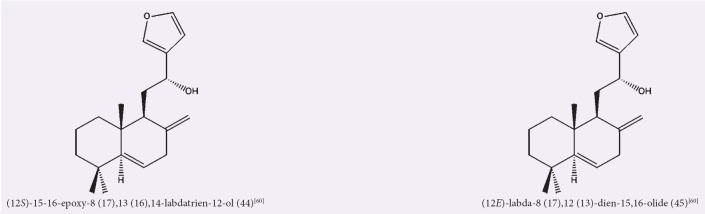Abstract
The rhizomes of Alpinia officinarum Hance have been used conventionally for the treatment of various ailments, triggering a wide interest from the scientific research community on this ethnomedicinal plant. This review summarizes the phytochemical and pharmacological properties of the extracts and fractions from A. officinarum, a plant species of the Zingiberaceae family. Different parts of the plant – leaves, roots, rhizomes, and aerial parts – have been extracted in various solvents – methanol, ethanol, ethyl acetate, hexane, dichloromethane, aqueous, chloroform, and petroleum ether, using various techniques – Soxhlet extraction, maceration, ultrasonication, and soaking, whereas fractionation of the plant extracts involves the solvent–solvent partition method. The extracts, fractions, and isolated compounds have been studied for their biological activities – antioxidant, antibacterial, anti-inflammatory, anticancer, antiproliferative, inhibition of enzymes, as well as the inhibition of nitric oxide production. More findings on A. officinarum are certainly important to further develop potential bioactive drug compounds.
Keywords: Alpinia officinarum, ethnomedicinal plant, lesser galangal, pharmacological, phytochemicals, Zingiberaceae
INTRODUCTION
Alpinia officinarum Hance, also known as lesser galangal, is indigenous to Southeast China (Guangdong, Guangxi, Hainan) and Indochina, and the plant is cultivated in the plains of West Bengal, Assam, and Eastern Himalayas in India.[1] A. officinarum belongs to the Zingiberaceae family. It is a perennial herb with thick, creeping reddish-brown rhizomes, lineolate acuminate ornamental leaves, and showy white flowers in racemes.[2] It has been used conventionally both in Ayurvedic and Chinese medicine since the very early times and in Europe since the Middle Ages.[1,3] The rhizome has been used in China for relieving stomach ache, treating colds, invigorating the circulatory system, and reducing swelling.[4] The dry root and rhizome have been used for their antioxidant, antidiabetic, antiulcer, antidiarrhea, anti-emetic, analgesic, anti-inflammatory, and anticoagulation effects.[5,6,7]
Different solvents are available to extract the bioactive compounds from natural products.[8] Various methods such as sonication, heating under reflux, Soxhlet extraction, maceration, and modern extraction techniques including supercritical fluid extraction are commonly used for plant sample extraction.[9,10,11] Alcoholic (methanol or ethanol) solutions frequently provide satisfactory results for the extraction process.[12] It is a common practice when isolating bioactive compounds that a number of different separation techniques such as thin layer chromatography, column chromatography, flash chromatography, Sephadex chromatography, and high-performance liquid chromatography (LC) are used to obtain pure compounds for the determination of structure and biological activity. Besides that, non-chromatographic techniques such as phytochemical screening assay can also be used to obtain and facilitate the identification of the bioactive compounds.[8] These compounds have been reported to possess biological activities due to the presence of various potentially active groups in their molecular structure.[12]
Diarylheptanoid (DAH) is a group of compounds found to have the potential in the development of natural products, with a special characteristic of bearing the 1,7-diphenylheptane skeleton.[13] There have been numerous DAH compounds isolated and reported for their structural characterization and biological activities.[13,14,15,16,17,17,19,20,21] Another group of compounds, polyphenols and flavonoids, are of interest because of their ability to scavenge reactive oxygen species (ROS).[22] The reduction capability of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals is determined by the decrease in their absorbance at 517 nm induced by antioxidants.[23] Many antioxidants that react quickly with peroxyl radicals may react slowly or may even be inert to DPPH.[24]
Carrageenan paw edema test is used to screen anti-inflammatory drugs as it involves the inhibition of the release and/or action of several mediators – histamine, serotonin, kinin, and prostaglandin.[25,26] The bioactive compounds within A. officinarum may also be responsible for the antiproliferative activity, which have shown to exert anticancer effects on numerous cancer cell lines.[27,28,29,30] It was reported that the galangal extracts could penetrate into the bacterial cell, causing the bacterial membrane to rupture, and resulted in bacterial death.[31] Herein we report the phytochemical and biological activities exerted by the different solvent extracts and fractions and the identified compounds of A. officinarum.
SOLVENT EXTRACTS/FRACTIONS AND ISOLATED COMPOUNDS OF ALPINIA OFFICINARUM
Methanol
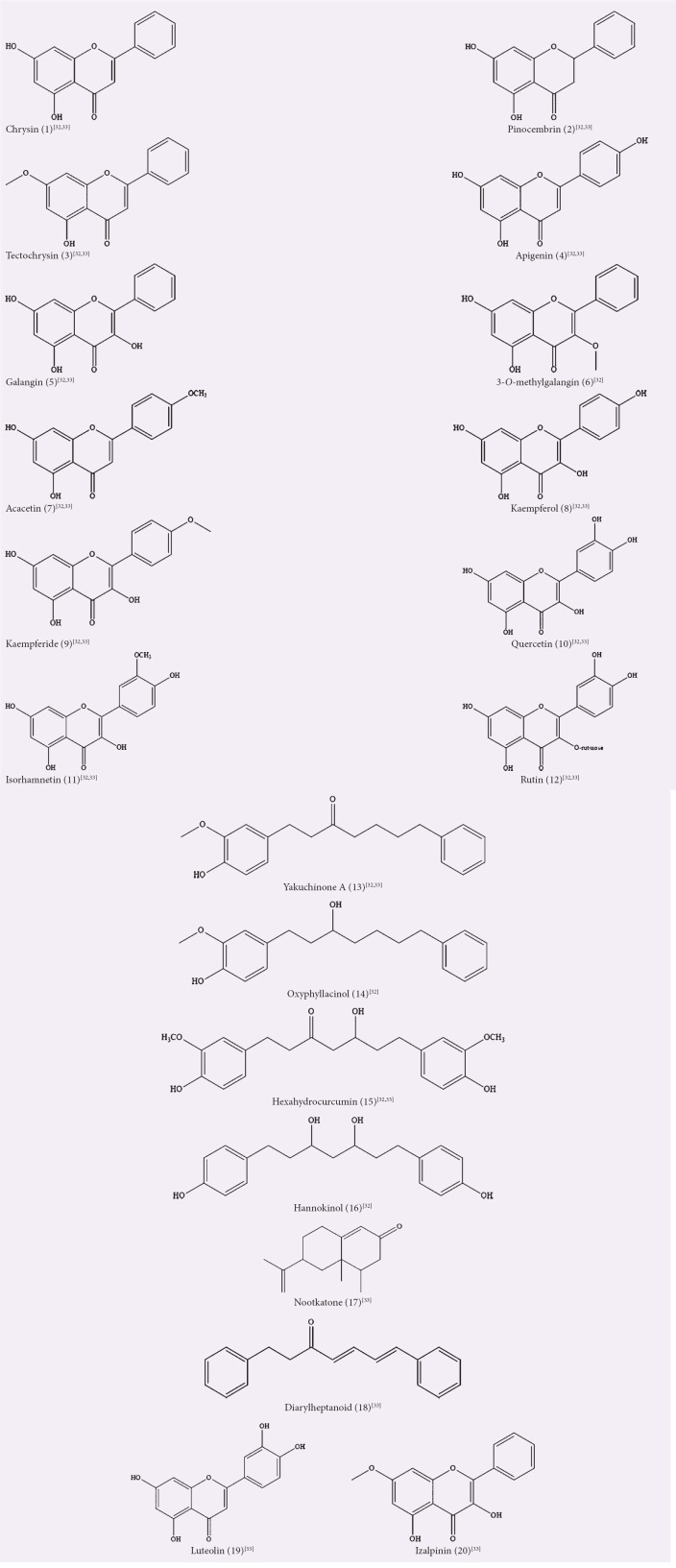
Tan et al. identified 16 chemicals consisting of 12 flavonoids and 4 DAHs from a methanol extract of A. officinarum leaves using LC-mass spectrometry (MS)/MS with a selected reaction monitoring mode.[32] The 12 flavonoids included chrysin (1), pinocembrin (2), tectochrysin (3), apigenin (4), galangin (5), 3-O-methylgalangin (6), acacetin (7), kaempferol (8), kaempferide (9), quercetin (10), isorhamnetin (11), and rutin (12). The four DAHs were yakuchinone A (13), oxyphyllacinol (14), hexahydrocurcumin (15), and hannokinol (16). In another study, they identified 17 components in the aerial parts and rhizome of a 3-year-old A. officinarum.[33] They extracted the plant material by maceration and ultrasonic extraction in methanol, and an aliquot was injected into the LC-MS/MS system. The 17 plant metabolites were compounds 1–5, 7–13, 15, nootkatone (17), DAH (18), luteolin (19), and izalpinin (20). Their study concluded that the contents of these compounds, except for compound 10, were higher in the rhizomes than in the aerial parts, and the six major constituents for both the aerial parts and rhizomes were compounds 1, 2, 5, 9, 11, 15.
Dried rhizomes of A. officinarum were extracted by maceration in methanol and were subsequently screened for in vivo anti-inflammatory and in vitro antioxidant activity.[34] The extract showed inhibition of right hind paw edema on carrageenan-induced inflammation in rats and promising free radical scavenging effect of DPPH in a concentration-dependent manner up to a concentration of 100 μg/ml. Ghil reported the ability of A. officinarum rhizome methanolic extract to inhibit cell proliferation in a dose- and time-dependent manner against human breast cancer cell line MCF-7, by promoting cell cycle arrest, hence triggering cell apoptosis.[35] In another study on the antiproliferative activity on A. officinarum leaf and rhizome, the 100% methanol extracts at a concentration of 2 mg/ml were tested against the AMoL cell line THP-1 and were reported to have significantly higher anti-proliferative activity for the leaf extract compared to the rhizome extract, with the solvent 100% methanol considered to be the least toxic extraction solvent on the cell culture, among other extraction solvents (hexane, chloroform, dichloromethane, acetone and aqueous), when tested in vitro against the cell culture.[36] Chang et al. prepared the methanol extract of A. officinarum dried rhizomes by ultrasonic extraction, which has demonstrated good antioxidant activity based on the scavenging effect on DPPH assay.[37] The roots of A. officinarum were extracted at 80°C in 70% methanol for 3 h, also displayed high DPPH radical scavenging activity in a dose-dependent manner, and effectively inhibited the lipid peroxidation in H2O2-treated V79-4 cells.[38] The summary of activities from methanol extracts/fractions and the isolated compounds, as well as their chemical structures, is shown in Table 1 and Figure 1, respectively.
Table 1.
Summary of activities from methanol extracts/fractions of Alpinia officinarum
Figure 1.
Isolated compounds from methanol extracts/fractions of Alpinia officinarum
Ethyl acetate
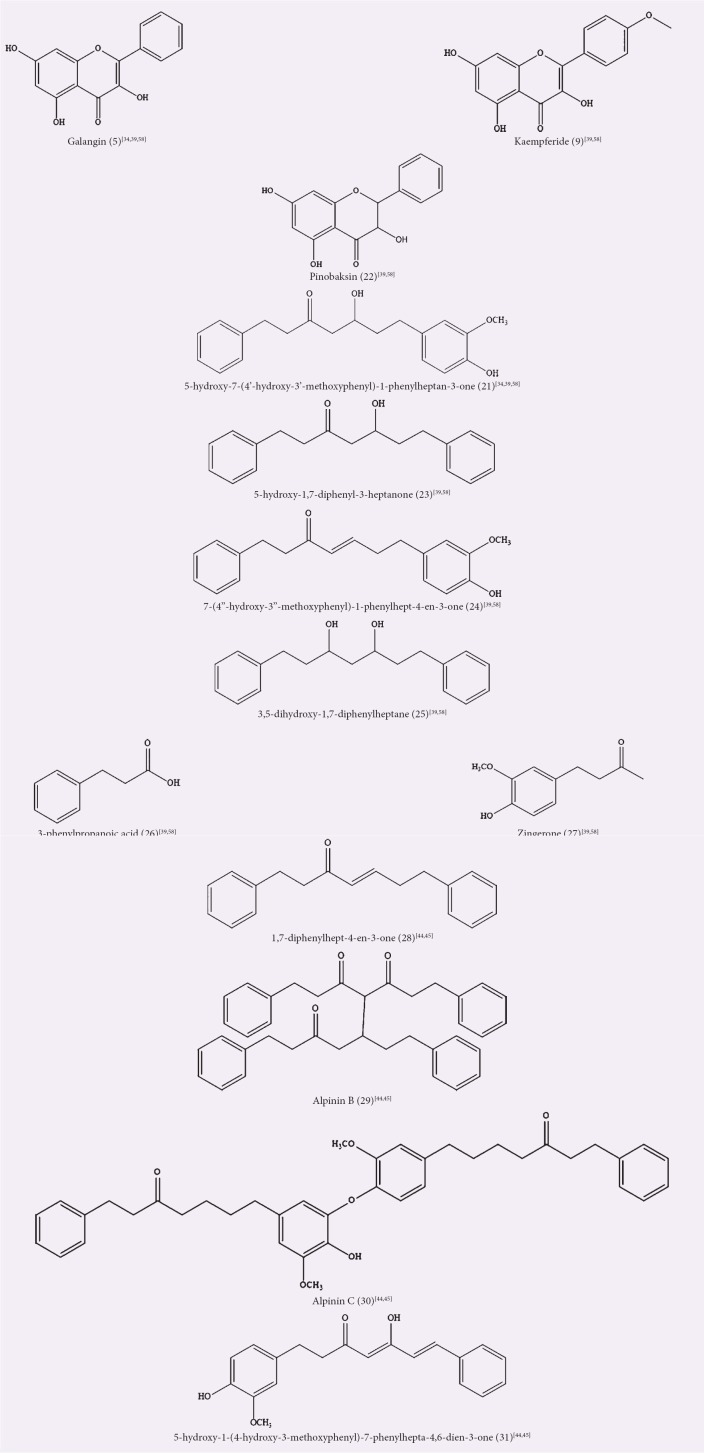
The screening of crude methanol extract of A. officinarum rhizomes for in vivo anti-inflammatory and in vitro antioxidant activity showed promising results, and the extract was further fractionated to isolate its marker compounds.[34] The ethyl acetate fraction from this extract had isolated compound 5 and 5-hydroxy-7-(4’-hydroxy-3’-methoxyphenyl)-1-phenylheptan-3-one (21) that has shown its effectiveness in acute inflammatory animal model, comparable to a clinical non-steroidal anti-inflammatory drug, diclofenac, that acts as the positive control. The compounds have displayed a significant inhibition of the increase in the carrageenan-induced paw edema in a time-dependent manner, as well as in vitro scavenging activity in a concentration-dependent manner.[34] In another study, the ethyl acetate fraction of acetone crude extract showed a more potent activity compared to other solvent extracts (acetone and aqueous) and was responsible for the isolation of compounds 5, 9, 21, pinobaksin (22), 5-hydroxy-1,7-diphenyl-3-heptanone (23), 7-(4’’-hydroxy-3’’-methoxyphenyl)-1-phenylhept-4-en-3-one (24), 3,5-dihydroxy-1,7-diphenylheptane (25), 3-phenylpropanoic acid (26), and zingerone (27). Only compounds 5, 24, and 25 showed the inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-induced murine RAW 264.7 macrophage cell line while, as melanogenesis inhibitors, compounds 5, 9, 21, 23, 24, and 25 substantially inhibited melanogenesis in theophylline-stimulated B16 melanoma 4A5 cells, and in addition, compounds 5, 9, and 24 inhibited the enzyme activity of mushroom tyrosinase.[39]
Zhao and Liu et al. reported several new DAH compounds isolated from the ethyl acetate fraction of A. officinarum dried and powdered rhizomes ethanolic extract.[40,41,42,43,44,45,46] All of the compounds were evaluated for their in vitro cytotoxic activity against several cancer cell lines. Compound 1,7-diphenylhept-4-en-3-one (28) showed cytotoxicity against the human glioblastoma T98G cell line with IC50 of 27 μmol/L, while compound alpinin B (29) was inactive against the cell lines tested (human glioblastoma T98G and B16-F10 murine melanoma cell lines).[44] Compound alpinin C (30) showed selective cytotoxicity against human breast cancer MCF-7 and human glioblastoma T98G cell lines, and compound 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylhepta-4,6-dien-3- one (31) showed significant cytotoxicity to human hepatoma HepG2, human breast cancer MCF-7, human glioblastoma T98G, and human murine melanoma B16-F10 cell lines with IC50 values of 8.46, 12.37, 22.68, and 4.44 μmol/L, respectively.[45] Table 2 and Figure 2 summarize the activities of ethyl acetate extracts/fractions and the isolated compounds and chemical structures.
Table 2.
Summary of activities from ethyl acetate extracts/fractions of Alpinia officinarum
Figure 2.
Isolated compounds from ethyl acetate extracts/fractions of Alpinia officinarum
Ethanol
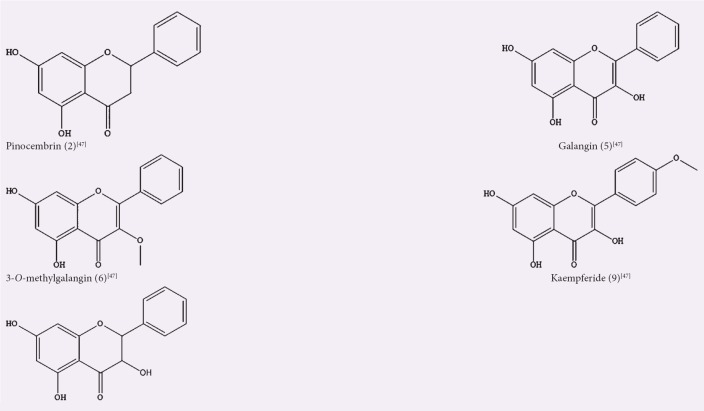
Zhang et al. identified five flavonoids which were compounds 2, 5, 6, 9, and 22 from an ethanol extract of the aerial parts of A. officinarum.[47] A Chinese patent (CN104138368A) provided a protocol for producing a purified extract from the aerial parts by ethanol extraction and subsequent purification via macroporous adsorptive resins.[48] The extract displayed antiproliferative activity via a mitochondrial pathway-induced cell apoptosis. It has been reported that A. officinarum rhizome ethanolic extract possessed potent anti-inflammatory, anticarcinogenic, antinociceptive, and antipsychiatric activities in animal model of carrageenan-induced paw edema due to the presence of DAHs.[6,49,50] Dried rhizomes of A. officinarum were powdered and extracted with 50% ethanol by either hot or cold maceration, with the former found to contain more phenol and flavonol compared to the latter.[51] The hot macerated ethanolic extract showed better antibacterial activity against Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli, compared to the cold macerated ethanolic extract. The former also showed better antioxidant activity by inhibiting DPPH-free radical with moderate reducing power when compared to ascorbic acid, as the antioxidant reference standard. However, both extracts did not show any antifungal activity against Aspergillus niger and Candida albicans.[51]
An A. officinarum ethanol extract prepared in 95% ethanol at 70°C for 6 h was shown to have more total phenolic and flavonoid contents compared to the aqueous extract although with lower free radical scavenging activity when studied for antioxidant activity using the DPPH assay.[52] In another study, A. officinarum rhizomes were soaked in 40% ethanol for 2 h, and the extract was found to inhibit the reaction of bacterial fatty acid synthase, β-ketoacyl-ACP reductase enzyme (FabG). It also showed effective inhibition against the proliferation of Gram-positive bacterial strains – S. aureus, α-Hemolytic streptococcus, β-Hemolytic streptococcus, and Streptococcus pneumoniae.[53]
Suja and Chinnaswamy prepared ethanolic extracts of A. officinarum dried roots using a Soxhlet extractor.[54] In their study, the ethanolic extract revealed the highest activity on an MTT analysis against a prostate cancer cell line PC-3, compared to the other solvent extracts (petroleum ether, chloroform, and aqueous), with the ethanolic extract displaying an effective reduction in the growth of the cancer cells. The list of activities of ethanol extracts/fractions and the isolated compounds and chemical structures are summarized in Table 3 and Figure 3, respectively.
Table 3.
Summary of activities from ethanol extracts/fractions of Alpinia officinarum
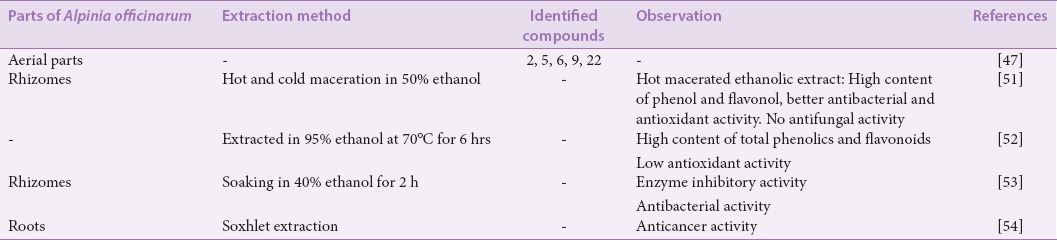
Figure 3.
Isolated compounds from ethanol extract/fraction of Alpinia officinarum
Hexane
An MTS-assay-based antiproliferative study on A. officinarum leaf and rhizome extracts, which were tested against the AMoL cell line THP-1, showed that the hexane leaf extract had distinctly higher antiproliferative activity at a concentration of 2 mg/ml compared to the hexane rhizome extract. However, when the A. officinarum leaf extract was diluted to a concentration of 0.1 mg/ml, its antiproliferative activity was reduced dramatically.[36]
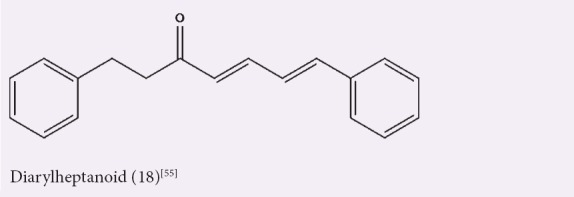
A study on the anti-inflammatory activity of A. officinarum rhizome hexane extract and its isolated compound 18 revealed the inhibition of NO production in LPS-induced murine RAW 264.7 macrophage cell line, which was found to be mediated by the inhibition of the transcriptional activity of nuclear factor-κβ, a gene regulator involved in cell proliferation, cell adhesion, and inflammatory responses.[55] The activities of hexane extracts/fractions and the chemical structures of the isolated compounds are shown in Table 4 and Figure 4, respectively.
Table 4.
Summary of activities from hexane extracts/fractions of Alpinia officinarum

Figure 4.
Isolated compounds from hexane extract/fraction of Alpinia officinarum
Dichloromethane
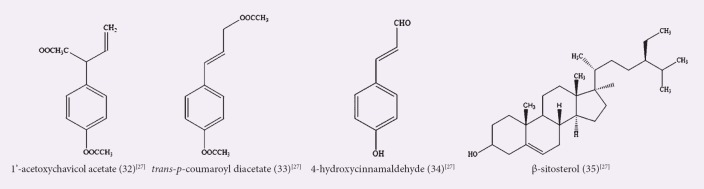
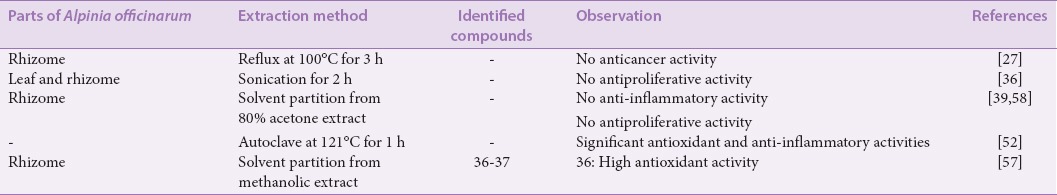
An A. officinarum dichloromethane extract showed a highly significant antiproliferative activity against AMoL THP-1 cell line using the MTS assay, with 100% cell death from both leaf and rhizome extracts, at both tested concentrations of 0.1 and 2 mg/mL and within 24 h.[36] Lee and Houghton studied the anticancer activity of A. officinarum dichloromethane rhizome extract using sulforhodamine B assay, and the extract exhibited the highest cytotoxicity against human nonsmall lung cancer COR L23 cell line following 48 h treatment, with an IC50 value of 5.4 ± 0.51 μM.[27] A number of pure compounds were isolated from the extract, which were 1’-acetoxychavicol acetate (32), trans-p-coumaroyl diacetate (33), 4-hydroxycinnamaldehyde (34), and β-sitosterol (35), and were also tested for their cytotoxic activities. Compound 32 demonstrated the highest activities, with IC50 values of 5.8 ± 0.2 μM and 8.6 ± 0.0 μM, against the COR L23 and human breast adenocarcinoma MCF7 cancer cell lines, respectively. When tested against a noncancer MCF5 cell line, compound 32 showed higher cancer cell selectivity toward the COR L23 cell line compared to the MCF7 cell line, with the selectivity factor of 2.83 and 1.91, respectively, whereas compound 35 displayed no cytotoxic activity toward all the cell lines tested.[27,56] The summary of activities displayed by dichloromethane extracts/fractions are shown in Table 5, and the chemical structures of the isolated compounds are shown in Figure 5.
Table 5.
Summary of activities from dichloromethane extracts/fractions of Alpinia officinarum

Figure 5.
Isolated compounds from dichloromethane extracts/fractions of Alpinia officinarum
Aqueous
An aqueous fraction of methanolic A. officinarum rhizome extract prepared by Ly et al. had isolated p-coumaryl alcohol (36) and 1,5-bis-(4-hydroxyphenyl)-2-(hydroxymethyl)-4-penten-1-ol (37), with the structures of the compounds as shown in Figure 6.[57] The compounds were studied to determine the antioxidant activity by autoxidation of methyl linoleate, and it was found that compound 36 has higher antioxidant activity than compound 37. Omoregie et al. reported that the ultrasonication-derived aqueous extracts of A. officinarum leaves and rhizomes, which was administered at both concentrations of 0.1 and 2 mg/mL, had no distinct antiproliferative activity against the AMoL THP-1 cell line within 24 and 48 h, in comparison with the other solvent extracts – methanol, hexane, chloroform, dichloromethane, and acetone.[36] In addition, the boiled aqueous extract of A. officinarum rhizomes was also prepared considering the traditional practice of brewed A. officinarum rhizome tea. However, the extract did not show any significant antiproliferative activity against the AMoL THP-1 cell line within 24 h. Similarly, a refluxed aqueous extract of A. officinarum rhizomes collected in Songkla, Thailand, only displayed <50% inhibition of growth against human nonsmall cell lung cancer COR L23 cell line and human breast adenocarcinoma MCF7 cell line, when tested at the highest concentration of 25 μg/mL and at 48 h exposure.[27]
Figure 6.
Isolated compounds from aqueous extracts/fractions of Alpinia officinarum
Matsuda et al. studied an aqueous fraction of A. officinarum acetone extract and reported to have no anti-inflammatory activity as there was no inhibition of NO production on LPS-induced murine RAW 264.7 macrophage cell line,[58] as well as no inhibition of melanogenesis and proliferation in B16 melanoma 4A5 cell line.[39] However, in another antioxidant and anti-inflammatory study of A. officinarum aqueous extract prepared by autoclaving with deionized water, the aqueous extract was found to have significant activities compared to the A. officinarum ethanol extract.[52] Table 6 shows the summary of activities by aqueous extracts/fractions and the isolated compounds.
Table 6.
Summary of activities from aqueous extracts/fractions of Alpinia officinarum
Chloroform
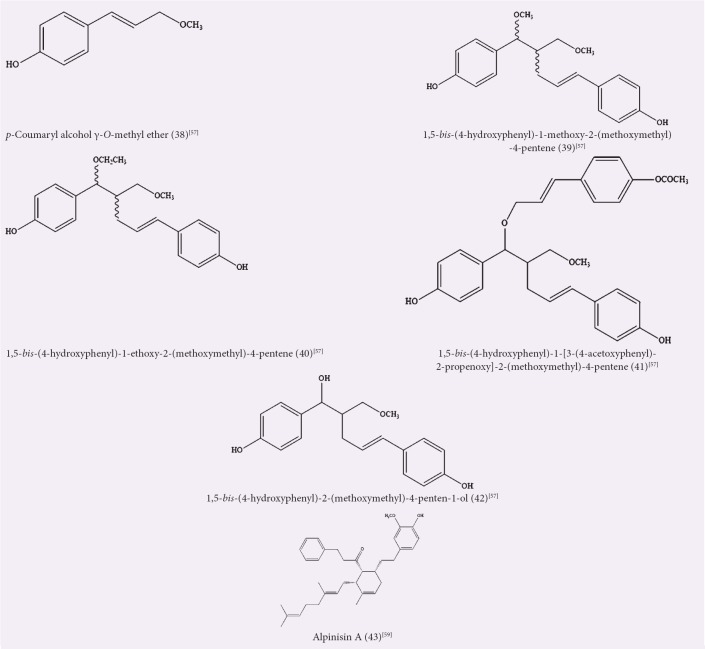
Ly et al. reported the isolation of compounds from the chloroform fraction of methanolic A. officinarum rhizome extract, which were used for antioxidant studies by autoxidation of methyl linoleate.[57] These compounds were p-coumaryl alcohol g-O-methyl ether (38), 1,5-bis-(4-hydroxyphenyl)-1-methoxy-2-(methoxymethyl)-4-pentene (39),1,5-bis-(4-hydroxyphenyl)-1-ethoxy-2-(methoxymethyl)-4- pentene(40),1,5-bis-(4-hydroxyphenyl)-1-[3-(4-acetoxyphenyl)- 2-propenoxy]- 2-(methoxymethyl)-4-pentene (41), and 1,5-bis- (4-hydroxyphenyl)-2-(methoxymethyl)-4-penten-1-ol (42). Compound 38 was shown to have the highest antioxidant, whereas compounds 39–42 exhibited lower antioxidant activities than that of a-tocopherol, an antioxidant reference standard. Figure 7 shows the structure of the compounds isolated from the chloroform fraction.
Figure 7.
Isolated compounds from chloroform extracts/fractions of Alpinia officinarum
Wei et al. isolated a new DAH compound, alpinisin A (43), from the chloroform fraction of A. officinarum rhizome ethyl acetate extract.[59] The cytotoxicity of the compound was tested by MTT assay against human gastric cancer SGC-7901, human breast cancer MCF-7, and cervical carcinoma Caski cell lines, and it was shown that the compound possessed anticancer activities and had a significant inhibitory effect with IC50 values of 11.42, 15.14, and 14.78 μM, respectively. In a separate study, chloroform extracts of A. officinarum leaves and rhizomes showed a very high antiproliferative activity against AMoL THP-1 cell line with 100% cell death, at both concentrations of 0.1 and 2 mg/mL within 24 h.[36] Table 7 summarizes the activities observed from chloroform extracts/fractions and the isolated compounds.
Table 7.
Summary of activities from chloroform extracts/fractions of Alpinia officinarum

Petroleum ether
Wen et al. isolated two novel diterpene compounds, as shown in Figure 8, from the rhizomes of A. officinarum through a petroleum ether fraction of 95% ethanol rhizome extract.[60] Compounds (12S)-15–16-epoxy-8 (17),13 (16),14-labdatrien-12-ol (44), and (12E)-labda-8 (17),12 (13)-dien-15,16-olide (45) were shown to exhibit strong anti-inflammatory effect and antioxidant activity in vitro. The activities and chemical structures are summarized in Table 8 and Figure 8, respectively.
Figure 8.
Isolated compounds from petroleum ether extracts/fractions of Alpinia officinarum
Table 8.
Summary of activities from petroleum ether extracts/fractions of Alpinia officinarum

DISCUSSION AND FUTURE DIRECTIONS
A. officinarum has been traditionally used for the treatment of various ailments. This review has presented a wide range of supporting scientific results to validate the traditional usage of A. officinarum as herbal medicine. The screening of A. officinarum solvent extracts revealed a high proportion of various biological activities that include antioxidant, antibacterial, anti-inflammatory, anti-cancer, anti-proliferative, enzyme inhibition, as well as, the inhibition of NO production. This proves that A. officinarum is enriched with bioactive chemical constituents, laying a solid foundation for its pharmacological research.
Many studies have focused on the rhizomes of A. officinarum, due to its known traditional uses for medicinal purposes such as to relieve fever and stomach ache. Consequently, other parts of the plant, which may have valuable potential, are being disposed of as waste while only its rhizomes are being collected. Therefore, to maximize the use of this medicinal plant species, it would be useful to carry out studies on the other parts of the plant such as the aerial parts, roots, and leaves, which could also contain potential bioactive metabolites. Several previous studies have successfully identified the bioactive compounds in A. officinarum as were summarized in this review. Higher percentage of compounds were found in the rhizomes than in the aerial parts of A. officinarum.[33] The identified compounds in this review are mainly found to be DAH, flavonoids, phytosterols, and terpenes. A few recent studies on novel dimeric DAH compounds 29 and 30 were reported showing only selective or inactive cytotoxicity that could be due to the lack of its α, β-unsaturation in its molecular structure.[45] An α, β-unsaturated unit is defined as the pi bond between the α and β carbons adjacent to the carbonyl (=CO) group[61] and is often employed as an active moiety in the design of enzyme inhibitors.[62] Further biological studies on the novel compounds are essential to reveal other potential bioactivities of the new dimeric DAH compounds.
Molecules that consist of phenolic hydroxyl groups are believed to act as antioxidants due to their hydrogen donating ability[23,63,64,65,66,67,68] and as prooxidants that contributes towards their anticancer activities.[69,70] A study in this review revealed the differences in the antioxidant behavior of phenylpropanoid compounds 36–42, and the results suggested that their activities might be influenced by the number of hydroxyl groups that were present in the molecule.[57] Galangin, compound 5, a flavonol of flavonoids, appears to be the predominant constituent in all parts of A. officinarum – leaves, aerial parts, and rhizomes, showing anti-inflammatory, antioxidant, and anticancer properties, as well as the inhibition of NO production and enzymes.[32,33,34,39,47,58,71,72,73] There have been much studies on the compound galangin;[74,75,76,77,78,79,80,81] however, its molecular mechanism is still unknown. It has been reported that flavonoids and terpenes act by inhibiting the cytoplasmic membrane functions, such as altering the influx of calcium, hence promoting the disruption of the cellular membrane.[21,82,83,84,85] It has also been reported that phenolics and flavonoids are able to enter the hydrophobic layer of the cell membrane, causing the disruption of the membrane’s lipid packing.[85,86,87] It would be useful to carry out further studies to investigate the efficacy of galangin and elucidate its mechanism (s) of action that underlie the observed pharmacological effects, as well as to reflect the traditional uses of A. officinarum.
Some of the A. officinarum isolated compounds even showed discriminatory tolerance against normal cells, especially compound 32, isolated from the rhizome dichloromethane extract of A. officinarum,[27] in which the 1’-acetoxyl group in the chavicol analog was found to majorly contribute toward its cytotoxic activity.[88,89] The ideal drug candidate would be those that are found to be selective and only target a specific region within the human body, as well as to not cause genetic and chromosomal aberrations that could lead to toxicity and unwanted side effects. This finding is, therefore, a promising step in the search for a safe treatment and management of patients in cancer therapy. By understanding the mechanisms of the biologically active compounds toward their respective therapeutic potentials, it will be able to support in preventing the possible adverse effects of the compounds, hence maximizing their medicinal benefit.
Bioassay-guided fractionation and isolation have been the most widely used approach for evidence-based pharmacological in vitro and in vivo studies, where each solvent extract/fraction is investigated for their potential biological activities. The studies on the different solvent extracts/fractions may lead to the identification of novel compounds in the field of pharmaceutical medicine. Various solvents were used for the extraction of A. officinarum as reported in this review. It can be seen that methanol is found to be the most preferred solvent used as the initial crude solvent extraction before they are further fractionated using other solvents. Methanol is also considered to be the least toxic extraction solvent toward an in vitro cell culture line,[36] indicating that the observed anticancer activities were not due to the interference from the methanol solvent itself.
Many studies have also been done on the aqueous A. officinarum extracts as to mimic the traditional practice of brewing the rhizomes of the plant for tea consumption; however, in contrast to the methanol extracts, the aqueous extracts were shown to exert the least biological activity, showing no presence of anticancer, antiproliferative, and anti-inflammatory activities. Previous studies have shown that some plant species extracted using organic solvents were found to give more consistent scientific results when compared to their aqueous extracts.[90] Furthermore, some water-soluble compounds, such as flavonoids and phenolics, only showed either selective or no significant biological activities at all.[91,92] However, depending on the extraction method,[93] significant antioxidant and anti-inflammatory activity could also be observed.[52] It was revealed that the plant materials extracted by either shaking or refluxing in a hydroalcoholic solvent system gave higher yields, with higher phenolic contents and better antioxidant activities compared to when using a 100% aqueous or 100% alcoholic solvent.[6,38,51,53,94,95] The results could also suggest that the aqueous extracts might contain different components of bioactive compounds compared to the contents of the other solvent extracts, or the various extracts may contain similar compounds, however, with different concentrations, hence leading to different values of their biological activities.
There has not been much work specifically on A. officinarum petroleum ether extract, and also the studies on the isolation of diterpene compounds are very rare. Further studies could be done on the solvent extract to further clarify the chemical compositions of A. officinarum, especially diterpenes, as well as to discover new biologically active compounds. Two novel labdane diterpenes have been shown to have strong anti-inflammatory activities that could be linked with the inhibition of ROS.[60] The production of ROS can cause damage to cells and tissues, activating oxidative stress, and triggering inflammation.[96,97,98] This leads to several disorders that include inflammatory, cardiovascular, and neurodegenerative diseases that have shown a significant increase in their occurrence worldwide.[99,100,101] This finding warrants further study on the two labdane diterpenes, as well as other bioactive plant constituents in A. officinarum especially in relation to both antioxidant and anti-inflammatory activities due to its potential application in disease treatment. Moreover, toxicity and immunological studies of A. officinarum are also beneficial to further authenticate the traditional claims of the uses of the plant species, as well as for the potential of clinical drug development.
CONCLUSION
We have reviewed the phytochemical and biological activities exerted by the different solvent extracts and fractions, as well as the isolated and identified compounds of A. officinarum. Most solvent extracts had shown significant biological activities, and a few novel compounds had been successfully isolated. There have been much studies on the methanol extract of this plant species, which were used for crude extraction before further fractionation using other solvents. The scientific results have provided evidence to support the traditional uses of A. officinarum in the treatments of various diseases, as well as to offer new therapeutic possibilities, such as antioxidant, anti-inflammatory, anticancer, and antimicrobial activities. These pharmacological activities are mainly exerted by the bioactive metabolites of the plant species. Flavonoids, DAHs, and terpenes were among the compounds isolated, and some were found to have significant biological activities, as well as being selective that shows good potential as natural drug candidates.
Financial support and sponsorship
The study was supported by the Department of Economic Planning and Development (JPKE) through Brunei Research Council grant (UBD/BRC/6) and Universiti Brunei Darussalam.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lim TK. Edible Medicinal and Non-Medicinal Plants: Modified Stems, Roots, Bulbs. Vol. 12. London: Springer; 2002. p. 178. [Google Scholar]
- 2.Daniel M. Medicinal Plants: Chemistry and Properties. Enfield: Science Publishers; 2006. p. 63. [Google Scholar]
- 3.Bown D. Encyclopedia of Herbs and Their Uses. London: Dorling Kindersley; 1995. p. 424. [Google Scholar]
- 4.Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Press; 2005. The State Pharmacopoeia Commission of the People’s Republic of China; p. 202. [Google Scholar]
- 5.Mayachiew P, Devahastin S. Antimicrobial and antioxidant activities of Indian gooseberry and galangal extracts. Food Sci Technol. 2008;41:1153–9. [Google Scholar]
- 6.Lee J, Kim KA, Jeong S, Lee S, Park HJ, Kim NJ, et al. Anti-inflammatory, anti-nociceptive, and anti-psychiatric effects by the rhizomes of Alpinia officinarum on complete Freund’s adjuvant-induced arthritis in rats. J Ethnopharmacol. 2009;126:258–64. doi: 10.1016/j.jep.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Xie ZS, Xu XJ, Xie CY, Huang JY, Yang M, Yang DP. Volatile components of rhizoma Alpinia officinarum using three different extraction methods combined with gas chromatography-mass spectrometry. J Pharm Anal. 2013;3:215–20. doi: 10.1016/j.jpha.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handa SS, Khanuja SP, Longo G, Rakesh DD. Extraction Technologies for Medicinal and Aromatic Plants. Italy: ICS-UNIDO; 2008. [Google Scholar]
- 9.Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’extracts. Afr J Tradit Complement Altern Med. 2011;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Jones WP, Kinghorn AD. Extraction of plant secondary metabolites. Methods Mol Biol. 2012;864:341–66. doi: 10.1007/978-1-61779-624-1_13. [DOI] [PubMed] [Google Scholar]
- 11.Azwanida NN. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants. 2015;4:196. [Google Scholar]
- 12.Xu R, Ye Y, Zhao W. Introduction to Natural Products Chemistry. Florida: Taylor and Francis Group; 2012. [Google Scholar]
- 13.Lv H, She G. Naturally occurring diarylheptanoids. Nat Prod Commun. 2010;5:1687–708. [PubMed] [Google Scholar]
- 14.Itokawa H, Morita M, Mihashi S. Two new diarylheptanoids from Alpinia officinarum. Chem Pharm Bull. 1981;29:2383–5. [Google Scholar]
- 15.Itokawa H, Morita H, Midorikawa I, Aiyama R, Morita M. Diarylheptanoids from the rhizome of Alpinia officinarum Hance. Chem Pharm Bull. 1985;33:4889–93. [Google Scholar]
- 16.Uehara SI, Yasuda I, Akiyama K, Morita H, Takeya K, Itokawa H. Diarylheptanoids from the rhizomes of Curcuma xanthorrhiza and Alpinia officinarum. Chem Pharm Bull. 1987;35:3298–304. [Google Scholar]
- 17.Claeson P, Tuchinda P, Reutrakul V. Naturally occurring 1,7-diarylheptanoids. J Indian Chem Soc. 1994;71:509–21. [Google Scholar]
- 18.Claeson P, Claeson UP, Tuchinda P, Reutrakul V. Occurrence, structure and bioactivity of 1,7-diarylheptanoids. Stud Nat Prod Chem. 2002;26:881–908. [Google Scholar]
- 19.Keserü GM, Nógrádi M. The chemistry of natural diarylheptanoids. Stud Nat Prod Chem. 1995;17:357–94. [Google Scholar]
- 20.Lv H, She G. Naturally occurring diarylheptanoids – A supplementary version. Rec Nat Prod. 2012;6:321–33. [Google Scholar]
- 21.Reid K, Wright V, Omoregie S. Anticancer properties of Alpinia officinarum (lesser galangal) – A mini review. Int J Adv Res. 2016;4:300–6. [Google Scholar]
- 22.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 23.Baumann J, Wurn G, Bruchlausen FV. Prostaglandin synthetase inhibiting O2-radical scavenging properties of some flavonoids and related phenolic compounds. Naunyn Schmiedebergs Arch Pharmacol. 1979;308:27–32. [Google Scholar]
- 24.Ghaisas M, Navghare V, Takawale A, Zope V, Deshpande A. In vitro antioxidant activity of Tectona grandis Linn. Pharmacol Online. 2008;3:296–305. [Google Scholar]
- 25.Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 26.Mazzanti G, Braghiroli L. Analgesic anti-inflammatory action of pfaffia paniculata (martius) kuntze. Phytother Res. 1994;8:413–6. [Google Scholar]
- 27.Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100:237–43. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 28.An N, Zou ZM, Tian Z, Luo XZ, Yang SL, Xu LZ. Diarylheptanoids from the rhizomes of Alpinia officinarum and their anticancer activity. Fitoterapia. 2008;79:27–31. doi: 10.1016/j.fitote.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Tabata K, Yamazaki Y, Okada M, Fukumura K, Shimada A, Sun Y, et al. Diarylheptanoids derived from Alpinia officinarum induce apoptosis, S-phase arrest and differentiation in human neuroblastoma cells. Anticancer Res. 2009;29:4981–8. [PubMed] [Google Scholar]
- 30.Zhang HT, Wu J, Wen M, Su LJ, Luo H. Galangin induces apoptosis in hepatocellular carcinoma cells through the caspase 8/t-Bid mitochondrial pathway. J Asian Nat Prod Res. 2012;14:626–33. doi: 10.1080/10286020.2012.682152. [DOI] [PubMed] [Google Scholar]
- 31.Rawlings M, Cronan JE., Jr The gene encoding Escherichia coli acyl carrier protein lies within a cluster of fatty acid biosynthetic genes. J Biol Chem. 1992;267:5751–4. [PubMed] [Google Scholar]
- 32.Tan YF, Li HL, Li YB, Li YH, Lai WY, Wang Y, et al. Identification of chemical constituents occurring in leaves of Alpinia officinarum. Chin J Exp Tradit Med Formulae. 2015;3:37–40. [Google Scholar]
- 33.Zhang JQ, Wang Y, Li HL, Wen Q, Yin H, Zeng NK, et al. Simultaneous quantification of seventeen bioactive components in rhizome and aerial parts of Alpinia officinarum Hance using LC-MS/MS. Anal Methods. 2015;7:4919. [Google Scholar]
- 34.Honmore VS, Kandhare AD, Kadam PP, Khedkar VM, Sarkar D, Bodhankar SL, et al. Isolates of Alpinia officinarum Hance as COX-2 inhibitors: Evidence from anti-inflammatory, antioxidant and molecular docking studies. Int Immunopharmacol. 2016;33:8–17. doi: 10.1016/j.intimp.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Ghil S. Antiproliferative activity of Alpinia officinarum extract in the human breast cancer cell line MCF-7. Mol Med Rep. 2013;7:1288–92. doi: 10.3892/mmr.2013.1305. [DOI] [PubMed] [Google Scholar]
- 36.Omoregie SN, Omoruyi FO, Wright VF, Jones L, Zimba PV. Antiproliferative activities of lesser galangal (Alpinia officinarum Hance Jam1), turmeric (Curcuma longa L.). and ginger (Zingiber officinale Rosc.) against acute monocytic leukemia. J Med Food. 2013;16:647–55. doi: 10.1089/jmf.2012.0254. [DOI] [PubMed] [Google Scholar]
- 37.Chang CL, Lin CS, Lai GH. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/984295. 984295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–79. doi: 10.1016/s0024-3205(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 39.Matsuda H, Nakashima S, Oda Y, Nakamura S, Yoshikawa M. Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg Med Chem. 2009;17:6048–53. doi: 10.1016/j.bmc.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L, Liang JY, Zhang JY, Chen Y. A novel diarylheptanoid bearing flavonol moiety from the rhizomes of Alpinia officinarum Hance. Chin Chem Lett. 2010;21:194–6. [Google Scholar]
- 41.Zhao L, Qu W, Fu JQ, Liang JY. A new diarylheptanoid from the rhizomes of Alpinia officinarum. Chin J Nat Med. 2010;8:241–3. doi: 10.1016/S1875-5364(14)60022-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, Liang JY, Qu W. A novel dimeric diarylheptanoid from the rhizomes of Alpinia officinarum. Chem Nat Compd. 2012;48:8368. doi: 10.1016/S1875-5364(14)60022-4. [DOI] [PubMed] [Google Scholar]
- 43.Liu D, Qu W, Zhao L, Liang JY. A novel dimeric diarylheptanoid from the rhizomes of Alpinia officinarum. Chin Chem Lett. 2012;23:189–92. doi: 10.1016/S1875-5364(14)60022-4. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Qu W, Zhao L, Guan FQ, Liang JY. A new dimeric diarylheptanoid from the rhizomes of Alpinia officinarum. Chin J Nat Med. 2014;12:139–41. doi: 10.1016/S1875-5364(14)60022-4. [DOI] [PubMed] [Google Scholar]
- 45.Liu D, Liu YW, Guan FQ, Liang JY. New cytotoxic diarylheptanoids from the rhizomes of Alpinia officinarum Hance. Fitoterapia. 2014;96:76–80. doi: 10.1016/j.fitote.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Liu D, Liang JY, Liu YW. A new diarylheptanoid from the rhizomes of Alpinia officinarum. Chem Nat Compd. 2016;52:824–6. [Google Scholar]
- 47.Zhang H, Xu LX, Wu P, Wei XY. Flavonoids from the Aerial Parts of Alpinia officinarum. J Trop Subtrop Bot. 2014;22:89–92. [Google Scholar]
- 48.CN104138368A. Preparation method and cancer treatment effect of rhizoma Alpiniae officinarum aboveground part AO-95;12 November. 2014 [Google Scholar]
- 49.Yadav PN, Liu Z, Rafi MM. A diarylheptanoid from lesser galangal (Alpinia officinarum) inhibits pro-inflammatory mediators viainhibition of mitogen-activated protein kinase, p44/42, and transcription factor nuclear factor-kappa B. J Pharmacol Exp Ther. 2003;305:925–31. doi: 10.1124/jpet.103.049171. [DOI] [PubMed] [Google Scholar]
- 50.Yasukawa K, Sun Y, Kitanaka S, Tomizawa N, Miura M, Motohashi S. Inhibitory effect of the rhizomes of Alpinia officinarum on TPA-induced inflammation and tumor promotion in two-stage carcinogenesis in mouse skin. J Nat Med. 2008;62:374–8. doi: 10.1007/s11418-008-0243-2. [DOI] [PubMed] [Google Scholar]
- 51.Srividya AR, Dhanabal SP, Misra VK, Suja G. Antioxidant and antimicrobial activity of Alpinia officinarum. Indian J Pharm Sci. 2010;72:145–8. doi: 10.4103/0250-474X.62233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern Med. 2012;12:173. doi: 10.1186/1472-6882-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H, Wu D, Tian WX, Ma XF, Wu XD. Antimicrobial effect by extracts of rhizome of Alpinia officinarum Hance may relate to its inhibition of beta-ketoacyl-ACP reductase. J Enzyme Inhib Med Chem. 2008;23:362–8. doi: 10.1080/14756360701622099. [DOI] [PubMed] [Google Scholar]
- 54.Suja S, Chinnaswamy P. Inhibition of in vitro cytotoxic effect evoked by Alpinia galanga and Alpinia officinarum on PC – 3 cell line. Anc Sci Life. 2008;27:33–40. [PMC free article] [PubMed] [Google Scholar]
- 55.Rajaganapathy BR, Thirugnanam K, Shanmuganathan MV, Singaravelu A, Subadhra LB. Molecular basis of the anti-inflammatory potential of a diarylheptanoid in murine macrophage RAW 264.7 cells. Adv Biol Chem. 2013;3:541–8. [Google Scholar]
- 56.Jackson SJ, Houghton PJ, Retsas S, Photiou A. In vitro cytotoxicity of norviburtinal and isopinnatal from Kigelia pinnata against cancer cell lines. Planta Med. 2000;66:758–61. doi: 10.1055/s-2000-9778. [DOI] [PubMed] [Google Scholar]
- 57.Ly TN, Shimoyamada M, Kato K, Yamauchi R. Isolation and characterization of some antioxidative compounds from the rhizomes of smaller galanga (Alpinia officinarum Hance) J Agric Food Chem. 2003;51:4924–9. doi: 10.1021/jf034295m. [DOI] [PubMed] [Google Scholar]
- 58.Matsuda H, Ando S, Kato T, Morikawa T, Yoshikawa M. Inhibitors from the rhizomes of Alpinia officinarum on production of nitric oxide in lipopolysaccharide-activated macrophages and the structural requirements of diarylheptanoids for the activity. Bioorg Med Chem. 2006;14:138–42. doi: 10.1016/j.bmc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Wei N, Zhou Z, Wei Q, Wang Y, Jiang J, Zhang J, et al. Anovel diarylheptanoid-bearing sesquiterpene moiety from the rhizomes of Alpinia officinarum. Nat Prod Res. 2016;30:2344–9. doi: 10.1080/14786419.2016.1185716. [DOI] [PubMed] [Google Scholar]
- 60.Wen T, Wang XK, Liu C, Liu H. Two anti-inflammatory diterpenes from the rhizomes of Alpinia officinarum Hance. J Pharm Biomed Sci. 2016;6:479–82. [Google Scholar]
- 61.Smith MB, March J. Advanced Organic Chemistry: Reactions, Mechanisms and Structure. 6th ed. New York: Wiley-Interscience; 2007. [Google Scholar]
- 62.Janser RF, Meka RK, Bryant ZE, Adogla EA, Vogel EK, Wharton JL, et al. Ethacrynic acid analogues lacking the alpha, beta-unsaturated carbonyl unit – potential anti-metastatic drugs. Bioorg Med Chem Lett. 2010;20:1848–50. doi: 10.1016/j.bmcl.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crouzet J, Chassagne D. Glycosidically bound volatiles in plants. In: Ikan R, editor. Naturally Occurring Glycosides. Chichester, England: John Wiley and Sons Ltd; 1999. pp. 225–74. [Google Scholar]
- 64.Ly TN, Yamauchi R, Shimoyamada M, Kato K. Isolation and structural elucidation of some glycosides from the rhizomes of smaller galanga (Alpinia officinarum Hance) J Agric Food Chem. 2002;50:4919–24. doi: 10.1021/jf025529p. [DOI] [PubMed] [Google Scholar]
- 65.Brewer MS. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf. 2011;10:221–47. [Google Scholar]
- 66.Ali HM, Abo-Shady A, Sharaf Eldeen HA, Soror HA, Shousha WG, Abdel-Barry OA, et al. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem Cent J. 2013;7:53. doi: 10.1186/1752-153X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bendary E, Francis RR, Ali HM, Sarwat MI, El Hady S. Antioxidant and structure-activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci. 2013;58:173–81. [Google Scholar]
- 68.Nimse SB, Pal D. Free radicals, natural antioxidants and their reaction mechanisms. RSC Adv. 2015;5:27986–8006. [Google Scholar]
- 69.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–46. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han M, Song Y, Zhang X. Quercetin suppresses the migration and invasion in human colon cancer Caco-2 cells through regulating toll-like receptor 4/nuclear factor-kappa B pathway. Pharmacogn Mag. 2016;12(Suppl 2):S237–44. doi: 10.4103/0973-1296.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li BH, Tian W. Presence of fatty acid synthase inhibitors in the rhizome of Alpinia officinarum hance. J Enzyme Inhib Med Chem. 2003;18:349–56. doi: 10.1080/1475636031000118419. [DOI] [PubMed] [Google Scholar]
- 72.Deng YF, Feng LN, Luo H. Determination of the content of galangin in Alpinia officinarum Hance harvested in different months by RP-HPLC. Chin Pharm J. 2010;45:1593–6. [Google Scholar]
- 73.Zhai HL, Li Q, Wang H, Liang DJ, Zeng YB, Cai CH, et al. Analysis of active constituents of Alpinia officinarum Hance from different localities of Hainan province. J Trop Biol. 2014;2:188–93. [Google Scholar]
- 74.Lu YH, Lin-Tao Wang ZT, Wei DZ, Xiang HB. Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J Enzyme Inhib Med Chem. 2007;22:433–8. doi: 10.1080/14756360601141562. [DOI] [PubMed] [Google Scholar]
- 75.Lee YS, Kang OH, Choi JG, Oh YC, Chae HS, Kim JH, et al. Synergistic effects of the combination of galangin with gentamicin against methicillin-resistant Staphylococcus aureus. J Microbiol. 2008;46:283–8. doi: 10.1007/s12275-008-0012-7. [DOI] [PubMed] [Google Scholar]
- 76.Guo AJ, Xie HQ, Choi RC, Zheng KY, Bi CW, Xu SL, et al. Galangin, a flavonol derived from rhizoma Alpiniae officinarum inhibits acetylcholinesterase activity in vitro. Chem Biol Interact. 2010;187:246–8. doi: 10.1016/j.cbi.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 77.Ha TK, Kim ME, Yoon JH, Bae SJ, Yeom J, Lee JS. Galangin induces human colon cancer cell death via the mitochondrial dysfunction and caspase-dependent pathway. Exp Biol Med (Maywood) 2013;238:1047–54. doi: 10.1177/1535370213497882. [DOI] [PubMed] [Google Scholar]
- 78.Zhang W, Lan Y, Huang Q, Hua Z. Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology. 2013;65:447–55. doi: 10.1007/s10616-012-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huo SX, Liu XM, Ge CH, Gao L, Peng XM, Zhao PP, et al. The effects of galangin on a mouse model of vitiligo induced by hydroquinone. Phytother Res. 2014;28:1533–8. doi: 10.1002/ptr.5161. [DOI] [PubMed] [Google Scholar]
- 80.Zhu L, Luo Q, Bi J, Ding J, Ge S, Chen F. Galangin inhibits growth of human head and neck squamous carcinoma cells in vitro and in vivo. Chem Biol Interact. 2014;224:149–56. doi: 10.1016/j.cbi.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 81.Cao J, Wang H, Chen F, Fang J, Xu A, Xi W, et al. Galangin inhibits cell invasion by suppressing the epithelial-mesenchymal transition and inducing apoptosis in renal cell carcinoma. Mol Med Rep. 2016;13:4238–44. doi: 10.3892/mmr.2016.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spencer JP. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2:257–73. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mendanha SA, Moura SS, Anjos JL, Valadares MC, Alonso A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol In Vitro. 2013;27:323–9. doi: 10.1016/j.tiv.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 84.Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem. 2015;22:132–49. doi: 10.2174/0929867321666140916113443. [DOI] [PubMed] [Google Scholar]
- 85.Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn Rev. 2016;10:84–9. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erlejman AG, Verstraeten SV, Fraga CG, Oteiza PI. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res. 2004;38:1311–20. doi: 10.1080/10715760400016105. [DOI] [PubMed] [Google Scholar]
- 87.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013. 2013 doi: 10.1155/2013/162750. 162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Itokawa H, Morita H, Sumitomo T, Totsuka N, Takeya K. Antitumour principles from Alpinia galanga. Planta Med. 1987;53:32–3. doi: 10.1055/s-2006-962611. [DOI] [PubMed] [Google Scholar]
- 89.Murakami A, Toyota K, Ohura S, Koshimizu K, Ohigashi H. Structure-activity relationships of (1-S’)-1’-acetoxychavicol acetate, a major constituent of a Southeast Asian condiment plant Languas galangal on the inhibition of tumor-promoter-induced Epstein-Barr virus activation. J Agric Food Chem. 2000;48:1518–23. doi: 10.1021/jf990528r. [DOI] [PubMed] [Google Scholar]
- 90.Parekh J, Jadeja D, Chanda S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk J Biol. 2005;29:203–10. [Google Scholar]
- 91.Yamaji K, Ishimoto H, Usui N, Mori S. Organic acids and water-soluble phenolics produced by Paxillus sp 60/92 together show antifungal activity against Pythium vexans under acidic culture conditions. Mycorrhiza. 2005;15:17–23. doi: 10.1007/s00572-003-0287-9. [DOI] [PubMed] [Google Scholar]
- 92.Nang HL, May CY, Ngan MA, Hock CC. Extraction and identification of water soluble compounds in palm pressed fiber by SC-CO2and GC-MS. Am J Environ Sci. 2007;3:54–9. [Google Scholar]
- 93.Das K, Tiwari RK, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J Med Plants Res. 2010;4:104–11. [Google Scholar]
- 94.Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghosh S, Rangan L. Alpinia: The gold mine of future therapeutics. 3 Biotech. 2013;3:173. doi: 10.1007/s13205-012-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rimessi A, Previati M, Nigro F, Wieckowski MR, Pinton P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int J Biochem Cell Biol. 2016;81(Pt B):281–93. doi: 10.1016/j.biocel.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 97.Urquiza-Martinez MV, Navarro BF. Antioxidant capacity of food. Free Radic Antioxid. 2016;6:1–12. [Google Scholar]
- 98.Ojo OA, Ajiboye B, Fadaka A, Taro P, Shariati MA. Nrf2-Keap1 activation, a promising strategy in the prevention of cancer. Frees Radic Antioxid. 2017;7:1–7. [Google Scholar]
- 99.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–67. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015. 2015 doi: 10.1155/2015/610813. 610813. [DOI] [PMC free article] [PubMed] [Google Scholar]