Abstract
Background
Malvidin (alvidin-3-glucoside) is a polyphenol that belongs to the class of natural anthocyanin, which is abundantly found in red wines, colored fruits, and the skin of red grapes. Therefore, the current investigation was intended to evaluate the effect of malvidin against myocardial infarction induced by isoproterenol in the rats.
Material/Methods
The cardioprotective effects was assessed by determining the effect of malvidin on the activities of endogenous antioxidants – catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH) – and on the levels of lipid peroxidation and serum marker enzymes. The serum levels of IL-6 and TNF-α were also determined using an enzyme-linked immunosorbent assay (ELISA) kit.
Result
The present study demonstrated a significant cardioprotective effect of malvidin by restoring the defensive activities of endogenous antioxidants – catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH) – and by reducing the levels of lipid peroxidation and serum marker enzymes lactate dehydrogenase (LD) and creatine kinase (CK). Malvidin significantly ameliorated the histopathological changes and impaired mitochondria in the cardiac necrosis stimulated with isoproterenol. Additionally, the results also demonstrated that nuclear translocation of Nrf-2 and subsequent HO-1 expression might be associated with nuclear factor kappa B (NF-κB) pathway activation.
Conclusions
Our findings suggest that malvidin exerts cardioprotective effects that might be due to possible strong antioxidant and anti-inflammatory activities. Therefore, this study provides the basis for the development of malvidin as a safe and effective treatment of myocardial infarction.
MeSH Keywords: Heart Diseases, Myocardial Infarction, Phytochemicals
Background
Globally, myocardial infarction (MI) is a leading cause of morbidity and mortality in developed as well as in developing countries [1,2]. MI is an important acute disease of myocardial necrosis, manifested by an imbalance between myocardial blood demand and the coronary blood delivery [3,4]. This imbalance leads to cardiac ischemia and degeneration of cardiomyocytes [5,6]. The damage to heart tissues because of ischemia eventually causes irreversible cardiac injury or death [7]. The changes in histopathology of cardiac tissues and biochemical alterations [8] associated with mitochondrial impairment, lipid peroxidation, and endogenous antioxidants in the myocardium, as well as serum markers, is the hallmark of cardiac ischemia [9].
Isoproterenol hydrochloride (ISP) is a structurally synthetic catecholamine. It is widely known as a potent beta-adrenergic agonist that produces extensive biochemical, functional, and histological alterations in the heart [10,11]. ISP is primarily applied to induce MI in experimental animals at larger doses owing to the production of substantially toxic free radicals and lipid peroxidation via an auto-oxidation process. ISP-induced MI animals show excessive generation of free radicals followed by production of oxidative stress as a result of reduced endogenous antioxidant activity [12]. The excessive production of ROS (e.g., hydroxyl radicals and superoxide anions) in the ischemic tissues causes oxidative injury to membrane proteins, lipids, and carbohydrates [13]. Several preclinical studies suggested that during myocardial ischemia, the level of oxidative stress is significantly enhanced via reactive oxygen species (ROS) and influences the development of MI [14,15]. Therefore, there is great need for a defensive antioxidant therapeutic approach to safely avert ischemic heart complications.
Several drugs have been found to be effective against cardiovascular disease, but their use is associated with adverse reactions. Therefore, many researchers have focused on nutraceuticals based on antioxidants to prevent CVD diseases with fewer adverse effects [16]. There has been great interest in the commercial exploration of dietary natural polyphenols that extensively exert antioxidant activities to promote health and well-being. Numerous studies have suggested that dietary polyphenols substantially prevent the prevalence of ischemic heart disease [17]. Moreover, moderate consumption of red wine has been associated with decreased risk of CVD and cancer [18]. This health benefit in CVD is principally associated with the presence of therapeutic polyphenols of red wine. Thus, the possible protective role of malvidin (a red wine polyphenol) was investigated in the isoproterenol-induced myocardial infarction in rats. Malvidin (Malvidin-3-glucoside) is a polyphenol and a natural anthocyanin abundant in red wines, colored fruits, and the skin of red grapes [19]. Various researchers have reported that the natural antioxidant activity of anthocyanins is closely linked to its conjugated double bond and hydroxyl groups on the B-ring [20]. Malvidin has been reported in many previous studies to be a powerful medicinal agent, and it is reported to protect human fibroblast cells against stress-induced premature senescence [21–23]. However, the cardioprotective effect of malvidin in isoproterenol-induced myocardial infarction in rats has not been documented to date. Therefore, the present study aimed to elucidate the potential cardioprotective role of malvidin in rats stimulated with ISP. The protective effect of malvidin was evaluated by assessing the marker enzymes of cardiac injury, lipid peroxidation, endogenous antioxidants, and mitochondrial impairment, as well as histopathological alterations.
Material and Methods
Animals
The current study was carried out with 36 male albino Wistar rats weighing about 150–200 g. The rats were housed in a polypropylene laboratory cage under optimum temperature (25±2°C) and humidity (50±5%) and 12: 12 h light–dark (LD) cycles. They were provided with standard laboratory rat food and water ad libitum before the experiments. All experimental procedures were carried out as per standard protocol evaluated and approved by the I.A.E.C. (Institutional Animal Ethics Committee) of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China.
Drugs, chemicals, and treatment schedule
Isoproterenol (ISP) hydrochloride, enalapril and malvidin (malvidin-3-glucoside chloride) were procured from Sigma-Aldrich Chemicals Pvt. Ltd. (St. Louis, MO, USA). All the biochemical and chemical reagents used in this study were of analytical grade (>90% pure).
A total of 36 male rats were randomly distributed into 6 groups, 6 animals in each and all groups of animal treated in the following manner:
Group I – Saline/Normal control-
Rats were administered with normal saline (2 ml/kg, p.o. per day) for 21 days.
Group II – Isoproterenol/ISP control-
Rats were administered with normal saline (2 ml/kg, p.o. per day) for 21 days and challenged with ISP (85 mg/kg, s.c.) on the 20th and 21st day.
Group III – Malvidin (100 mg/kg+ISP)-
Rats were treated with malvidin (100 mg/kg, p.o. per day) for 21 days and challenged with ISP (85 mg/kg, s.c.) on the 20th and 21st day.
Group IV – Malvidin (200 mg/kg+ISP)-
Rats were treated with malvidin (200 mg/kg, p.o. per day) 21 days and challenged with ISP (85 mg/kg, s.c.) on the 20th and 21st day.
Group V – Enalapril (10 mg/kg+ISP)-
Rats were treated with enalapril (10 mg/kg p.o. per day) for 21st days and challenged with ISP (85 mg/kg, s.c.) on the 20th and 21st day.
Group VI – Malvidin (200 mg/kg) alone group-
Rats were treated with malvidin (200 mg/kg p.o. per day) for 21st days.
Biochemical estimation in serum
The animal blood samples were taken from the retro-orbital plexus and allowed to clot at room temperature for 30 min. The serum was extracted from the blood via centrifugation at 3000 rpm (15 min at 30°C) and used for the assessment of marker enzymes, including lactate dehydrogenase (LD) and creatine kinase (CK).
Estimation of cardiac injury markers LD and CK in serum
The levels of lactate dehydrogenase (LD) and creatine kinase (CK) were estimated with kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) in serum. The procedure was performed according to the instructions provided by the supplier. The results are presented as IU/L.
Biochemical estimation in heart
Hearts were isolated from the sacrificed rats, rinsed in isotonic saline, and weighed. The myocardial tissues were homogenized with 0.1 M ice-cold phosphate buffer, pH 7.4. Postnuclear supernatant was formed by centrifugation of 10% w/v homogenates of myocardial tissues at 12000×g (20 min at 4°C). Afterward, separated supernatant aliquot was used for the biochemical estimation.
Lipid Peroxidation (LPO) quantification
The amount of lipid peroxidation (LPO) was estimated by 2-Thiobarbituric Acid Reactive Substances (TBARS) assay using the method described by Ohkawa [24]. In this assay, malondialdehyde (MDA) generated during lipid peroxidation was quantified via thiobarbituric acid (TBA) reactions. The value of TBARS is presented as nmol of MDA per mg of protein.
Estimation of catalase (CAT), reduced glutathione (GSH), and superoxide dismutase (SOD)
The extent of CAT enzyme was determined using the procedure described by Luck [25]. The procedure involves measurement of the breakdown of H2O2, and results are denoted as μmole of H2O2 decomposed/min/mg of protein. GSH enzyme was estimated using the procedure of Ellman [26], and the results are reported as μmole of the GSH/mg of protein. The level of SOD was determined according to the procedure of Kono [27]. The results are denoted as unit/mg of protein.
Estimation of mitochondrial complex
Isolation of the heart mitochondria
The heart mitochondria were isolated by standard methods of Takasawa [28]. In this procedure, the heart tissues were placed in the fresh ice-cold buffer (50 mM Tris-HCl, pH 7.4) containing the sucrose (0.25 M) and then homogenized. The formed homogenate was centrifuged at 700×g for 20 min to obtain the supernatant, which was further centrifuged at 9000×g for 15 min. The mitochondrial pellets were washed and re-suspended in Tris-hydrochloric buffer (ice-cold, 10 mM, pH 7.8), containing the sucrose (0.25 M) The obtained heart mitochondrial fractions were stored at −80°C until use for complete evaluation of enzymatic complex.
NADH dehydrogenase activity (Complex – 1)
This activity of complex-I was determined according to the method described by King and Howard [29]. Briefly, this process involves the conversion of NAD+ from NADH via catalytic oxidation along with the successive reduction of cytochrome-c.
Succinate dehydrogenase activity (Complex – 2)
Succinate dehydrogenase was determined according to the method described by King (30). The method is associated with oxidation of succinate by potassium ferricyanide.
MTT ability (Complex – 3)
According to the method of Liu (31), the reduction rate of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-H-tetrazolium bromide) by hydrogenase activity was measured in the isolated mitochondrial samples.
Cytochrome-c oxidase assay (Complex – 4)
The level of cytochrome-c oxidase was measured by the procedure of Sotocassa [32]. Briefly, the procedure involves the estimation of cytochrome-c oxidase activity in the isolated mitochondrial samples.
Histopathology examination of myocardial tissue
Hearts were extracted from sacrificed rats, washed, immediately fixed in 10% NBF (neutral buffered formalin), and embedded in paraffin. We made 5-mm-thick sections of myocardial tissues, stained them with hematoxylin and eosin (H&E), and then examined them under a light microscopic for histopathological changes.
Estimation of pro-inflammatory cytokines
The serum levels of IL-6, TNF-α were determined using an enzyme-linked immunosorbent assay (ELISA) kit (R&D System Inc, USA).
Western blot analysis
The isolated heart tissue was homogenized in ice-cold RIPA buffer (0.1% phenyl methyl sulfonyl fluoride), containing protease inhibitor (Roche Applied Science, Germany), and the supernatant was collected after centrifugation at 12000 rpm for 20 min. The protein concentration was determined using the BCA protein assay kit. The protein extracts were loaded and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the resolved proteins were transferred onto PVDF membranes. The membranes were blocked with 5% non-fat milk in Tris buffer saline and incubated at 4°C overnight with separate primary antibodies, anti-Nrf-2 (Abcam, 1: 1000), antieHOe1 (Abcam, 1: 2000), anti-p-NF-κB (CST, 1: 1000), anti-NF-κB (CST,1: 1000), anti-p-IκBα (CST, 1: 1000), and anti-IKKβ (CST, 1: 1000).
Statistical analysis of data
All results are presented as the mean ±SEM. The statistical results were evaluated using one-way ANOVA followed by post-hoc analysis using Tukey’s multiple comparison test using GraphPad Prism software. P<0.05 was considered a statistically significant difference.
Results
Effects of malvidin on LD and CK in serum
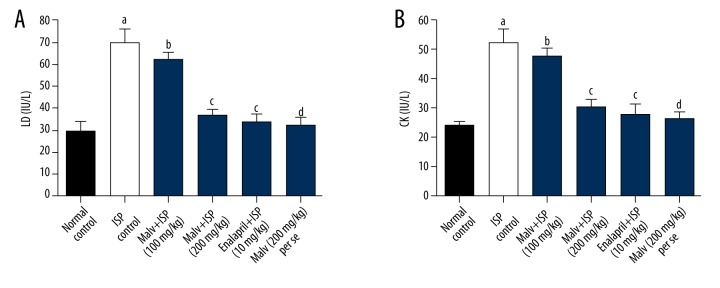
Results showed that ISP-administered rats exhibited a significant (p<0.05) rise in myocardial injury markers such as lactate dehydrogenase (LD) and creatine kinase (CK) in blood serum in comparison to saline control rats. However, the treatment with malvidin (200 mg/kg) to ISP-challenged rats caused significant (p<0.05) reduction in the levels of LD and CK in serum in comparison to ISP control rats. Further, enalapril-treated rats also exhibited a significant decrease in LD and CK in comparison to ISP control, while no significant alterations in LD and CK were noticed in the malvidin (200 mg/kg) alone group compared to saline control rats (Figure 1).
Figure 1.
Effects of malvidin on myocardial injury marker enzymes in ISP-induced MI in rats. (A) Level of lactate dehydrogenase (LD) and (B) level of creatinine kinase (CK) were measured in serum. Values are presented as mean ±S.E.M. a p<0.05, d p>0.05, compared to saline control (normal control); c p<0.05, b p>0.05, compared to ISP control. ISP – isoproterenol; Malv – malvidin; LD – lactate dehydrogenase; CK – creatinine kinase.
Effect of malvidin on oxidative damage and endogenous antioxidants in the heart
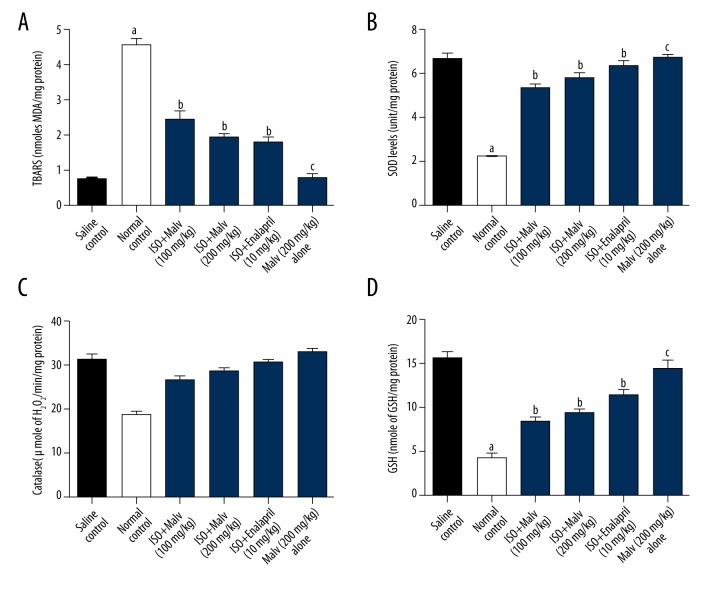
ISP administration in rats significantly (p<0.05) increased the lipid peroxidation, as indicated by the elevated level of MDA (a marker of oxidative damage), in the heart compared with saline control rats. However, malvidin (100 and 200 mg/kg) treatment showed significant (p<0.05) reduction in ISP-induced oxidative damage, as demonstrated by decreased levels of malondialdehyde (MDA), compared with saline control rats.
Furthermore, we noticed that isoproterenol (ISP) markedly (p<0.05) diminished the myocardial antioxidant enzymes CAT, SOD, and GSH, thereby indicating myocardial damage, when compared with the saline control group. Treatment with malvidin (100 and 200 mg/kg) significantly (p<0.05) reduced myocardial damage by restoring the beneficial antioxidant enzymes CAT and SOD. Additionally, GSH level was also significantly up-regulated by malvidin (100 and 200 mg/kg) treatment. Moreover, rats in the malvidin (200 mg/kg) alone group did not show significant changes in the levels of CAT, SOD, GSH, or MDA content when compared to saline control rats (Figure 2).
Figure 2.
Effects of malvidin on antioxidant enzymes in ISP-induced MI in rats. The levels of antioxidants were measured in the heart of rats. Values are presented as mean ±S.E.M. a p<0.05, c p>0.05, compared to saline control (normal control); b p<0.05, compared to ISP control. ISP – isoproterenoll Malv – malvidin; MDA – malondialdehyde; SOD – superoxide dismutase; CAT – catalase; GSH – glutathione.
Effects of malvidin on heart mitochondrial enzyme complex
The results show that ISP administration caused a notable mitochondrial dysfunction by significant (p<0.05) depletion of succinate dehydrogenase, NADH dehydrogenase, cytochrome-c oxidase enzymes activities, and MTT ability in heart mitochondria compared to saline control rats. However, we observed that ISP-induced mitochondrial dysfunction was significantly (p<0.05) decreased by treatment with malvidin (200 mg/kg) and enalapril (10mg/kg). The malvidin (200 mg/kg) alone group did not show any distinct change in heart mitochondrial complex activity or MTT ability compared to saline control rats (Figure 3).
Figure 3.
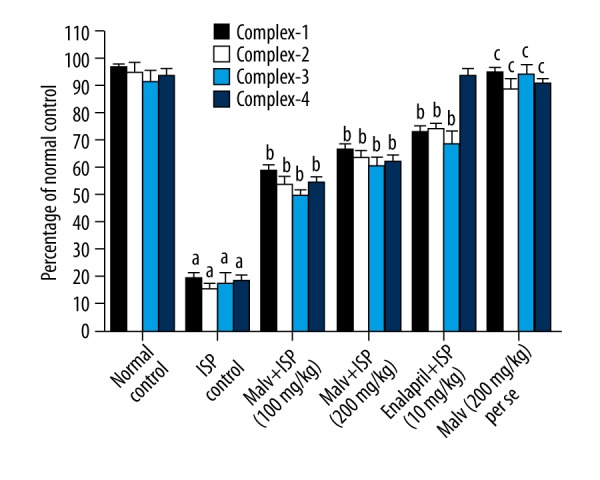
Effect of malvidin on mitochondrial enzyme complex 1, 2, 3, and 4 in heart. The levels of mitochondrial enzyme complex 1, 2, 3, and 4 were measured in the hearts of rats. Values are presented as mean ±S.E.M. a p<0.05, c p>0.05, compared to saline control (normal control); b p<0.05, compared to ISP control. ISP – isoproterenol; Malv – malvidin; Mitochondrial enzymes (complex1 – NADH dehydrogenase, complex2 – succinate dehydrogenase, complex3 – MTT ability, and complex-4 – cytochrome-c oxidase).
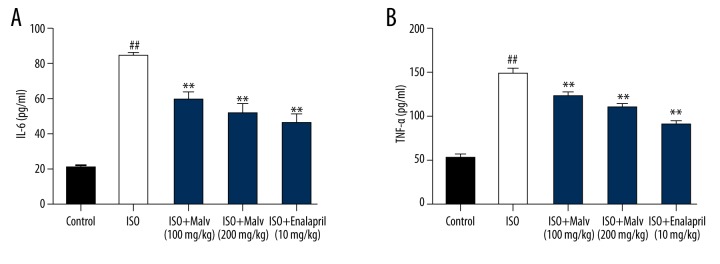
Effect of malvidin on serum pro-inflammatory cytokines
As shown in Figure 4, the serum level of IL-6 and TNF-α was significantly elevated in the ISO-treated group compared with the controls (p>0.01). Moreover, treatment with malvidin caused a significant decline in the level these pro-inflammatory cytokines in a dose-dependent manner.
Figure 4.
Effect of malvidin on the serum level of pro-inflammatory cytokines IL-6 (A) and TNF-α (B) in ISO-induced myocardial infarction in rats. Values are expressed as means ±S.E.M. Compared with control: # P<0.05; ## P<0.01; ### P<0.001; Compared with model: * P<0.05; ** P<0.01; *** P<0.001.
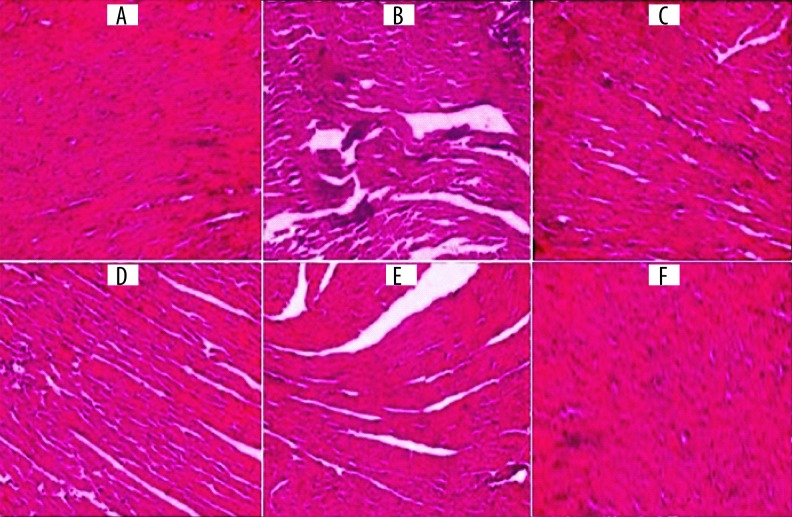
Effect of malvidin treatment on histopathological changes
In this histopathological examination, normal saline control rats showed a clear, intact homogeneous structure of the myocardium, with no sign of edema or inflammation. On the other hand, rats administered with ISP had moderate-to-marked myocardium necrosis and edema, along with infiltration of inflammatory cells like lymphocytes and macrophages. Further, the rats treated with malvidin (100 and 200 mg/kg) and enalapril (10 mg/kg), compared to ISP-challenged rats, showed markedly improved myocardial necrosis, with decreased edema and infiltration of lymphocyte cells and macrophage cells. However, malvidin (200 mg/kg) treatment showed more improvement in the damaged myocardial tissue compared to malvidin (100 mg/kg). The malvidin (200 mg/kg) alone group presented similar histoarchitecture of the myocardium as in normal rats (Figure 5).
Figure 5.
Effect of malvidin treatment on histopathological changes in myocardium (cardiac tissue light micrograph at magnification 100×). (A) Saline control (normal control) rats showed clearly normal histoarchitecture of the myocardium; (B) ISP (85 mg/kg) control group presented moderate-to-marked myocardium necrosis, edema, and infiltration of inflammatory cells; (C) Malvidin (100 mg/kg) group presented moderate myocardium necrosis with lesser edema and infiltration of inflammatory cells compared to the ISP control group; (D) The malvidin (200 mg/kg) group presented mild myocardial necrosis with a significant decrease in edema and infiltration of inflammatory cells compared to the ISP control group; (E) The enalapril (10 mg/kg) group presented mild necrosis with a significant decrease in edema and focal infiltration of inflammatory cells compared to the ISP control group; (F) The malvidin (200 mg/kg) alone group presented similar histoarchitecture of myocardium as in the saline control (normal control) rats.
Determination of malvidin on HO-1 and Nrf-2 protein expressions in the heart
As shown in Figure 6, the ISO-treated rats showed significant decline in HO-1 level compared to the control group (P<0.01). Malvidin treatment caused restoration of the ISO-induced HO-1 level. However, the level of Nrf-2 was significantly decreased after administration of ISO, and the expression was reversed in a concentration-dependent manner after the introduction of malvidin.
Figure 6.
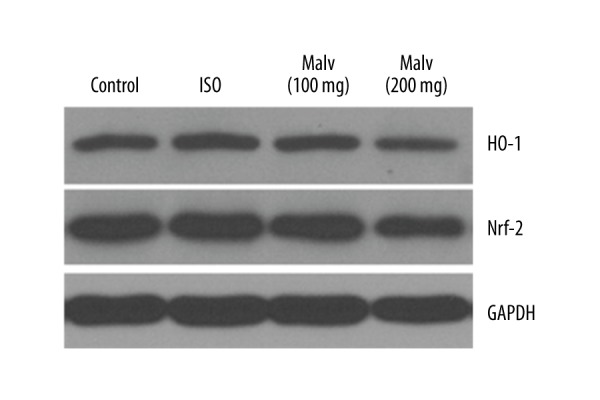
Effect of malvidin treatments significantly increased HO-1 induction and activated nuclear respiratory factor 2 (Nrf-2) translocation in ISO-induced myocardial infarction in rats. Representative immunoblots for HO-1 and Nrf-2.
Effect of malvidin on NF-κB signalling pathway
As presented in Figure 7, we found that malvidin caused significant inhibition of phosphorylated IκB-α and NF-κB expression in comparison to the myocardial tissues following challenge by ISO.
Figure 7.
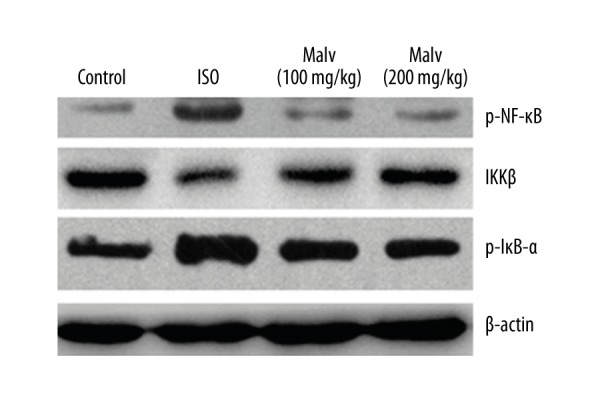
Effect of malvidin on IκB-α and NF-κB phosphorylation determined by Western blot analysis.
Discussion
Globally, CVD is a critical health problem. It is predicted that by 2023, CVD will cause more than 20 million deaths annually [33]. Myocardial infarction (MI), a serous CVD, is a prominent cause of morbidity and mortality throughout the world [34]. Cardiotoxicity caused by isoproterenol (ISP) at a larger dosage in an animal model exhibits a better discernment in the pathology of myocardial damage and clearly indicates involvement of oxidative stress. Several studies have proven the protective function of dietary polyphenols in the treatment of hypertension, cardiovascular disease (CVD), and diabetes [35]. Dietary natural polyphenols extensively exert antioxidant activities and are widely reported to prevent ischemic heart disease [17]. Moreover, a variety of studies have suggested that malvidin exhibits considerable antioxidant activity [22,23,36]. Collectively, on the basis of these findings, we intended to investigate the cardioprotective effects of malvidin (a red wine polyphenol) in isoproterenol-induced myocardial infarction in rats. The current investigation showed that malvidin exerts potential cardioprotective effects in ISP-induced oxidative cardiac damage as indicated by reduced levels of marker enzymes of cardiac injury and lipid peroxidation and by restoring endogenous antioxidant activities. Moreover, malvidin effectively ameliorated the mitochondrial dysfunction and histological changes in the myocardium. During the investigation, we observed that serum enzymes LD and CK activities were significantly increased in the ISP-treated rats, suggesting the leakage of marker enzymes LD and CK from necrotic cardiac cells into blood circulation. The release of LDH1 and LDH2 (cardiac specific isoenzymes) in the blood circulation indicates necrosis caused by ISP stimulation [37]. The higher concentration of malvidin (200 mg/kg) treatment to ISP-challenged rats drastically reduced the marker enzymes LD and CK in serum and indicates a cardioprotective action of malvidin. A severe cellular injury to cardiac tissues induced via ISP is commonly manifested by the elevated MDA content (a bioactive marker of lipid peroxidation), which is closely linked to the enzyme inactivation and changed membrane structure. Subsequently, it promotes the necrosis of myocardial membranes and eventually leads to severe, irreversible damage to the myocardium [38].
In this study, the results reveal that ISP significantly raised the level of MDA (a marker of oxidative damage) and increased production of lipid peroxidation along with excessive generation of free radicals. These effects are most likely due to auto-oxidation of ISP. On the other hand, malvidin (100 and 200 mg/kg) treatment significantly decreased the MDA levels in ISP-induced MI, thereby showing good protection against myocardial oxidative damage. Similarly, a previous study also supported that malvidin prevents lipid peroxidation and oxidative stress in mouse forebrain homogenates, possibly due to its ROS scavenging properties [39]. An antioxidant system of endogenous enzymes containing hydroxyl radical and superoxide anions and composed of CAT, SOD, and GSH is the prominent cellular safeguard against oxidative stress, and this system also helps to avert ROS generation [40]. Our study shows that the endogenous marker enzymes CAT, SOD, and GSH were significantly reduced in ISP-induced infarcted rats.
This indicates oxidative damage of cardiac tissue due to excessive formation of free radicals induced by isoproterenol. However, malvidin treatment in ISP-induced infarcted rats remarkably restored the levels of CAT, SOD, and GSH, which may be correlated with the antioxidant effects of malvidin by quenching of free radicals. Mitochondria are a major intracellular source of ROS formation and thus are a target for oxidative injury mediated by ROS in the heart. Increased production of free radicals through excessive ROS generation leads to damage of cell membranes, fatty acid oxidation, and deactivation of TCA. Thus, an alteration in enzyme activity of the TCA cycle involves free radical-mediated cardiac damage. Several studies have demonstrated that cardiac dysfunction due to abnormal mitochondrial membrane structure and its altered content are elemental characteristics of myocardial infarction [41,42]. We found that malvidin treatment noticeably up-regulated the mitochondrial complexes such as succinate dehydrogenase, NADH dehydrogenase, cytochrome oxidase, and MTT ability in ISP-induced infarcted rats. Our results reveal that malvidin effectively restored the normal levels of mitochondrial enzymes, possibly due to its free radical neutralization activities, thereby suggesting preventive effects against mitochondrial dysfunction.
During our histopathological investigation, normal control saline rats exhibited a clear, intact homogeneous structure of myocardium with no sign of edema and inflammation. In contrast, ISP-induced infarcted rats presented moderate-to-marked myocardium necrosis with edema and infiltration of inflammatory cells (lymphocytes and macrophages). Moreover, rats treated with malvidin had decreased necrosis with edema and infiltration of lymphocyte cells and macrophage cells. This finding presents a marked improvement in myocardium necrosis, possibly due to the antioxidant effects of malvidin. It is extensively reported that the distinct patterns of hydroxylation and methoxylation, basically on the B-ring, regulate particular antioxidant attributes of polyphenols [20,43]. Thus, the polyphenolic structural activity of malvidin may contribute strong antioxidant effects. The conversion of heme to its end-products is the rate-limiting step catalyzed by the stress protein HO-1 in response to oxidative stress. The transcription factor Nrf-2 has been considered as a key molecule regulating the activity of GSH, GPx, ROS, and HO-1. Thus, once activated, the Nrf-2 moves into the nucleus and binds to the antioxidant responsive element (ARE) positioned in the promoter region of antioxidant and phase II enzymes to augment antioxidant capability and re-establish redox homeostasis [44]. In the present study, Nrf-2 levels were found to be significantly lower in the heart tissue of ISO-treated rats, reflecting a reduced antioxidant and homeostatic capacity. Malvidin treatment increased the HO-1 expression and Nrf-2 translocation in the myocardium, suggesting that its cardioprotective effects were through activating the Nrf-2/HO-1 pathway. The critical factor in cardiovascular disease is the generation of inflammatory response; therefore, anti-inflammatory agents play a significant role in attenuation of these responses. Studies have shown that, in myocardial insults, pro-inflammatory cytokines (e.g., TNF-α and IL-6) are found in elevated concentrations and are greatly dependent upon the activation of NF-κB [45]. In dormant cells, NF-κB is not able to bind in the cytoplasm by interacting with the inhibitory kappa B (IκB) family. Thus, after simulation, IκBa is phosphorylated and degraded by the IκB kinase (IKK) complex, which allows NF-κB to be translocated from the cytosol to the nucleus, where it can bind to the promoter region of target genes. We found that, after ISO insult, the levels of TNF-α and IL-6 were elevated. However, in the malvidin-treated group, the levels of these cytokines were significantly decreased in the serum, which might be the cause of its cardioprotective effects via anti-inflammatory properties. The results further indicated that malvidin treatment inhibited the upstream IKK and IκBa phosphorylation, as well as IκBa degradation. Moreover, we also found that ISO-induced nuclear translocation of NF-κB p65 was significantly attenuated by malvidin. Collectively, our observations support the benefit of moderate consumption of red wine as a way to decrease risk of cardiovascular disease, and we hope our results contribute to the development of safe and effective nutraceuticals based on malvidin.
Conclusions
Our results suggest that malvidin exhibits strong cardioprotective effects against isoproterenol-stimulated myocardial infarction in rats. The protective effects of malvidin occur by restoring the defensive activities of endogenous antioxidants CAT, SOD, and GSH by reducing the levels of lipid peroxidation and serum marker enzymes LD and CK. Additionally, malvidin significantly ameliorated the histopathological changes and impaired mitochondria in the cardiac necrosis produced by isoproterenol. Overall, our findings suggest that the cardioprotective effects of malvidin be due to its antioxidant properties. Therefore, we hope that the present investigation offers information that will be useful in developing a safe and effective malvidin-based therapy, which could be an effective treatment of cardiovascular disease. Nevertheless, more molecular-level studies are needed for further validation.
Footnotes
Conflict of interest
The authors have declared no conflict of interest.
Source of support: Departmental sources
References
- 1.Steffens S, Montecucco F, Mach F. The inflammatory response as a target to reduce myocardial ischaemia and reperfusion injury. Thromb Haemost. 2009;102:240–47. doi: 10.1160/TH08-12-0837. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah MH, Arnaout S, Karrowni W, Dakik HA. The management of acute myocardial infarction in developing countries. Int J Cardiol. 2006;111:189–94. doi: 10.1016/j.ijcard.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Li G, Zhao W, Hu Y. Inhibition of MiR92a may protect endothelial cells after acute myocardial infarction in rats: Role of KLF2/4. Med Sci Monit. 2016;22:2451–62. doi: 10.12659/MSM.897266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang F, Liu W, Yan X, et al. Effects of mir-21 on cardiac microvascular endothelial cells after acute myocardial infarction in rats: Role of phosphatase and tensin homolog (PTEN)/vascular endothelial growth factor (VEGF) signal pathway. Med Sci Monit. 2016;22:3562–75. doi: 10.12659/MSM.897773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temesszentandrasi G, Vörös K, Márkus B, et al. Human fetuin-A Rs4918 polymorphism and its association with its association with obesity in healthy persons and in patients with myocardial infarction in two hungarian cohorts. Med Sci Monit. 2016;22:2742–50. doi: 10.12659/MSM.896232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou C, Cui Q, Su G, et al. MicroRNA-208b Alleviates post-infarction myocardial fibrosis in a rat model by inhibiting GATA4. Med Sci Monit. 2016;22:1808–16. doi: 10.12659/MSM.896428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98:1355–57. doi: 10.1161/01.cir.98.14.1355. [DOI] [PubMed] [Google Scholar]
- 8.Zou Y, Lin L, Xiao H, Xiang D. A rare case of toxic myocarditis caused by bacterial liver abscess mimicking acute myocardialinfarction. Am J Case Rep. 2016;17:1–5. doi: 10.12659/AJCR.895350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basha RH, Priscilla DH. An in vivo and in vitro study on the protective effects of N-acetylcysteine on mitochondrial dysfunction in isoproterenol treated myocardial infarcted rats. Exp Toxicol Pathol. 2013;65:7–14. doi: 10.1016/j.etp.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Prabhu S, Jainu M, Sabitha KE, et al. Cardioprotective effect of mangiferin on isoproterenol induced myocardial infarction in rats. Indian J Exp Biol. 2006;44:209–15. [PubMed] [Google Scholar]
- 11.Manju V, Murugesan M, Revathi R. Cardioprotective effect of fenugreek on isoproterenol-induced myocardial infarction in rats. Indian J Pharmacol. 2011;43:516–19. doi: 10.4103/0253-7613.84957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathore N, Kale M, John S, Bhatnagar D. Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat erythrocytes. Indian J Physiol Pharmacol. 2000;44:161–66. [PubMed] [Google Scholar]
- 13.McMichael M, Moore RM. Ischemia-reperfusion injury pathophysiology, part I. J Vet Emerg Crit Care. 2004;14:231–41. [Google Scholar]
- 14.Hill MF, Palace VP, Kaur K, et al. Reduction in oxidative stress and modulation of heart failure subsequent to myocardial infarction in rats. Exp Clin Cardiol. 2005;10:146–53. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Zhou Y, Zhang Y, et al. Antioxidant probucol attenuates myocardial oxidative stress and collagen expressions in post-myocardial infarction rats. J Cardiovasc Pharmacol. 2009;54:154–62. doi: 10.1097/FJC.0b013e3181af6d7f. [DOI] [PubMed] [Google Scholar]
- 16.Alissa EM, Ferns GA. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J Nutr Metab. 2012;2012:569486. doi: 10.1155/2012/569486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginter E, Simko V. Plant polyphenols in prevention of heart disease. Bratislava Med J. 2012;113:476–80. doi: 10.4149/bll_2012_105. [DOI] [PubMed] [Google Scholar]
- 18.Arranz S, Chiva-Blanch G, Valderas-Martínez P, et al. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients. 2012;4:759–81. doi: 10.3390/nu4070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintieri AM, Baldino N, Filice E, et al. Malvidin, a red wine polyphenol, modulates mammalian myocardial and coronary performance and protects the heart against ischemia/reperfusion injury. J Nutr Biochem. 2013;24:1221–31. doi: 10.1016/j.jnutbio.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Sekher Pannala A, Chan TS, O’Brien PJ, Rice-Evans CA. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem Biophys Res Commun. 2001;282:1161–68. doi: 10.1006/bbrc.2001.4705. [DOI] [PubMed] [Google Scholar]
- 21.Seo HR, Choi MJ, Choi JM, et al. Malvidin protects WI-38 human fibroblast cells against stress-induced premature senescence. J Cancer Prev. 2016;21:32–40. doi: 10.15430/JCP.2016.21.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azevedo J, Teixeira N, Oliveira J, et al. Effect of sugar acylation on the antioxidant properties of Vitis vinifera red grape malvidin-3-glucoside. Int J Food Sci Technol. 2011;46:343–49. [Google Scholar]
- 23.Bognar E, Sarszegi Z, Szabo A, et al. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS One. 2013;8(6):e65355. doi: 10.1371/journal.pone.0065355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–58. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Luck H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Academic Press; New York: 1971. pp. 885–93. [Google Scholar]
- 26.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 27.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 28.Takasawa M, Hayakawa M, Sugiyama S, et al. Age-associated damage in mitochondrial function in rat hearts. Exp Gerontol. 1993;28:269–80. doi: 10.1016/0531-5565(93)90034-b. [DOI] [PubMed] [Google Scholar]
- 29.King TE, Howard RL. Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. In: Estabrook RW, editor. Methods in enzymology. Vol. 10. Academic Press; 1967. pp. 275–94. [Google Scholar]
- 30.King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. In: Estabrook RW, editor. Methods in enzymology. Vol. 10. Academic Press; 1967. pp. 322–31. [Google Scholar]
- 31.Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69:581–93. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- 32.Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A. An electron transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967;32:415–38. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:2011–30. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denollet J, Sys SU, Brutsaert DL. Personality and mortality after myocardial infarction. Psychosom Med. 1995;57:582–91. doi: 10.1097/00006842-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Pandey KB, Rizvi SI. Current understanding of dietary polyphenols and their role in health and disease. Curr Nutr Food Sci. 2009;5:249–263. [Google Scholar]
- 36.Rossetto M, Vanzani P, Mattivi F, Lunelli M, Scarpa M, Rigo A. Synergistic antioxidant effect of catechin and malvidin 3-glucoside on free radical-initiated peroxidation of linoleic acid in micelles. Arch Biochem Biophys. 2002;408:239–45. doi: 10.1016/s0003-9861(02)00561-1. [DOI] [PubMed] [Google Scholar]
- 37.Priscilla DH, Prince PSM. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact. 2009;179:118–24. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Madhesh M, Vaiyapuri M. Luteolin a dietary flavonoid attenuates isoproterenol-induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed Prev Nutr. 2013;3:159–64. [Google Scholar]
- 39.Matsunaga N, Imai S, Inokuchi Y, et al. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol Nutr Food Res. 2009;53:869–77. doi: 10.1002/mnfr.200800394. [DOI] [PubMed] [Google Scholar]
- 40.Rathore N, John S, Kale M, Bhatnagar D. Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol Res. 1998;38:297–303. doi: 10.1006/phrs.1998.0365. [DOI] [PubMed] [Google Scholar]
- 41.Kornfeld OS, Hwang S, Disatnik M-H, et al. Mitochondrial reactive oxygen species at the heart of the matter: New therapeutic approaches for cardiovascular diseases. Circ Res. 2015;116:1783–99. doi: 10.1161/CIRCRESAHA.116.305432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao L, Laude K, Cai H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am – Small Anim Pract. 2008;38:137–55. doi: 10.1016/j.cvsm.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roleira FMF, Tavares-Da-Silva EJ, Varela CL, et al. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015;183:235–58. doi: 10.1016/j.foodchem.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, An C, Gao Y, et al. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]