Abstract
Osteoarthritis (OA) is a chronic inflammatory degenerative process that affects joints such as the hands, hips, shoulders, feet, spine, and especially knees in millions of people worldwide. Some authors have shown that Curcuma longa components may exhibit benefic effects in the treatment of degenerative diseases as OA. This plant belongs to the family Zingiberaceae and it is popularly known as turmeric or saffron. This review intended to perform a retrospective search to identify studies involving humans and animal models. This review was based on articles linking OA and C. longa. Databases as Medline, Science Direct, and Lilacs were consulted and a retrospective search was carried out in order to identify studies involving humans and animal models. The curcuminoids from C. longa exhibit actions at different locations in the pathogenesis of OA once it may play an important role as anti-inflammatory, down-regulating enzymes as phospholipase A2, cyclooxygenase-2, and lipoxygenases, and reducing tumor necrosis factor-alpha-and interleukins such as interleukin-1β (IL-1β), IL-6, and IL-8. They also act as inducer of apoptosis in synoviocytes, decreasing the inflammation process and may also reduce the synthesis of reactive oxygen species. For these reasons, new pharmaceutical technology and pharmacological studies should be proposed to determine the dose, the best delivery vehicle, pharmaceutical formulation and route of administration of this plant so its use as an adjunct in the treatment of OA may become a reality in clinical practice.
Keywords: Curcuma longa, curcumin, inflammation, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is a chronic inflammatory degenerative disease that affects the joints of the body in millions of people worldwide.[1,2] The number of people affected by this pathology increases with age, and can reach several joints such as the hands, hips, shoulders, feet, spine, and especially knees, resulting in inflammation and pain.[1,3,4] OA has no cure and the conventional treatment is restricted primarily to the use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, and analgesics. However, therapy with NSAIDs and analgesics such as acetaminophen can lead to adverse effects as gastrointestinal and cardiovascular problems, especially if it is used for long periods. This situation shows the need for new agents that treat pain and reduce the progression of the disease.[1,2,5]
C. longa is a plant belonging to Zingiberaceae family and it is popularly known as turmeric or saffron.[6,7,8] Its therapeutic potential has been widely studied for the treatment of several diseases including cancer, HIV, and Alzheimer’s.[9,10,11] Several studies suggest that it may also exhibit hypocholesterolemic, anti-apoptotic, neuroprotective, anti-inflammatory, and anti-proliferative effects[6,9,11,12,13,14,15,16] and some authors have shown that C. longa components may exhibit benefic effects in the treatment of degenerative diseases as OA.[2,2,3,4,5]
Due to the difficulties in the conventional treatment of OA, the aim of this review was to survey up the effects of C. longa in this inflammatory condition.
METHODS
This survey was based on articles linking OA and C. longa. Databases as Medline, Science Direct, and Lilacs were consulted and a retrospective search was carried out in order to identify studies involving humans and animal models.
CURCUMA LONGA
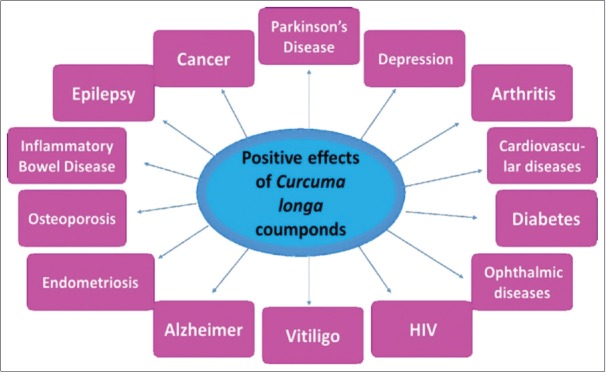
The C. longa is commonly used in cooking (the rhizome) as a seasoning and in traditional medicine in Asia and India.[16,17] It possesses three main components named curcumin, demethoxycurcumin, and bisdemethoxycurcumin, that are curcuminoids known to produce different medicinal properties and therefore have been widely studied.[7,18,19] The curcumin is a natural polyphenolic compound of low toxicity and is considered the main compound of the rhizome.[20,21,22] Thanks to its different pharmacological actions, curcumin and its analogs have been employed in different studies involving several pathologies such as cardiovascular and ophthalmic diseases, diabetes, depression, HIV, vitiligo, Alzheimer’s disease, endometriosis, osteoporosis, inflammatory bowel disease, epilepsy, Parkinson’s disease, and cancer [Figure 1].[8,9,10,21,23,24,25,26,27,28,29,30]
Figure 1.
Curcuma longa compounds may positively influence several pathologies
Its pharmacological actions occur by different mechanisms in different cells.[21] Among the various pathways, the curcumin can reduce inflammation due to its capacity of decreasing the production of interleukin-1 (IL-1), IL-6, IL-8, IL-12 and tumor necrosis factor-alpha (TNF-α), inhibiting the activation of nuclear factor kappa-B (NF-κβ) and declining the synthesis of reactive oxygen species.[13,18,22]
Due to its pharmacological potential, several authors point out a feature of curcumin and also bisdemethoxycurcumin that could jeopardize its use: They are highly hydrophobic. Molecules with this characteristic has a low oral bioavailability.[19] Therefore, to improve this property of curcumin and bisdemethoxycurcumin, researchers have used delivery systems, including nanoparticles, micelles, liposomes, and phospholipid complexes.[22,31] Szymusiak et al.[19] showed that when the curcumin is formulated in stable polymeric nanoparticles it is necessary to use a dose 20 times lower to achieve the same plasma concentration.
There are numerous reports in the literature indicating that curcuminoids and its analogs can have positive effects on OA.
PATHOPHYSIOLOGY OF OSTEOARTHRITIS
Commonly, the chondrocytes possess an equilibrium among the production and degradation of extracellular matrix such as Type II collagen and aggrecan, which are the main proteoglycan in the articular cartilage.[32]
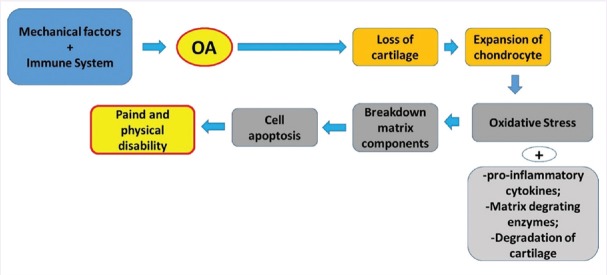
OA occurs due to multiple factors, which include genetic and environmental factors.[33,34] The pathophysiology of OA is not completely understood but it is believed that different pathways could lead to the disease. The pathogenesis involves processes as oxidative stress and inflammation, osteoclastogenesis and proteolytic degradation of cartilage.[11,35,36,37] Figures 2 and 3 show some aspects involved in OA.
Figure 2.
In the Osteoarthritis it is possible to observe a disruption in the matrix homeostasis and consequent loss of cartilage, expansion of chondrocytes leading to increase in the release of pro-inflammatory cytokines and increase in the production of reactive oxygen species. This scenario is related to the breakdown of matrix components and cell apoptosis resulting in pain and physical disability. Modified from Lee et al.[32]
Figure 3.
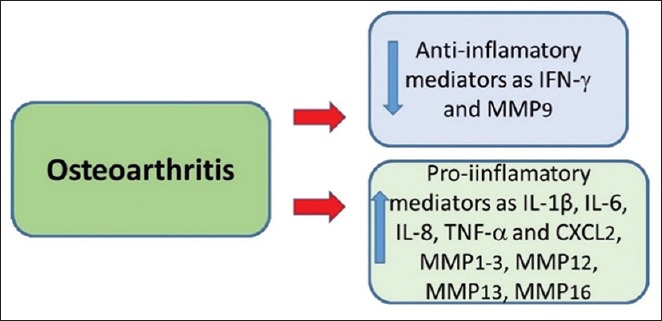
Osteoarthritis is related to the decrease of anti-inflammatory (as IFN-γ and MMP9) and increase in pro-inflammatory mediators (as MMP1-3, MMP 12, MMP 13, MMP 16; TNF-α, IL-1β, IL 6, IL-8 and CXCL2). MMP = Matrix metalloproteinase, IL = Interleukin, TNF-α = Tumor necrosis factor, CXCL = Chemokine (C-X-C motif) ligand, INF-γ = Interferon gamma. Modified from Wang et al.[37]
Osteoclasts are derived from hematopoietic cells that also originate macrophages and monocytes. The Receptor Activator NF-kb Ligand (RANKL) is produced by osteoblasts, stromal cells, and chondrocytes. After RANK biding to RANKL in the membrane of osteoclasts precursor cells, there is activation of I Kappa B Kinase (IKK), phosphorylation, and degradation of I Kappa B alpha (IkBα); thus, the Activator of NF-κB is activated. Then, these precursor cells differentiate into osteoclasts, which are activated by starting the osteoclastogenesis.[11,35,36,38,39]
To prevent the activation of osteoclasts, the osteoprotegerin (OPG), also produced by osteoblasts acts as a “decoy receptor” and binds to RANKL, thereby preventing RANKL binding to RANK, and thus avoids the osteoclastogenesis. The OPG also has a role in inducing apoptosis of mature osteoclasts. Thus, the RANKL/OPG ratio is a good index to analyze the occurrence of osteogenesis or osteoclastogenesis. When there is an increase in RANKL/OPG ratio there is predominance of bone destruction, and when there is a decrease of this index, there is protection of subchondral bones.[11,40,41,42] In OA, various interleukins and cytokines such as IL-1β, IL-6, IL-11, IL-17, and TNF-α lead to increased formation of RANKL and decreased production of OPG resulting in bone lost.[38,39,40,41]
In addition, cathepsin K, importantly expressed in mature osteoclasts, is the main agent in osteoclastogenesis and degrades type II collagen, thus destroying cartilage.[11,43,44] Portions of collagen cleaved by this protease can be labeled in OA as demonstrated by the study of Mort et al.[43] Kozawa et al.[45] showed that people who do not have the disease have a lower activation and expression of cathepsin K when compared to cartilage of patients with OA.
Furthermore, in the OA process are involved proteases degrading enzymes such as matrix metalloproteinases (MMPs) MMP-3, MMP-9, MMP-13, tartrate-resistant acid phosphatase (TRAP), a disintegrin, and metalloproteinase with thrombospondin Motifs (ADAMTS). IL-1 acts on chondrocytes b, resulting in the induction of NF-κB and activator protein 1 (AP-1) and the production of MMPs, enzymes that breakdown collagen.[15,46] Among the metalloproteinase, MMP-13 is more potent in cleavage of type II collagen. The ADAMTS acts in cleaving aggrecan molecules (another component of cartilage). In OA these proteases are increased, leading to an abnormal destruction of cartilage[11,40,46,47] IL-6 also acts on chondrocyte decreasing the production of type II collagen.[33,48] Probably TNF-α acts in synergy with these interleukins in the inhibition of proteoglycan synthesis and increasing cartilage resorption.[49,50]
Authors believe that this environment full of inflammatory cytokines and proteases leads to death of chondrocytes and synoviocytes stimulation, which secrete inflammatory cytokines and recruitment of mononuclear and polymorphonuclear factors generating more cartilage destruction.[15,33]
Furthermore, various substances such as IL-1β, IL-6, IL-11, IL-17, prostaglandin-2 (PGE-2), TNF-α and the presence of reactive oxygen and nitrogen species were related to the pathogenesis of the disease.[11,37,46,47] Figure 4 resumes the modification of osteoblasts and production of pro-inflammatory cytokines released in the synovial liquid.
Figure 4.
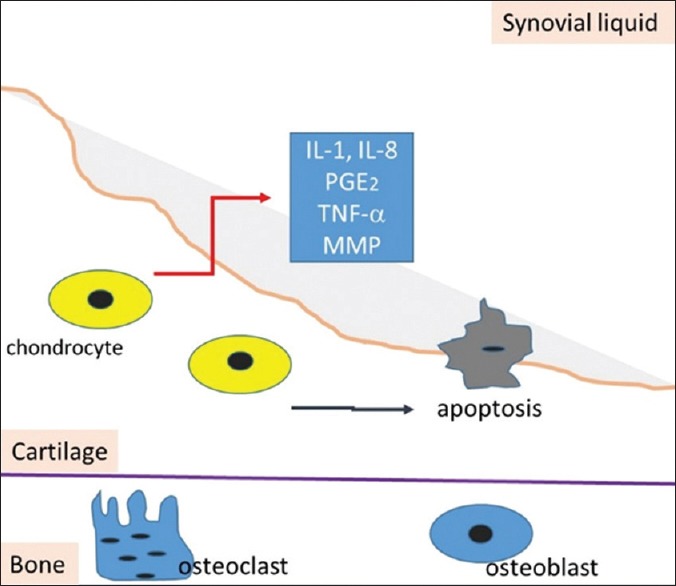
Scheme showing the production of pro-inflammatory cytokines as IL-1, IL-8, PGE-2, TNF-α and MMP by aberrantly activated chondrocytes in synovial liquid. In osteoarthritic state, the production of these mediators and catabolic growth factors as PGE-2 induces the catabolic effects. Apoptosis also occur resulting in cartilage loss. IL = Interleukin, PGE-2 = Prostaglandin 2, TNF-α = Tumor necrosis factor, MMP = Matrix metalloproteinases (Modified from Lee et al., 2013)[32]
CURCUMA LONGA AND OSTEOARTHRITIS
The curcuminoids seem to act at different locations in the pathogenesis of OA. The curcumin inhibits RANKL-induced osteoclastogenesis and TNF-α.[11,13,39] Bharti et al.[38] showed that macrophage cell line RAW 264.7 stimulated in vitro with RANKL and curcumin formed osteoclasts less than when stimulated only in the presence of RANKL. Moreover, according to studies by Yeh et al.,[11] these macrophages when incubated with curcumin or bisdemethoxycurcumin loaded liposomes (Cur-Lip or BDMC-Lip) with RANKL become smaller and with a reduced number of nuclei when compared to the cells with only the presence of RANKL and LPS. These findings support the effects of curcuminoids on the osteoclastogenesis.
Authors believe that the effects of curcumin on osteoclastogenesis occurs early in the signaling because its effects are greatly reduced when added 2 days after RANKL and it is maximum if it is added 2 h before or concomitantly with RANKL. An agent that can inhibit the action of RANKL can suppress bone destruction.[38]
The curcumin inhibits the signaling that occurs through NF-κB. With the stop in the activation of IKK, there is no phosphorylation and degradation of IkBa, without consequently activation of NF-κB. Furthermore, it acts by reducing the activation of NF-κB by inflammatory cytokines.[38] Moreover, as demonstrated by Yeh et al.[11] the inhibition of the expression of TRAP and cathepsin K leads to decrease of TRAP activity and consequent suppression of osteoclastogenesis. When using these curcuminoids, the OPG/RANKL rate increases, suggesting bone development. In addition to the inhibition of osteoclastogenesis, curcumin may also inhibit the pit formation.[38]
Besides decreasing bone degradation, curcumin has chondroprotective effects once it is able to inhibit the production of MMP-1, MMP-3, MMP-9, MMP-13 by inhibiting the AP-1 pathway, the NF-κB and Jun N-terminal kinase.[1,11,15] Studies conducted by Yeh et al.[11] showed that levels of MMP-1 mRNA, MMP-3, and MMP-13 in human chondrocytes receiving curcumin for 6 h were decreased compared with the control group, showing the reduction of the metalloproteinases synthesis caused by curcumin. Curcumin also restart the production of type II collagen and glycosaminoglycan and has anti-apoptotic effect on chondrocytes (inhibits caspase-3 activation).[1,15,51] Another effect is the inhibition of expression of ADAMT-5. Aggrecanase-mediated aggrecan degradation is a relevant situation that occurs in early-stage OA. Aggrecanases belonging to the metalloproteinase and disintegrin with ADAMTS family of proteinases play a significant role in aggrecan depletion in osteoarthritic cartilage and increased expression of CITED2 gene (Cbp/P300 Interacting Trans-activator With Glu/Asp Rich Carboxy-Terminal Domain 2), that appears to be involved with inhibition of the activity of NF-κB.[11]
The curcumin also play anti-inflammatory role. Several studies showed its action down-regulating phospholipase A2, cyclooxygenase-2, lipoxygenases, PGEs and reducing TNFa-and interleukins such as IL-1b, IL-6, and IL-8.[11,15,51,52] It also acts as inducer of apoptosis in synoviocytes decreasing the inflammation process. This compound may reduce the synthesis of reactive oxygen species and nitrogen in vitro, but further studies are necessary to find out if it could help to slow the progression of the disease or in the pain.[11,15] Other effects of this plant in OA may be found in Table 1.
Table 1.
Biological effects of Curcuma sp.
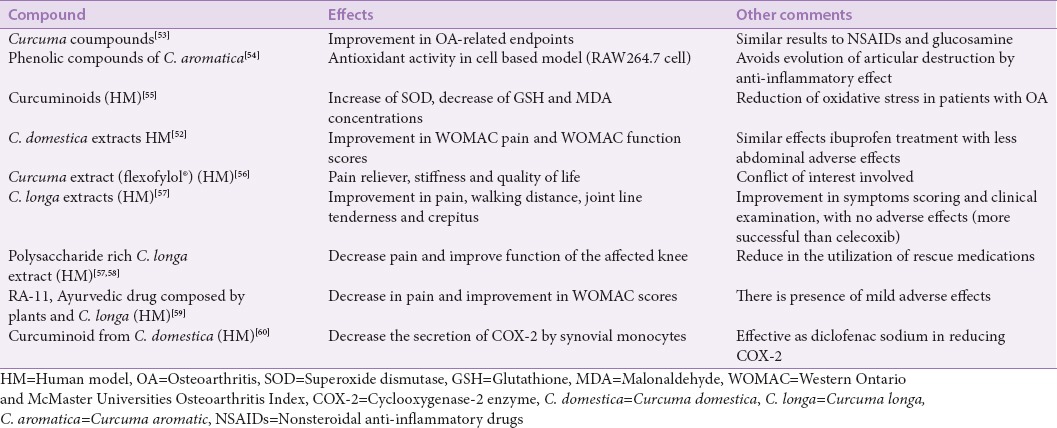
Chandrasekaran et al.[61] and Madhu et al.[58] studied the effects of an aqueous based extract of C. longa (devoid curcuminoids, standardized to polysaccharides) and found it may act as anti-inflammatory and may modulate immune-stimulatory properties. These properties showed important effects in primary painful knee and joint pain.
CONCLUSION
C. longa is a plant with promising pharmacological properties in various diseases due to its multiple action. The study of curcumin and its derivatives in cells, animals and humans models have confirmed their benefit in the use of OA. Studies show that in addition to the improvement in the symptomatology of the patients with OA, there may be a slowing progression of the disease by reducing inflammation and cartilage and bone destruction.
For these reasons, new pharmaceutical technology and pharmacological studies should be proposed in order to determine the dose, the best delivery vehicle, pharmaceutical formulation and route of administration of this plant so its use as an adjunct in the treatment of OA may become a reality in clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yimam M, Lee YC, Jiao P, Hong M, Nam JB, Brownell L, et al. UP1306, a botanical composition with analgesic and anti-inflammatory effect. Pharmacognosy Res. 2016;8:186–92. doi: 10.4103/0974-8490.182918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa Y, Mukai S, Yamada S, Matsuoka M, Tarumi E, Hashimoto T, et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19:933–9. doi: 10.1007/s00776-014-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo JJ, Wu K, Guan H, Zhang L, Ji C, Yang H, et al. Three-year follow-up of conservative treatments of shoulder osteoarthritis in older patients. Orthopedics. 2016;39:e634–41. doi: 10.3928/01477447-20160606-02. [DOI] [PubMed] [Google Scholar]
- 4.Peters MJ, Ramos YF, den Hollander W, Schiphof D, Hofman A, Uitterlinden AG, et al. Associations between joint effusion in the knee and gene expression levels in the circulation: A meta-analysis. F1000Res. 2016;5:109. doi: 10.12688/f1000research.7763.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH International NSAID Consensus Group. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis – An expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:55. doi: 10.1186/s12916-015-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Sripradha R, Badhe B. Curcumin inhibits hyperlipidemia and hepatic fat accumulation in high-fructose-fed male Wistar rats. Pharm Biol. 2016;54:2857–63. doi: 10.1080/13880209.2016.1187179. [DOI] [PubMed] [Google Scholar]
- 7.Yadav SK, Sah AK, Jha RK, Sah P, Shah DK. Turmeric (curcumin) remedies gastroprotective action. Pharmacogn Rev. 2013;7:42–6. doi: 10.4103/0973-7847.112843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirzabeigi P, Mohammadpour AH, Salarifar M, Gholami K, Mojtahedzadeh M, Javadi MR. The effect of curcumin on some of traditional and non-traditional cardiovascular risk factors: A pilot randomized, double-blind, placebo-controlled trial. Iran J Pharm Res. 2015;14:479–86. [PMC free article] [PubMed] [Google Scholar]
- 9.Devi VK, Jain N, Valli KS. Importance of novel drug delivery systems in herbal medicines. Pharmacogn Rev. 2010;4:27–31. doi: 10.4103/0973-7847.65322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali A, Banerjea AC. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci Rep. 2016;6:27539. doi: 10.1038/srep27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh CC, Su YH, Lin YJ, Chen PJ, Shi CS, Chen CN, et al. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des Devel Ther. 2015;9:2285–300. doi: 10.2147/DDDT.S78277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doshi GM, Une HD, Shanbhag PP. Rasayans and non-rasayans herbs: Future immunodrug – Targets. Pharmacogn Rev. 2013;7:92–6. doi: 10.4103/0973-7847.120506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Gupta SC, Sung B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169:1672–92. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors. 2013;39:69–77. doi: 10.1002/biof.1066. [DOI] [PubMed] [Google Scholar]
- 15.Henrotin Y, Clutterbuck AL, Allaway D, Lodwig EM, Harris P, Mathy-Hartert M, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141–9. doi: 10.1016/j.joca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Mauren FM, Yanti, Lay BW. Efficacy of oral curcuminoid fraction from Curcuma xanthorrhiza and curcuminoid cider in high-cholesterol fed rats. Pharmacognosy Res. 2016;8:153–9. doi: 10.4103/0974-8490.181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JJ, Lee JH, Cho WK, Han JH, Ma JY. Herbal composition of Cinnamomum cassia Pinus densiflora Curcuma longa and Glycyrrhiza glabra prevents atherosclerosis by upregulating p27 (Kip1) expression. BMC Complement Altern Med. 2016;16:253. doi: 10.1186/s12906-016-1224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymusiak M, Hu X, Leon Plata PA, Ciupinski P, Wang ZJ, Liu Y. Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin. Int J Pharm. 2016;511:415–23. doi: 10.1016/j.ijpharm.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Cifuentes M, Weiss-Lopez B, Santos LS, Araya-Maturana R. Heterocyclic curcumin derivatives of pharmacological interest: Recent progress. Curr Top Med Chem. 2015;15:1663–72. doi: 10.2174/1568026615666150427111837. [DOI] [PubMed] [Google Scholar]
- 21.Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014;80:249–54. doi: 10.1055/s-0033-1351074. [DOI] [PubMed] [Google Scholar]
- 22.Zamarioli CM, Martins RM, Carvalho EC, Freitas Luis AP. Nanoparticles containing curcuminoids (Curcuma longa): Development of topical delivery formulation. Rev Bras Farmacognosia. 2015;25:53–60. [Google Scholar]
- 23.Kumar B, Singh V, Shankar R, Kumar K, Rawal RK. Synthetic and medicinal prospective of structurally modified curcumins. Curr Top Med Chem. 2017;17:148–61. doi: 10.2174/1568026616666160605050052. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann FN, Gazal M, Bastos CR, Kaster MP, Ghisleni G. Curcumin in depressive disorders: An overview of potential mechanisms, preclinical and clinical findings. Eur J Pharmacol. 2016;784:192–8. doi: 10.1016/j.ejphar.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Cao H, Yu Z, Peng HY, Zhang CJ. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iran J Reprod Med. 2013;11:415–22. [PMC free article] [PubMed] [Google Scholar]
- 26.Kaur H, Patro I, Tikoo K, Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem Int. 2015;89:40–50. doi: 10.1016/j.neuint.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Penagini F, Dilillo D, Borsani B, Cococcioni L, Galli E, Bedogni G, et al. Nutrition in pediatric inflammatory bowel disease: From etiology to treatment. A systematic review. Nutrients. 2016 doi: 10.3390/nu8060334. 8. pii: E334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Xue J, Shen T, Mu S, Fu Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int J Mol Med. 2016;37:329–38. doi: 10.3892/ijmm.2015.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandareesh MD, Shrivash MK, Naveen Kumar HN, Misra K, Srinivas Bharath MM. Curcumin monoglucoside shows improved bioavailability and mitigates rotenone induced neurotoxicity in cell and drosophila models of Parkinson’s disease. Neurochem Res. 2016;41:3113–28. doi: 10.1007/s11064-016-2034-6. [DOI] [PubMed] [Google Scholar]
- 30.Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (Curcuma longa) on skin health: A systematic review of the clinical evidence. Phytother Res. 2016;30:1243–64. doi: 10.1002/ptr.5640. [DOI] [PubMed] [Google Scholar]
- 31.Mirzaei H, Naseri G, Rezaee R, Mohammadi M, Banikazemi Z, Mirzaei HR, et al. Curcumin: A new candidate for melanoma therapy?Int J Cancer. 2016;139:1683–95. doi: 10.1002/ijc.30224. [DOI] [PubMed] [Google Scholar]
- 32.Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, van Wijnen AJ, et al. Acurrent review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–7. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limagne E, Lançon A, Delmas D, Cherkaoui-Malki M, Latruffe N. Resveratrol Interferes with IL1-ß-induced pro-inflammatory paracrine interaction between primary chondrocytes and macrophages. Nutrients. 2016;8 doi: 10.3390/nu8050280. pii: E280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynard LN. Analysis of genetics and DNA methylation in osteoarthritis: What have we learnt about the disease? Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.04.017. pii: S1084-952130121-5. [DOI] [PubMed] [Google Scholar]
- 35.TenBroek EM, Yunker L, Nies MF, Bendele AM. Randomized controlled studies on the efficacy of antiarthritic agents in inhibiting cartilage degeneration and pain associated with progression of osteoarthritis in the rat. Arthritis Res Ther. 2016;18:24. doi: 10.1186/s13075-016-0921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamma R, Zallone A. Osteoblast and osteoclast crosstalks: From OAF to Ephrin. Inflamm Allergy Drug Targets. 2012;11:196–200. doi: 10.2174/187152812800392670. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Guan PP, Guo C, Zhu F, Konstantopoulos K, Wang ZY. Fluid shear stress-induced osteoarthritis: Roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J. 2013;27:4664–77. doi: 10.1096/fj.13-234542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004;172:5940–7. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 39.Wei S, Teitelbaum SL, Wang MW, Ross FP. Receptor activator of nuclear factor-kappa b ligand activates nuclear factor-kappa b in osteoclast precursors. Endocrinology. 2001;142:1290–5. doi: 10.1210/endo.142.3.8031. [DOI] [PubMed] [Google Scholar]
- 40.Liu YD, Yang HX, Liao LF, Jiao K, Zhang HY, Lu L, et al. Systemic administration of strontium or NBD peptide ameliorates early stage cartilage degradation of mouse mandibular condyles. Osteoarthritis Cartilage. 2016;24:178–87. doi: 10.1016/j.joca.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu L, Guo H, Li C, Xu J, Fang W, Long X. A time-dependent degeneration manner of condyle in rat CFA-induced inflamed TMJ. Am J Transl Res. 2016;8:556–67. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S, Huang Y, Chen W, Wu G, Liao N, Li X, et al. Protective effects of the Tougu Xiaotong capsule on morphology and osteoprotegerin/nuclear factor-kB ligand expression in rabbits with knee osteoarthritis. Mol Med Rep. 2016;13:419–25. doi: 10.3892/mmr.2015.4547. [DOI] [PubMed] [Google Scholar]
- 43.Mort JS, Beaudry F, Théroux K, Emmott AA, Richard H, Fisher WD, et al. Early cathepsin K degradation of type II collagen in vitro and in vivo in articular cartilage. Osteoarthritis Cartilage. 2016;24:1461–9. doi: 10.1016/j.joca.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Troen BR. Molecular mechanisms underlying osteoclast formation and activation. Exp Gerontol. 2003;38:605–14. doi: 10.1016/s0531-5565(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 45.Kozawa E, Cheng XW, Urakawa H, Arai E, Yamada Y, Kitamura S, et al. Increased expression and activation of cathepsin K in human osteoarthritic cartilage and synovial tissues. J Orthop Res. 2016;34:127–34. doi: 10.1002/jor.23005. [DOI] [PubMed] [Google Scholar]
- 46.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014. 2014 doi: 10.1155/2014/561459. 561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Leong DJ, Xu L, He Z, Wang A, Navati M, et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res Ther. 2016;18:128. doi: 10.1186/s13075-016-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 48.Porée B, Kypriotou M, Chadjichristos C, Beauchef G, Renard E, Legendre F, et al. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem. 2008;283:4850–65. doi: 10.1074/jbc.M706387200. [DOI] [PubMed] [Google Scholar]
- 49.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322:547–9. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Séguin CA, Bernier SM. TNFalpha suppresses link protein and type II collagen expression in chondrocytes: Role of MEK1/2 and NF-kappaB signaling pathways. J Cell Physiol. 2003;197:356–69. doi: 10.1002/jcp.10371. [DOI] [PubMed] [Google Scholar]
- 51.Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Curcumin mediated suppression of nuclear factor-kB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther. 2010;12:R127. doi: 10.1186/ar3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, Buntragulpoontawee M, Lukkanapichonchut P, Chootip C, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin Interv Aging. 2014;9:451–8. doi: 10.2147/CIA.S58535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perkins K, Sahy W, Beckett RD. Efficacy of Curcuma for treatment of osteoarthritis. J Evid Based Complementary Altern Med. 2017;22:156–65. doi: 10.1177/2156587216636747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anuthakoengkun A, Itharat A. Inhibitory effect on nitric oxide production and free radical scavenging activity of Thai medicinal plants in osteoarthritic knee treatment. J Med Assoc Thai. 2014;97(Suppl 8):S116–24. [PubMed] [Google Scholar]
- 55.Panahi Y, Alishiri GH, Parvin S, Sahebkar A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: Results of a randomized controlled trial. J Diet Suppl. 2016;13:209–20. doi: 10.3109/19390211.2015.1008611. [DOI] [PubMed] [Google Scholar]
- 56.Appelboom T, Maes N, Albert A. A new Curcuma extract (flexofytol®) in osteoarthritis: Results from a belgian real-life experience. Open Rheumatol J. 2014;8:77–81. doi: 10.2174/1874312901408010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kizhakkedath R. Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol Med Rep. 2013;8:1542–8. doi: 10.3892/mmr.2013.1661. [DOI] [PubMed] [Google Scholar]
- 58.Madhu K, Chanda K, Saji MJ. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: A randomized placebo-controlled trial. Inflammopharmacology. 2013;21:129–36. doi: 10.1007/s10787-012-0163-3. [DOI] [PubMed] [Google Scholar]
- 59.Kertia N, Asdie AH, Rochmah W, Marsetyawan Ability of curcuminoid compared to diclofenac sodium in reducing the secretion of cycloxygenase-2 enzyme by synovial fluid’s monocytes of patients with osteoarthritis. Acta Med Indones. 2012;44:105–13. [PubMed] [Google Scholar]
- 60.Chopra A, Lavin P, Patwardhan B, Chitre D. A 32-week randomized, placebo-controlled clinical evaluation of RA-11, an Ayurvedic drug, on osteoarthritis of the knees. J Clin Rheumatol. 2004;10:236–45. doi: 10.1097/01.rhu.0000138087.47382.6d. [DOI] [PubMed] [Google Scholar]
- 61.Chandrasekaran CV, Sundarajan K, Edwin JR, Gururaja GM, Mundkinajeddu D, Agarwal A. Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharmacognosy Res. 2013;5:71–9. doi: 10.4103/0974-8490.110527. [DOI] [PMC free article] [PubMed] [Google Scholar]