320–5. doi: 10.1021/jf204118n. [DOI] [PubMed] [Google Scholar]
- 39.Lahogue V, Réhel K, Taupin L, Haras D, Allaume P. A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2010;118:870–5. [Google Scholar]
- 40.Hansen K, Adsersen A, Christensen SB, Jensen SR, Nyman U, Smitt UW. Isolation of an angiotensin converting enzyme (ACE) inhibitor from Olea europaea and Olea lancea. Phytomedicine. 1996;2:319–25. doi: 10.1016/S0944-7113(96)80076-6. [DOI] [PubMed] [Google Scholar]
- 41.Duncan AC, Jäger AK, van Staden J. Screening of Zulu medicinal plants for angiotensin converting enzyme (ACE) inhibitors. J Ethnopharmacol. 1999;68:63–70. doi: 10.1016/s0378-8741(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 42.Castro Braga F, Wagner H, Lombardi JA, de Oliveira AB. Screening the Brazilian flora for antihypertensive plant species for in vitro angiotensin-I-converting enzyme inhibiting activity. Phytomedicine. 2000;7:245–50. doi: 10.1016/s0944-7113(00)80011-2. [DOI] [PubMed] [Google Scholar]
- 43.Lacaille-Dubois MA, Franck U, Wagner H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine. 2001;8:47–52. doi: 10.1078/0944-7113-00003. [DOI] [PubMed] [Google Scholar]
- 44.Serra CP, Côrtes SF, Lombardi JA, Braga de Oliveira A, Braga FC. Validation of a colorimetric assay for the in vitro screening of inhibitors of angiotensin-converting enzyme (ACE) from plant extracts. Phytomedicine. 2005;12:424–32. doi: 10.1016/j.phymed.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Sentandreu MÁ, Toldrá F. A rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chem. 2006;97:546–54. [Google Scholar]
- 46.Lam le H, Shimamura T, Manabe S, Ishiyama M, Ukeda H. Assay of angiotensin I-converting enzyme-inhibiting activity based on the detection of 3-hydroxybutyrate with water-soluble tetrazolium salt. Anal Sci. 2008;24:1057–60. doi: 10.2116/analsci.24.1057. [DOI] [PubMed] [Google Scholar]
- 47.Lam le H, Shimamura T, Ishiyama M, Ukeda H. Flow injection analysis of angiotensin I-converting enzyme inhibitory activity with enzymatic reactors. Talanta. 2009;79:1130–4. doi: 10.1016/j.talanta.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 48.Lau CC, Abdullah N, Shuib AS, Aminudin N. Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J.E. Lange) Imbach identified by LC-MS/MS. Food Chem. 2014;148:396–401. doi: 10.1016/j.foodchem.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Tran HB, Yamamoto A, Matsumoto S, Ito H, Igami K, Miyazaki T, et al. Hypotensive effects and angiotensin-converting enzyme inhibitory peptides of reishi (Ganoderma lingzhi) auto-digested extract. Molecules. 2014;19:13473–85. doi: 10.3390/molecules190913473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li F, Yamaki K, Cheng Y, Fang Y. Assessment and separation of angiotensin I-converting enzyme inhibitory peptides in Chinese soypaste. Int J Food Eng. 2015;11:301–5. [Google Scholar]
- 51.Shalaby SM, Zakora M, Otte J. Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates. J Dairy Res. 2006;73:178–86. doi: 10.1017/S0022029905001639. [DOI] [PubMed] [Google Scholar]
- 52.Suetsuna K. Isolation and characterization of angiotensin I-converting enzyme inhibitor dipeptides derived from Allium sativum L (garlic) J Nutr Biochem. 1998;9:415–9. [Google Scholar]
- 53.Persson IA, Dong L, Persson K. Effect of Panax ginseng extract (G115) on angiotensin-converting enzyme (ACE) activity and nitric oxide (NO) production. J Ethnopharmacol. 2006;105:321–5. doi: 10.1016/j.jep.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 54.Loizzo MR, Saab AM, Tundis R, Menichini F, Bonesi M, Piccolo V, et al. In vitro inhibitory activities of plants used in Lebanon traditional medicine against angiotensin converting enzyme (ACE) and digestive enzymes related to diabetes. J Ethnopharmacol. 2008;119:109–16. doi: 10.1016/j.jep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Hajji M, Masmoudi O, Souissi N, Triki Y, Kammoun S, Nasri M. Chemical composition, angiotensin I-converting enzyme (ACE) inhibitory, antioxidant and antimicrobial activities of the essential oil from Periploca laevigata root barks. Food Chem. 2010;121:724–31. [Google Scholar]
- 56.Lucas-Filho MD, Silva GC, Cortes SF, Mares-Guia TR, Perpétua Ferraz V, Serra CP, et al. ACE inhibition by astilbin isolated from Erythroxylum gonocladum (Mart.). O.E. Schulz. Phytomedicine. 2010;17:383–7. doi: 10.1016/j.phymed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Rinayanti A, Radji M, Mun’im A, Suyatna FD. Screening angiotensin converting enzyme (ACE) inhibitor activity of antihypertensive Mmdicinal plants from Indonesia. Int J Pharm Teach Pract. 2013;4:527–32. [Google Scholar]
- 58.Xing Y, Liao J, Tang Y, Zhang P, Tan C, Ni H, et al. ACE and platelet aggregation inhibitors from Tamarix hohenackeri Bunge (host plant of Herba Cistanches) growing in Xinjiang. Pharmacogn Mag. 2014;10:111–7. doi: 10.4103/0973-1296.131021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simaratanamongkol A, Umehara K, Noguchi H, Panichayupakaranant P. Identification of a new angiotensin-converting enzyme (ACE) inhibitor from Thai edible plants. Food Chem. 2014;165:92–7. doi: 10.1016/j.foodchem.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 60.Simaratanamongkol A, Umehara K, Niki H. Angiotensin-converting enzyme (ACE) inhibitory activity of Solanum torvum and isolation of a novel methyl salicylate glycoside. J Funct Foods. 2014;11:557–62. [Google Scholar]
- 61.Chaudhary SK, De A, Bhadra S, Mukherjee PK. Angiotensin-converting enzyme (ACE) inhibitory potential of standardized Mucuna pruriens seed extract. Pharm Biol. 2015;53:1614–20. doi: 10.3109/13880209.2014.996820. [DOI] [PubMed] [Google Scholar]
- 62.Saputri F, Mun’im A, Lukmanto D, Aisyah S, Rinandy J. Inhibition of angiotensin converting enzyme (ACE) activity by some indonesia edible plants. Int J Pharm Sci Res. 2015;6:1054–9. [Google Scholar]
- 63.Kurniawan A, Saputri F, Ahmad I, Mun’im A. Isolation of angiotensin converting enzyme (ACE) inhibitory activity quercetin from Peperomia pellucida. Int J PharmTech Res. 2016;9:115–21. [Google Scholar]
- 64.Chang YW, Alli I. In silico assessment: Suggested homology of chickpea (Cicer arietinum L.). legumin and prediction of ACE-inhibitory peptides from chickpea proteins using BLAST and BIOPEP analyses. Food Res Int. 2012;49:477–86. [Google Scholar]
- 65.Men R, Li N, Xing Y, Tang Y, Tan C, Meng F, et al. Chemical constituents and ACE inhibitory activity of desert plant Suaeda physophora Pall. Acta Pharm Sin B. 2013;3:328–32. [Google Scholar]


