Abstract
Cultures of Azolla caroliniana Willd. free of the symbiotic blue-green alga, Anabaena azollae, were obtained by treatment of Azolla fronds with a regimen of antibiotics. These symbiontfree plants can be maintained only on medium containing a combined nitrogen source.
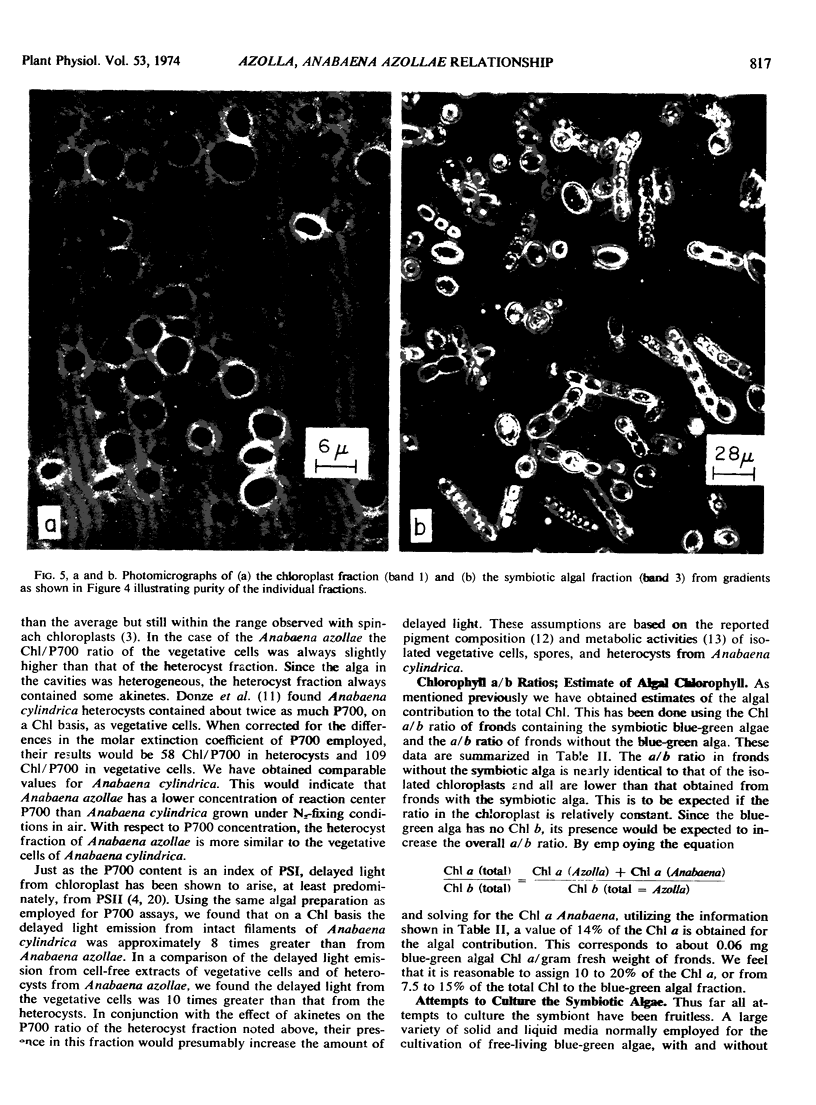
Morphological aspects of the symbiotic association show the confinement of the Anabaena azollae within the leaf cavity of the Azolla. Procedures were established for the isolation of pure preparations of Anabaena azollae and Azolla chloroplasts. It has not yet been possible to grow the isolated alga in independent culture.
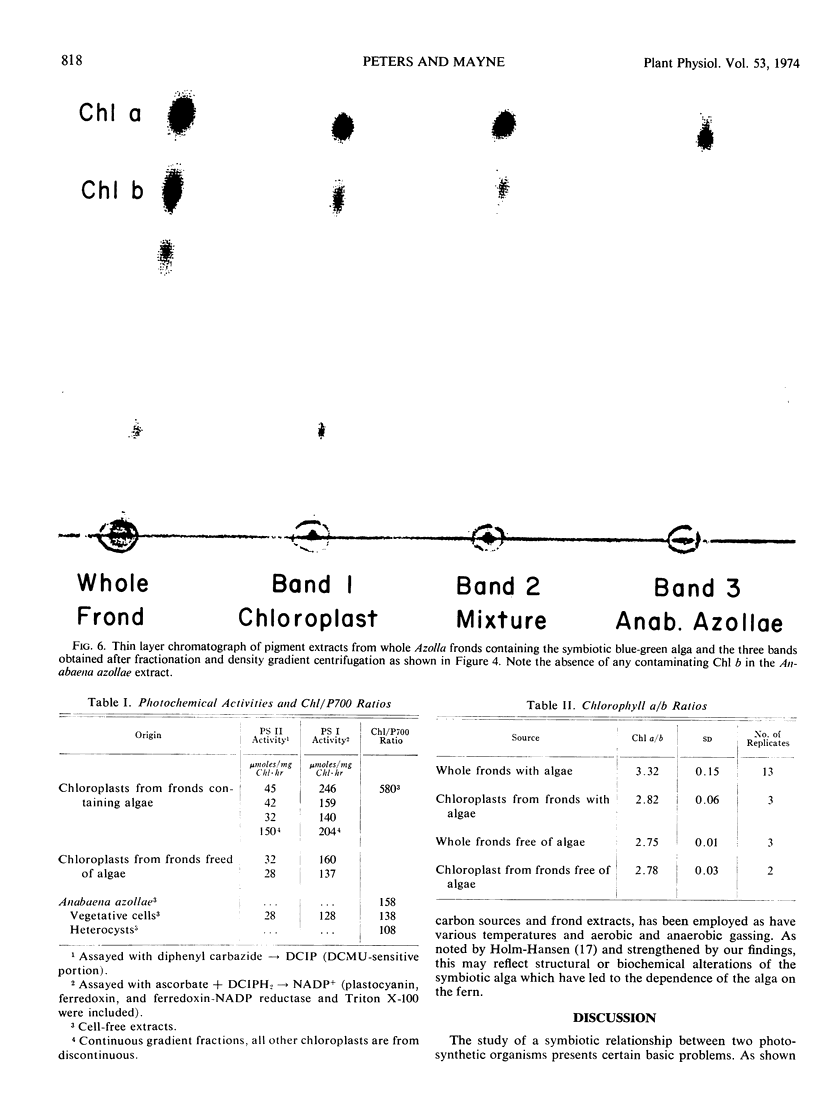
Photochemical activities of the isolated alga and fern chloroplasts were measured by spectrophotometric assays for photosystems I and II as well as by P700-content (photosystem I) and delayed light emission (photosystem II). In the algal fraction, both photosystems were repressed when compared to freeliving Anabaena cylindrica, but the relative ratio of photosystem I to photosystem II may be appreciably greater in Anabaena azollae. Azolla chloroplasts were generally comparable to spinach chloroplasts.
A comparison of the chlorophyll a and b content of Azolla fronds with and without the symbiotic alga resulted in an estimate that in the symbiotic association, the Anabaena azollae accounts for from 7.5 to 15% of the total chlorophyll.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Dilley R. A., Peters G. A., Shaw E. R. Photochemical activity and structural studies of photosystems derived from chloroplast grana and stroma lamellae. Biochim Biophys Acta. 1972 Jan 21;256(1):85–107. doi: 10.1016/0005-2728(72)90165-x. [DOI] [PubMed] [Google Scholar]
- Bertsch W., Azzi J. R., Davidson J. B. Delayed light studies on photosynthetic energy conversion. I. Identification of the oxygen-evolving photoreaction as the delayed light emitter in mutants of Scenedesmus obliquus. Biochim Biophys Acta. 1967 Jul 5;143(1):129–143. doi: 10.1016/0005-2728(67)90116-8. [DOI] [PubMed] [Google Scholar]
- Biggins J. Photosynthetic Reactions by Lysed Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1447–1456. doi: 10.1104/pp.42.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAYTON R. K. CHARACTERISTICS OF FLUORESCENCE AND DELAYED LIGHT EMISSION FROM GREEN PHOTOSYNTHETIC BACTERIA AND ALGAE. J Gen Physiol. 1965 Mar;48:633–646. doi: 10.1085/jgp.48.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., Lefort-Tran M. Fixation of phycobiliproteins to photosynthetic membranes by glutaraldehyde. Arch Mikrobiol. 1970;71(3):245–257. doi: 10.1007/BF00410158. [DOI] [PubMed] [Google Scholar]
- Donze M., Haveman J., Schiereck P. Absence of photosystem 2 in heterocysts of the blue-green alga Anabaena. Biochim Biophys Acta. 1972 Jan 21;256(1):157–161. doi: 10.1016/0005-2728(72)90170-3. [DOI] [PubMed] [Google Scholar]
- Fay P. Cell differentiation and pigment composition in Anabaena cylindrica. Arch Mikrobiol. 1969;67(1):62–70. doi: 10.1007/BF00413682. [DOI] [PubMed] [Google Scholar]
- Fay P., Walsby A. E. Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature. 1966 Jan 1;209(5018):94–95. doi: 10.1038/209094a0. [DOI] [PubMed] [Google Scholar]
- Grodzinski B., Colman B. Loss of photosynthetic activity in two blue-green algae as a result of osmotic stress. J Bacteriol. 1973 Jul;115(1):456–458. doi: 10.1128/jb.115.1.456-458.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Johnson G. V., Mayeux P. A., Evans H. J. A Cobalt Requirement for Symbiotic Growth of Azolla filiculoides in the Absence of Combined Nitrogen. Plant Physiol. 1966 May;41(5):852–855. doi: 10.1104/pp.41.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne B. C. Spectral, Physical, and Electron Transport Activities in the Photosynthetic Apparatus of Mesophyll Cells and Bundle Sheath Cells of Digitaria sanguinalis (L.) Scop. Plant Physiol. 1971 May;47(5):600–605. doi: 10.1104/pp.47.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
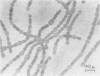
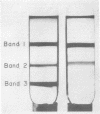
- Peters G. A., Mayne B. C. The Azolla, Anabaena azollae Relationship: II. Localization of Nitrogenase Activity as Assayed by Acetylene Reduction. Plant Physiol. 1974 Jun;53(6):820–824. doi: 10.1104/pp.53.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPORN M. B., WANKO T., DINGMAN W. The isolation of cell nuclei from rat brain. J Cell Biol. 1962 Oct;15:109–120. doi: 10.1083/jcb.15.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]