Abstract
Background
Gene polymorphisms are associated with sensitivity to platinum drugs. This study aimed to investigate the polymorphisms of GSTP1 rs1695 locus and ABCC2 rs717620 locus, and the sensitivity of patients with advanced non-small cell lung cancer (NSCLC) to platinum drugs in a Xinjiang Uygur population.
Material/Methods
The gene polymorphisms of GSTP1 rs1695 and ABCC2 rs717620 of Uygur NSCLC patients were assessed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The relationship between the prognosis of advanced NSCLC Uygur patients and the gene polymorphisms of GSTP1 rs1695 and ABCC2 rs717620 was analyzed using progression-free survival (PFS) and overall survival (OS) as the major outcome indicators.
Results
The median PFS of patients with advanced NSCLC was 6.9 months and the OS of Uygur patients with advanced NSCLC was 10.8 months. Kaplan-Meier survival analysis indicated that survival time of patients with GSTP1 AG + GG was significantly longer than in patients with AA gene (P<0.05), and survival time of patients with ABCC2 CT + TT was significantly longer than in patients with the CC gene (P<0.05).
Conclusions
Polymorphisms of GSTP1 rs1695 and ABCC2 rs717620 can be used to predict the outcomes of Uygur patients with advanced NSCLC who have received platinum-based chemotherapy. Additionally, this information could be used to guide the individualized treatment of Uygur patients with advanced NSCLC.
MeSH Keywords: Carcinoma in Situ; Carcinoma, Non-Small-Cell Lung; Platinum Compounds; Polymorphism, Genetic
Background
Morbidity and mortality from lung cancer are the highest of all cancers worldwide. Non-small cell lung cancer (NSCLC) affects 80% of all patients with lung cancer. In Xinjiang, China, different cultural customs, dietary habits, genetic backgrounds, and regional distributions lead to differences in the distribution of tumor diseases. However, research on Uygur patients with lung cancer has been scarce. Recent studies indicate that the incidence of lung cancer ranks sixth among malignant tumors within the Uygur population [1]. According to results of the ECOG1594 study, the combination of third-generation cytotoxic drugs with platinum drugs is the standard chemotherapy for advanced NSCLC [2]. However, the sensitivity of NSCLC patients to platinum drugs varies. Polymorphisms at lung cancer-related genes have a significant effect on lung cancer chemotherapy. Such genes include those involved in DNA repair, apoptosis, drug transport, and inflammatory signals.
Pharmacogenetics plays an important role in tumor chemotherapy. Prognosis of patients with NSCLC may be related to their genetic background, individual chemotherapy response, and tumor resistance to treatment. Drug resistance of tumor cells to platinum drugs is the main reason for the variability in chemotherapeutic effects.
Glutathione S transferase is an important cell defense system and is involved in the detoxification of a variety of chemotherapeutic agents, including platinum drugs. Glutathione S transferase P1 (GSTPl) is one of the major isozymes of GSTs in NSCLC, and is involved in the metabolism of anticancer drugs. Single-nucleotide polymorphisms (SNPs) at its gene are associated with lung cancer risk and survival [3,4]. ABCC2 is a protein that can transfer cisplatin (DDP)-glutathione conjugates and pumps glutathione bound with platinum ions out of cells. Up-regulated expression of ABCC2 can reduce the formation of platinum-DNA adducts and promote DDP drug-resistant mutant cells in the G2 phase. Consequently, the drug resistance of tumor cells to platinum drugs is enhanced. However, changes in amino acids caused by SNPs may attenuate this effect.
We speculated that polymorphisms of GSTP1 rs1695 and ABCC2 rs717620 are associated with outcomes in Uygur patients with advanced NSCLC treated with platinum-based chemotherapy. This study aimed to investigate the influence of polymorphisms at GSTP1 rs1695 and ABCC2 rs717620 on platinum-based chemotherapy in patients with advanced NSCLC from the Uygur population in Xinjiang, with the ultimate goal of providing guidance to individualized treatment of Uygur patients with advanced NSCLC.
Material and Methods
Patients and samples
Eighty-four patients with advanced NSCLC undergoing chemotherapy in the Internal Medicine Department of the Affiliated Tumor Hospital of Xinjiang Medical University from June 2011 to June 2014 were enrolled as the research subjects. Of the 59 men and 25 women, all had been pathologically diagnosed. Their median age was 61 years. There was a total of 22 cases of squamous carcinoma and 62 cases of adenocarcinoma. All patients were staged according to the 7th edition of the American Joint Committee on Cancer Staging system. Twenty-six cases were classified as IIIB stage and 58 as IV stage. According to the physical condition rating criteria formulated by the Eastern Cooperative Oncology Group (ECOG), 67 cases scored 0–1 and 17 cases scored 2. Characteristics of the patients are shown in Table 1. All patients were confirmed to have measurable tumor focuses on computed tomography (CT) or positron emission tomography (PET)-CT. All patients or their families signed informed consent forms. This study was approved by the Ethics Committee of the Tumor Hospital Affiliated to Xinjiang Medical University.
Table 1.
The characteristics of NSCLC patients.
| Characteristics | n | % |
|---|---|---|
| Age, years | ||
| Median | 61 | |
| Range | 34–75 | |
| Gender | ||
| Male | 59 | 70.2 |
| Female | 25 | 29.8 |
| Histologic type | ||
| Squamous cell | 22 | 26.2 |
| Adenocarcinoma | 62 | 73.8 |
| Stage | ||
| IIIB | 26 | 31.0 |
| IV | 58 | 69.0 |
| ECOG | ||
| 0 | 35 | 41.7 |
| 1 | 32 | 38.1 |
| 2 | 17 | 20.2 |
| Chemotherapy regimens | ||
| PP | 43 | 51.2 |
| TP | 19 | 22.6 |
| GP | 22 | 26.2 |
| GSTP1 | ||
| AA | 48 | 57.1 |
| AG | 25 | 29.8 |
| GG | 11 | 13.1 |
| ABCC2 | ||
| CC | 63 | 75.0 |
| CT | 17 | 20.2 |
| TT | 4 | 4.8 |
Chemotherapy regimen and therapeutic evaluation
All patients were treated with a joint chemotherapy regimen of DDP combined with third-generation cytotoxic drugs. More specifically, the DP regimen consisted of docetaxel 75 mg/m2, d1+ DDP 30 mg/m2, d2–4; GP regimen, gemcitabine 1 g/m2, d1, d8 + DDP30 mg/m2, d2–4; PP regimen, pemetrexed 500 mg/m2, d1 + DDP30 mg/m2, and d2–4. Three to 4 weeks constituted 1 cycle, and all patients were treated with at least 2 cycles. Among the patients, 19 were treated with TP, 22 with GP, and 43 with PP. All patients underwent CT or magnetic resonance imaging (MRI) after 2 cycles of chemotherapy to evaluate the therapeutic effect according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.0).
DNA extraction and genotype analysis
Before chemotherapy, 3 mL of peripheral venous blood was drawn from 84 patients with NSCLC into an ethylenediaminetetraacetic acid (EDTA) anticoagulant test tube. Following the manufacturer’s instruction manual, a DNA extraction kit was used to extract DNA, which was kept at −20°C. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used to determine genotypes of GSTP1 and ABCC2. The PCR composition was a total volume of 15 μL, including 1.5 μL 10× Taq buffer, 0.2 μL positive primer, 0.2 μL negative primer, 0.4 μL dNTP, 0.1 μL Taq enzyme, and 11 μL dH2O. The PCR reaction conditions were as follows: 95°C pre-denaturation for 5 min; 95°C for 30 s, 68°C for 45 s, and 72°C for 60 s, for a total of 20 cycles; 95°C for 30 s, 58°C for 30 s, 72°C for 40 s, for a total of 20 cycles; 72°C extension for 7 min; and then held at 4°C. The primer sequences are shown in Table 2. The amplified fragment sizes of GSTP1 rs1695 and ABCC2 rs717620 gene SNP were 383 bp and 139 bp, respectively. Electrophoresis detection was performed on 3% agarose gels, and the electrophoretograms are shown in Figures 1 and 2.
Table 2.
PCR primer sequence.
| Gene | SNP | Primer sequence |
|---|---|---|
| ABCC2 | C-24T | Positive primer: 5′-CCTGGACTGCGTCTGGATC-3′ |
| rs717620 | Reverse primer: 5′-GGTAGATAATTCCTGTTCCACTTTC-3′ | |
| GSTP1 | A342G | Positive primer: 5′-ATCCCCAGTGACTGTGTGTTGA-3′ |
| rs1695 | Reverse primer: 5′-CGTTACTTGGCTGGTTGATGTC-3′ |
Figure 1.
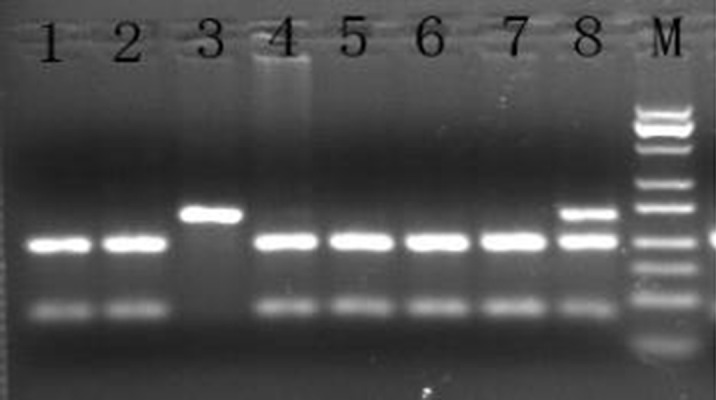
The GSTP1 gene rs1695 polymorphism in PCR-RFLP electrophoresis map. M – Marker; 1, 2, 4, 5, 6, 7 – CC; 8 – CT; 3 – TT.
Figure 2.
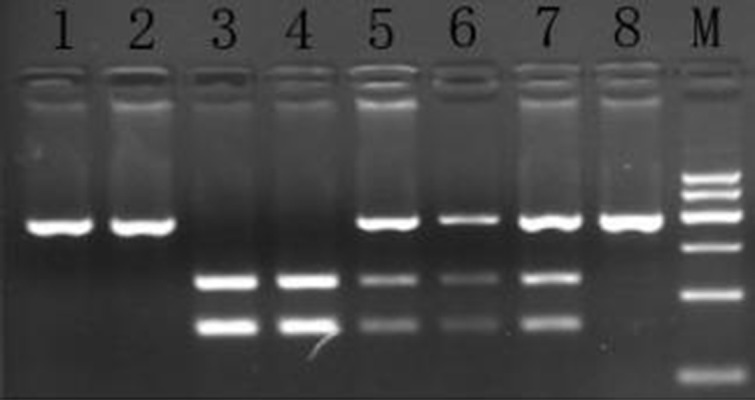
The ABCC2 gene rs717620 polymorphism in PCR-RFLP electrophoresis map. M – Marker; 1, 2, 4, 5, 6, 7 – CC; 8 – CT; 3 – TT.
Statistical analysis
SPSS 19.0 software was used for statistical analysis, and the rates were compared with the Pearson χ2 test. Theoretical frequencies less than 5 were analyzed with the Fisher exact probability method. The Kaplan-Meier method was utilized to calculate progression-free survival (PFS) and overall survival (OS). A cumulative survival function curve was drawn, and comparisons among groups were performed with log-rank tests. The Cox proportional hazards model was utilized to carry out the multiple-factor analysis for patient prognosis. In all statistical tests, a two-sided P<0.05 was defined as a difference that was statistically significant.
Results
Genotype distribution frequency and Hardy-Weinberg genetic equilibrium test
Of the 84 Uygur patients with advanced NSCLC, there were a total of 48 with the AA genotype for the GSTP1 gene (57.1%), 25 with the AG genotype (29.8%), and 11 with the GG genotype (13.1%). A total of 63 patients had the CC genotype for the ABCC2 gene (75%), 17 with the CT genotype (20.2%), and 4 with the TT genotype (4.8%). According to the Hardy-Weinberg genetic equilibrium rule test, the P value was larger than 0.05, which was consistent with genetic equilibrium of the population. The data were collected from the same Mendel group, and had good population representation, as shown in Table 3.
Table 3.
The Hardy-Weinberg genetic equilibrium test in NSCLC from Uygur.
| Genotype | Observation | Prediction | χ2 | P |
|---|---|---|---|---|
| GSTP1 rs1695 | 2.430 | 0.297 | ||
| AA | 48 | 43.5 | ||
| AG | 25 | 33.9 | ||
| GG | 11 | 6.6 | ||
| ABCC2 rs717620 | 1.120 | 0.571 | ||
| CC | 63 | 60.7 | ||
| CT | 17 | 21.4 | ||
| TT | 4 | 1.9 |
Progression-free survival (PFS)
The median follow-up period of the 84 patients was 15.5 months. According the calculation based on the Kaplan-Meier method, the median PFS of the 79 patients was 6.9 months (6.468–7.332 months, including 5 cases of censored data).
Polymorphism at GSTP1 rs1695 and PFS
The median PFS of patients with the AA genotype was 6.4 months (95% CI: 5.877–6.923) and the median PFS of the patients with the AG and the GG genotypes was 7.5 months (95% CI: 6.734–8.266). According to the log-rank test, the difference was statistically significant (χ2=6.245, P=0.040), as shown in Figure 3. The results of multi-factor Cox risk regression analysis indicated many possible contributing factors (GSTP1 genotype, age, stage, pathological type, ECOG score, sex, and chemotherapy regimen). Only the GSTP1 genotype was significantly associated with the PFS of the patients (OR=2.295, 95% CI: 1.332–3.954, P=0.003), as shown in Table 4.
Figure 3.
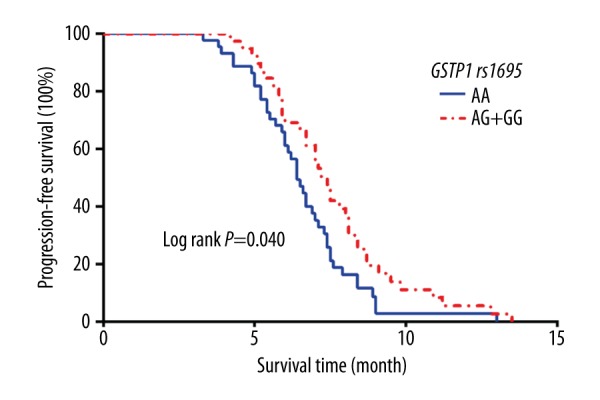
Kaplan-Meier curves of PFS stratified by patients with different numbers of risk alleles of GSTP1 rs1695.
Table 4.
Multivariate Cox proportional hazards regression analysis.
| Variable | B | SE | Wald | P | Exp(β) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| GSTP1 | 0.831 | 0.278 | 8.954 | 0.003 | 2.295 | 1.332 | 3.954 |
| Age | −0.019 | 0.016 | 1.374 | 0.241 | 0.981 | 0.950 | 1.013 |
| Stage | −0.419 | 0.257 | 2.656 | 0.103 | 0.657 | 0.397 | 1.089 |
| Histologic type | −0.373 | 0.356 | 1.096 | 0.295 | 0.689 | 0.343 | 1.384 |
| ECOG | 0.485 | 0.341 | 2.148 | 0.342 | 1.625 | 0.832 | 3.170 |
| Gender | −0.500 | 0.272 | 3.378 | 0.066 | 0.606 | 0.356 | 1.034 |
| Chemotherapy regimens | 0.353 | 0.296 | 1.607 | 0.448 | 1.423 | 0.797 | 2.541 |
Polymorphism at ABCC2 rs717620 and PFS
The median PFS of patients with the CC genotype was 6.5 months (95% CI: 6.080–6.920), and the median PFS of patients with the CT and the TT genotypes was 7.9 months (95% CI: 7.184–8.616). According to a log-rank test, the difference was statistically significant (χ2=6.808, P=0.009), as shown in Figure 4. The results of multi-factor Cox risk regression analysis indicated many possible contributing factors (ABCC2 genotype, age, stage, pathological type, ECOG score, sex, and chemotherapy regimen). Only the ABCC2 genotype was significantly associated with the PFS of the patients (OR=2.182, 95% CI: 1.252–3.805, P=0.006), as shown in Table 5.
Figure 4.
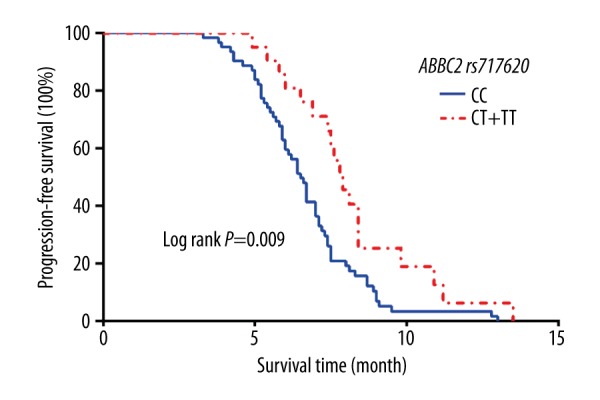
Kaplan-Meier curves of PFS stratified by patients with different numbers of risk alleles of ABCC2 rs717620.
Table 5.
Multivariate Cox proportional hazards regression analysis.
| Variable | B | SE | Wald | P | Exp(β) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| ABCC2 | 0.780 | 0.284 | 7.572 | 0.006 | 2.182 | 1.252 | 3.805 |
| Age | 0.000 | 0.013 | 0.000 | 0.997 | 1.000 | 0.975 | 1.026 |
| Stage | −0.295 | 0.268 | 1.216 | 0.270 | 0.744 | 0.440 | 1.258 |
| Histologic type | −0.151 | 0.345 | 0.191 | 0.662 | 0.860 | 0.438 | 1.690 |
| ECOG | −0.338 | 0.325 | 1.079 | 0.583 | 0.713 | 0.377 | 1.350 |
| Gender | −0.245 | 0.276 | 0.787 | 0.375 | 0.783 | 0.456 | 1.345 |
| Chemotherapy regimens | 0.477 | 0.302 | 2.826 | 0.243 | 1.611 | 0.892 | 2.909 |
Gene polymorphism and OS
Up to the final follow-up data, 4 cases among the 84 patients with advanced NSCLC were lost during the follow-up period, and the median follow-up period was 15.5 months. The median OS of the remaining 76 patients was 10.8 months (10.025–11.575 months), including 8 cases of censored data.
Polymorphism at GSTP1 rs1695 and OS
The median OS of patients with the AA genotype was 10.4 months (95% CI: 9.350–11.450), and the median OS of patients with the AG and the GG genotypes was 11.8 months (95% CI: 10.745–12.855). According to the log-rank test, the difference was statistically significant (χ2=5.862, P=0.016), as shown in Figure 5. The results of multi-factor Cox risk regression analysis indicated many possible contributing factors (GSTP1 genotype, age, stage, pathological type, ECOG score, sex, and chemotherapy regimen). Only GSTP1 genotype was significantly associated with the OS of the patients (OR=1.910, 95% CI: 1.161–3.144, P=0.011), as shown in Table 6.
Figure 5.
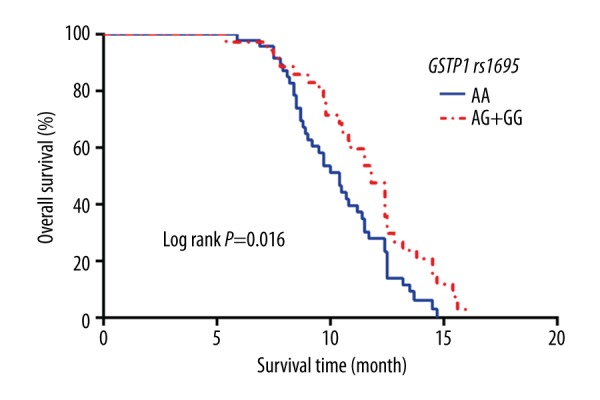
Kaplan-Meier curves of OS stratified by patients with different numbers of risk alleles of GSTP1 rs1695.
Table 6.
Multivariate Cox proportional hazards regression analysis.
| Variable | B | SE | Wald | P | Exp(β) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| GSTP1 | 0.647 | 0.254 | 6.482 | 0.011 | 1.910 | 1.161 | 3.144 |
| Age | 0.007 | 0.016 | 0.211 | 0.646 | 1.007 | 0.977 | 1.039 |
| Stage | −0.006 | 0.296 | 0.000 | 0.985 | 0.994 | 0.557 | 1.775 |
| Histologic type | −0.117 | 0.365 | 0.103 | 0.748 | 0.889 | 0.435 | 1.817 |
| ECOG | −0.038 | 0.337 | 0.582 | 0.747 | 0.962 | 0.497 | 1.863 |
| Gender | 0.173 | 0.277 | 0.391 | 0.532 | 1.189 | 0.691 | 2.044 |
| Chemotherapy regimens | 0.411 | 0.308 | 2.065 | 0.356 | 1.508 | 0.825 | 2.757 |
Polymorphism at ABCC2 rs1695 and OS
The median OS of patients with the CC genotype was 10.5 months (95% CI: 9.586–11.414), and the median OS of patients with the CT and the TT genotypes was 12.4 months (95% CI: 11.419–13.381). According to the log-rank test, the difference was statistically significant (χ2=5.683, P=0.017), as shown in Figure 6. The results of multi-factor Cox risk regression analysis indicated many possible contributing factors (ABCC2 genotype, age, stage, pathological type, ECOG score, sex, and chemotherapy regimen). Only the ABCC2 genotype was significantly associated with the OS of the patients (OR=2.019, 95% CI: 1.130–3.607, P=0.018), as shown in Table 7.
Figure 6.
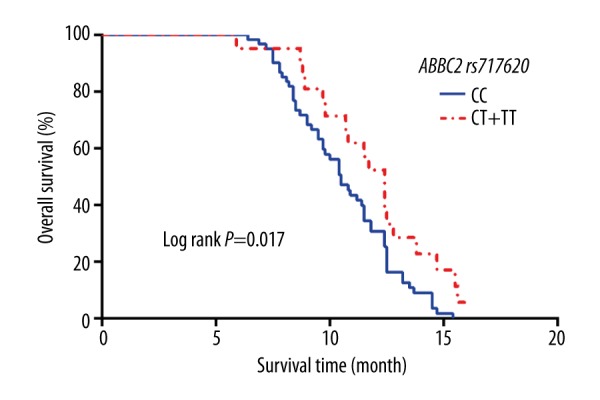
Kaplan-Meier curves of OS stratified by patients with different numbers of risk alleles of ABCC2 rs717620.
Table 7.
Multivariate Cox proportional hazards regression analysis.
| Variable | B | SE | Wald | P | Exp(β) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| ABCC2 | 0.703 | 0.296 | 5.630 | 0.018 | 2.019 | 1.130 | 3.607 |
| Age | −0.011 | 0.013 | 0.670 | 0.413 | 0.989 | 0.964 | 1.015 |
| Stage | −0.065 | 0.278 | 0.054 | 0.816 | 0.937 | 0.543 | 1.618 |
| Histologic type | −0.160 | 0.346 | 0.215 | 0.643 | 0.852 | 0.433 | 1.677 |
| ECOG | 0.188 | 0.347 | 1.269 | 0.530 | 1.207 | 0.611 | 2.384 |
| Gender | 0.257 | 0.273 | 0.883 | 0.347 | 1.293 | 0.757 | 2.209 |
| Chemotherapy regimens | 0.018 | 0.297 | 0.274 | 0.872 | 1.018 | 0.569 | 1.824 |
Discussion
With the continuous improvement in detection technology and treatment, the tumor treatment model has evolved from being guided by pathology to being guided by molecular detection that enables precise targeting. Despite the numerous therapeutic regimens, chemotherapy is still an indispensable cornerstone in treating advanced NSCLC in the era of targeted medical treatment.
At present, morbidity due to lung cancer is increasing worldwide and also within the Uygur population. Studies have shown that the EGFR gene, which has a high mutation rate among NSCLC patients in Asia, is rarely mutated among Uygur patients with NSCLC, with a mutated fraction of only around 7–17% [5]. This leads to restriction in the use of EGFR-tyrosine kinase inhibitor drugs within the Uygur population. Therefore, chemotherapy plays an important role in the treatment of Uygur patients with advanced NSCLC. However, there is a dramatic difference in the sensitivity of different individuals to chemotherapy drugs, which may be due to genetic differences between the metabolic enzymes and transporters that scavenge the drugs.
SNPs related to drug metabolism may also have a significant impact on the efficacy of chemotherapeutic agents. Therefore, we hypothesized that polymorphisms at genes coding for drug-metabolizing enzymes and drug transporters may affect the efficacy of platinum-based chemotherapy for patients with cancer.
From all types of cancer tissues, GSTP1 is the most frequently expressed GSTs isozyme. The change of GSTP1 rs1695 from A to G alters the coded amino acid. Specifically, isoleucine at the 105 locus is mutated into valine, decreasing the stability and function of GSTP1 by 2–3 fold [6,7]. The ability of GSTP1 to bind platinum is reduced, which further affects the efficacy of platinum chemotherapy and patient prognosis.
Li et al. studied and reported the association between polymorphism at the GSTP1 gene and prognosis of patients with advanced gastric cancer who were treated with platinum-based chemotherapy. Their results showed that the efficacy rate of chemotherapy for patients with the GG genotype was higher than that for patients with the AA and the AG genotypes. In addition, the median and overall survival times of gastric cancer patients with the AG and the GG genotypes were significantly longer than of those with the AA genotype [8]. Similar findings were also reported in lung cancer, colorectal cancer, and ovarian cancer [9–12].
Our previous research focusing on the GSTP1 gene polymorphisms of Uygur patients with advanced NSCLC and their sensitivity to platinum-based chemotherapy found a significant increase in the sensitivity of patients with the GSTP1 rs1695 mutant genotype to platinum drugs. Based on this previous result, we further investigated genetic polymorphisms and prognosis of Uygur patients with advanced NSCLC who underwent the platinum-based chemotherapy. According to the present findings, the median PFS and median OS of patients with the GSTP1 AG and the GG genotypes were significantly longer than those of the patients with the AA genotype. Therefore, we believe that polymorphism at the GSTP1 gene rs1695 locus can predict the efficacy of platinum-based chemotherapy for Uygur patients with advanced NSCLC.
ABCC2 is also known as multidrug resistance-associated protein 2 (MRP2). It plays a very important role in the process of drug absorption, distribution, and excretion, pumping substances out of the cell by active transport. Extensive domestic and foreign studies focusing on NSCLC patients from different ethnic groups have verified that a mutant genotype in the ABCC2 rs717620 (C-24T) locus can improve the efficacy of platinum-based regimens for the treatment of patients with NSCLC and prolong their PFS and OS [13,14].
In a previous study, we investigated the genetic polymorphisms of ABCC2 rs717620, rs2273697, and rs3740066, and the sensitivity of platinum-based protocols among patients with advanced NSCLC. Our results indicated that gene polymorphism at the ABCC2 rs717620 locus was associated with sensitivity to platinum drugs. Thus, the effective rate of chemotherapy for patients with the ABCC2 CT and the TT genotypes was higher than that of patients with the CC genotype. Based on the results of this study, we further investigated the relationship between the polymorphism at ABCC2 rs717620 and prognosis of patients with advanced NSCLC treated with platinum-based chemotherapy. Our results showed that the median PFS and median OS of patients with the ABCC2 CT and the TT genotypes were significantly longer than for those with the CC genotype. This means that the ABCC2 gene polymorphism can also be utilized to predict the sensitivity of Uygur patients with advanced NSCLC to chemotherapy.
This study takes full advantage of the unique geographical isolation of Xinjiang and studied the relationship between GSTP1/ABCC2 gene polymorphisms and the outcomes of advanced NSCLC patients undergoing platinum-based chemotherapy. Our results indicate that the GSTP1 and ABCC2 gene polymorphisms are associated with the prognosis of Uygur patients with advanced NSCLC who undergo platinum-based chemotherapy. The treatment effects for patients with the GSTP1 AG or the GG genotypes and patients with the ABCC2 CT or the TT genotypes were better, with longer survival time.
These genotypes may be used as indicators to predict the effect of platinum drugs on NSCLC, to guide the individualized use of platinum drugs, and even to achieve individualized treatment. After accounting for multiple factors that underlie tumors and for differences in gene polymorphism, race, nationality, and region, the influence of the GSTP1 and ABCC2 gene polymorphisms on chemotherapeutic effects and survival may be only one important factor to consider. In addition, the small sample size of this study may have led to some selection bias. The prognosis of patients with advanced NSCLC may also be affected by other known or unknown oncogenes and their polymorphisms. In future studies, a larger sample size should be used and a variety of related gene polymorphisms should be considered, with the goal of providing a more accurate basis for the treatment and prognostication of Uygur patients with advanced NSCLC.
Conclusions
Polymorphisms at GSTP1 rs1695 and ABCC2 rs717620 can be used to predict the outcomes of Uygur patients with advanced NSCLC who have received platinum-based chemotherapy. Additionally, this information might be used to guide individualized treatment of Uygur patients with advanced NSCLC.
Footnotes
Conflict of interest
The authors report no conflicts of interest in this work.
Source of support: This study was funded by grants from the Natural Science Foundation of Xinjiang Uygur Autonomous Region (No.2013211A074)
References
- 1.Liu X, Wang XM, Liu XY, et al. [Analysis on malignant tumor cases of different ethnic groups of Xinjiang from 2010 to 2014]. Chinese Medical Record. 2016;17(11):63–65. [in Chinese] [Google Scholar]
- 2.Sun Y, Wu YL, Zhou CC, et al. Second-linepemetrexedversusdocetaxel in Chinesepatients with locallyadvanced or metastaticnon-small cell lung cancer: A randomized, open-labelstudy. Lung Cancer. 2013;79(2):143–50. doi: 10.1016/j.lungcan.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Zhong H, Feng Y, Zheng G-X, et al. A meta-analysis of the association between glutathione S-transferase P1 gene polymorphism and the risk of adenocarcinomas of lung cancer. Cancer Biomark. 2013;29(4):29–35. doi: 10.3233/CBM-130322. [DOI] [PubMed] [Google Scholar]
- 4.Ramalhinho AC, Fonseca-Moutinho JA, Breitenfeld L. Glutathione S-transferase M1, T1, and P1 genotypes and breast cancer risk: A study in a Portuguese population. Mol Cell Biochem. 2011;355(1–2):265–71. doi: 10.1007/s11010-011-0863-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Ma L, Shan L. [Relationship between mutations of epidermal growth factor receptor gene and clinicopathological features of non-small cell lung cancer in Uygur patients]. Journal of Practical Oncology. 2013;28(5):469–72. [in Chinese] [Google Scholar]
- 6.Wang Y, Spitz MR, Schabath MB, et al. Association between glutathione S-transferase P1 polymorphisms and lung cancer risk in Caucasians: A case-control study. Lung Cancer. 2003;40(1):25–32. doi: 10.1016/s0169-5002(02)00537-8. [DOI] [PubMed] [Google Scholar]
- 7.Deenen MJ, Cats A, Beijnen JH, et al. Part3: Pharmacogenetic variability in phase II anticancer drug metabolism. Oncologist. 2011;16(7):992–1005. doi: 10.1634/theoncologist.2010-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li QF, Yao RY, Liu KW, et al. Genetic polymorphism of GSTP1: prediction of clinical outcome to oxaliplatin/5-FU-based chemotherapy in advanced gastric cance. J Korean Med Sci. 2010;25(6):846–52. doi: 10.3346/jkms.2010.25.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Xian L. The association between the GSTP1 A313G and GSTM1 null/present polymorphisms and the treatment response of the platinum-based chemotherapy in non-small cell lung cancer (NSCLC) patients: A meta-analysis. Tumor Biol. 2014;154(2):1866–74. doi: 10.1007/s13277-014-1866-4. [DOI] [PubMed] [Google Scholar]
- 10.Jang SH, Kim SY, Kim HJ, et al. Timing lof chemotherapy induced neutropenia is a prognostic factor in patients with metastatic non-small cel lung cancer: A retrospective anaylsis in gemcitabineplus-platinum-treated patients. J Cancer Res Clin Oncol. 2013;139(3):409–17. doi: 10.1007/s00432-012-1341-9. [DOI] [PubMed] [Google Scholar]
- 11.Shitara K, Matsuo K, Yokoat T, et al. Prognostic factors for metastatic colorectal cancer patients undergoing irinotecan-based second-line chemotherapy. Gastrointest Cancer Res. 2011;4(5/6):168–72. [PMC free article] [PubMed] [Google Scholar]
- 12.Khrunin AV, Moisseev A, Gorbunova V, et al. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010;10(1):54–61. doi: 10.1038/tpj.2009.45. [DOI] [PubMed] [Google Scholar]
- 13.Han JY, Lim HS, Yoo YK, et al. Associations of ABCB1, ABCC2 and ABCG2 polymorphisms with irinotecan pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110(1):138–47. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
- 14.Campa D, Müller P, Edler L, et al. A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis. Int J Cancer. 2012;131(3):2920–28. doi: 10.1002/ijc.27567. [DOI] [PubMed] [Google Scholar]


