Abstract
Balanites rotundifolia (BR) (Van Tiegh.) Blatter (Balanitaceae) has been used in Ethiopian folk medicine to treat malaria, despite the lack of scientific validation. Therefore, the present study was carried out to evaluate the antiplasmodial activity of 80% methanol leaf extract of BR in mice. Both the 4-day suppressive test and Rane’s test were employed. Three extract doses (BR100 mg/kg, BR200 mg/kg, and BR400 mg/kg/d) were given orally, and chloroquine was the standard drug administered through the same route. Outcome measures for evaluating antiplasmodial efficacy were parasitemia level, packed cell volume, survival time, and body temperature as well as body weight change. Moreover, preliminary phytochemical and acute toxicity studies were carried out. With the 4-day suppressive test, BR demonstrated dose-dependent significant reduction in parasitemia level at all test doses compared to the negative control: BR400 (67%, P<0.001), BR200 (42%, P<0.01), and BR100 (37%, P<0.05). With Rane’s test as well, BR significantly (P<0.001 for all test doses) reduced the parasitemia level by 38% (BR100), 45% (BR200), and 69% (BR400) in comparison to vehicle treatment. The crude extract was estimated to have oral median lethal dose higher than 2,000 mg/kg, and the presence of alkaloids and cardiac glycosides was confirmed. Therefore, this study for the first time validated the antiplasmodial activity of crude leaf extract of BR. Further investigations for isolating specific phytochemicals and elucidating mechanisms are needed to address the quest for novel antimalarial drugs.
Keywords: antimalaria, antiplasmodial, Balanites rotundifolia, in vivo, Plasmodium berghei, traditional medicine
Introduction
Despite a global fall in mortality rates since 2000, malaria remains a worldwide problem, with 198 million cases and 584,000 deaths in 2013 alone. The burden is high, particularly, in the African region, where 90% of all cases and deaths occur.1 The disease has been estimated to cost the continent more than US$12 billion every year in lost gross domestic product and an average loss of 1.3% of economic growth per year.2 It is prevalent in 75% of the land of Ethiopia, putting over 45 million people at risk and 4–5 million clinical cases each year.3 Plasmodium falciparum and Plasmodium vivax contribute to malaria morbidity in the nation in relative proportions of 60% and 40%, respectively.4
Management of malaria has been challenged by resistance development against the drugs. Chloroquine-resistant P. falciparum has spread explosively in sub-Saharan Africa, South East Asia, and South Asia.5 Resistance to sulfadoxine–pyrimethamine is also widespread, and mefloquine and quinine resistance has been reported.6 Recent evidence of failure of artesunate–mefloquine combination therapy on the Thailand–Cambodia border and of resistance to artemisinin have raised concerns about the failure of the last effective antimalarial drugs.7
Such resistance to antimalarial agents has been implicated in the spread of malaria to new areas and reemergence of malaria in areas where the disease had been eradicated.8 Therefore, new chemotherapeutic tools are urgently needed. This search can follow phytochemical investigation of medicinal plants used in traditional medicine.9 Worldwide, the traditional pharmacopeia has played and continues to play a very important role in the discovery of new molecules of therapeutic interest. Natural products are the origin of approximately two-thirds of all drugs introduced in the past 30 years.10 Majority of antimalarial drugs have been derived from medicinal plants or from structures modeled on plant-derived compounds. These include the quinoline-based antimalarials as well as artemisinin and its derivatives.11 As a result of this, interest in phytomedicine is renewed during the last decade, and many medicinal plant species are being screened for pharmacological activities with possible role in drug discovery.12 Despite the scientific advances made by modern medicine, the World Health Organization estimates that 80% of Africa’s population still uses traditional medicine for primary health care.13 The World Health Organization supports the use of medicinal plants, provided they are proven to be efficacious and safe.14
Malaria is known to be the leading cause of mortality and morbidity in the Afar Regional state of Ethiopia, which is the site for plant collection in the present study.15 In the region, malaria is one of those diseases that are commonly treated by herbal drugs, as reported by Seifu et al,16 signifying the presence of important indigenous knowledge regarding the treatment of malaria. The analysis of traditional medicines that are employed for the treatment of malaria represents a potential for discovery of lead molecules for the development of antimalarial drugs, as history of quinine and artemisinin derivatives confirmed.17
Balanites rotundifolia (locally known as Qaalayto in Afaraf and Kulen in Somali) (BR) belongs to the family Balanitaceae. The Balanitaceae family contains flowering plants of 1 genus and around 25 species. Most members of the family are small trees and shrubs.18 The plant has been widely used for treatment of malaria.16,19–21 It is also a folk remedy for pneumonia in animal,22 gastrointestinal illness and intestinal parasites,23 asthma, cough,21 eye infection, and it also functions as an emetic agent.24 Even though there are no secondary metabolites in the B. rotundifolia identified so far, there are biomolecules like oxime of 6-phenyl-2(H)-1,2,4-triazine-5-one, which is identified from the extract of similar family, Balanites aegyptiaca. Such compounds have only been extracted from B. aegyptiaca. The removal of this chemical from the extract was evidenced by 50% decreased inhibition of antiplasmodial activity.25 However, no data are available for validating the antimalarial use of B. rotundifolia. Hence, this study was designed to evaluate the antiplasmodial activity of B. rotundifolia against chloroquine-sensitive strain of Plasmodium berghei in mice.
Materials and methods
Collection of plant material
Fresh aerial parts of B. rotundifolia were collected in Sep-tember 2015 from the Afar region. These were then identified and authenticated at the National Herbarium, Addis Ababa University, Addis Ababa, Ethiopia, where a voucher specimen (AS 002) was deposited.
Preparation of plant extract
The leaves of B. rotundifolia were cleaned of extraneous matter and air-dried under shade at room temperature. It was then coarsely powdered using a mortar and pestle, and a weighed amount of the powder was macerated using 80% methanol for 72 h with occasional stirring. The resultant mixture was filtered using gauze and Whatman filter paper (Grade 1). The residue was further remacerated twice. Finally, the filtrates were combined together, concentrated using oven (Leaders Engineering, Hastings, UK) at 40°C, and the extract was placed in screw cup vials in refrigerator until usage. The extract was reconstituted in 2% Tween 80 at appropriate concentrations for the various experiments conducted. The 80% methanolic extract of leaves of B. rotundifolia using maceration technique resulted in 3.13% yield.
Experimental animals
Healthy male Swiss albino mice (aged 6–8 weeks, weighting 27–32 g) were obtained from the animal house of the Department of Pharmacology, Wollo University. They were maintained under standard conditions (temperature of 22°C±3°C, relative humidity of 40%–50%, and 12 h light/12 h dark cycle) with food and water ad libitum. The care and the handling of the mice were according to the international guidelines for the use and maintenance of experimental animals,26 and the institutional review board of Wollo University has approved the protocol. The mice were allowed 1-week acclimatization to the experimental environment prior to the study.
Parasite
Chloroquine-sensitive P. berghei ANKA strain was obtained from the Ethiopian Health Nutrition and Research Institute (EHNRI) and was subsequently maintained in the laboratory by serial blood passage from mouse to mouse on a weekly basis.
Phytochemical screening
The 80% methanol extract of leaves of B. rotundifolia was subjected to qualitative phytochemical screening for the presence of terpenoids, tannins, flavonoids, cardiac glycosides, and alkaloids according to the standard methods.27,28
Test for terpenoids
The dried 80% methanolic extract (100 mg) was dissolved in a mixture of methanol and water. Five milliliters of plant extract was mixed in 2 mL of chloroform, followed by the careful addition of 3 mL concentrated H2SO4. A layer of the reddish-brown coloration formed at the interface indicates a positive result for the presence of terpenoids.
Test for tannins
The 80% methanol extract (3 g) was heated in a test tube with 10 mL of distilled water on a water bath for 5 min. After cooling, the solution was filtered using a filter paper, and 5 mL of 2% sodium chloride was added to the clear filtrate. The suspension was filtered (Whatman No. 1), and 5 mL of 1% gelatin was added to the clear filtrate. Then, the filtrate was observed whether it gives a precipitate that disappears upon addition of excess gelatin solution, indicating the presence of tannins.
Test for flavonoids
The dried 80% methanolic extract (100 mg) was dissolved in a mixture of methanol and water. To 2 mL of the extract solution, three to five drops of 2% lead acetate solution were added. Then, it was observed whether it develops yellow or orange color that indicates the presence of flavonoids.
Keller–Killiani test for cardiac glycosides
Half a gram of dried extract was placed into a test tube. About 20 mL of distilled water was added, and after 24 h, the extract was filtered using a Whatman No. 1 filter paper. Thereafter, 5 mL of the extract was treated with 2 mL of concentrated glacial acetic acid and two drops of 0.1% ferric chloride solution. This mixture was then carefully added to 1 mL of the concentrated sulfuric acid. The interface was observed for coloration.
Test for alkaloids
Thoroughly ground material (2 g) was treated in a test tube with 10 mL of 1% HCl for 30 min in a water bath. The suspension was filtered into a test tube using cotton and was divided into two parts. To one part of the solution, five drops of Dragendorff’s reagent were added; to the other part, five drops of Mayer’s reagent were added. If alkaloids are present, the test with Dragendorff’s reagent should form a yellowish-orange precipitate or a whitish opalescence with Mayer’s reagent.
Acute toxicity testing
The acute toxicity median lethal dose (LD50) of B. rotundifolia extract was estimated orally in healthy female Swiss albino mice, following the 2008 Organization for Economic Cooperation and Development (OECD) guideline 425.29 Accordingly, five female albino mice of 6–8 weeks were used. All mice were fasted (food but not water) for 4 h before and 2 h after the administration of the extract. First, a sighting study was performed to determine the starting dose. For this, 2,000 mg/kg was given for single female mouse by oral gavage. No death was observed within 24 h. As a result, additional four mice were used and were administered the same dose of the extract. The animals were housed separately and observed continuously for 4 h with 30 min interval and then for 14 consecutive days with an interval of 24 h for the general signs and symptoms of toxicity, like food and water intake; mortality; changes in skin and fur, eyes, and mucous membranes; and respiratory and behavior patterns.
Grouping and dosing of animals
The mice were divided into negative control (NC), positive control, and three test groups of seven animals each. The NC received 2% Tween 80, and the positive control received chloroquine (25 mg/kg, Addis pharmaceutical company, Adigrat, Ethiopia), and the test groups received orally different doses of the 80% methanol leaf extract of B. rotundifolia (100, 200, and 400 mg/kg body weight [BWt]). These doses were selected according to the result obtained from the acute toxicity test study.
In vivo antiplasmodial activity
For testing the antimalarial activity of the extracts, 4-day suppressive test30 and Rane’s test31 were applied. Blood from a donor mouse, with a rising parasitemia of about 20%–30%, was diluted in normal saline treated with sodium citrate, so that each 0.2 mL contains about 107 infected red blood cells (RBCs). For the 4-day suppressive test and Rane’s test, the three groups were given BR100, BR200, and BR400, respectively. The remaining two groups were receiving 2% Tween 80 and the standard chloroquine 25 mg (CQ25). All treatments were given by oral gavage. For the 4-day suppressive test, treatment was started 2 h postinfection and was continued daily for 4 days. For Rane’s test, the administered doses are similar to the 4-day suppressive test, but the treatments were delayed for 72 h to allow parasitemia to establish. On the fifth day for the 4-day suppressive test and on the eighth day for Rane’s test, a drop of blood was taken from tail snip of each mouse on frosted slide, and smears were prepared, fixed with methanol, and stained with 10% Giemsa solution at pH 7.2 for 15 min. Then, five fields were randomly selected on each stained slide and examined under microscope with an oil immersion objective (×100 magnification power). The parasitemia level was determined by counting the number of parasitized erythrocytes on randomly selected fields of the slide. The parasite count was done by an experiment-blinded technician, and percent age parasite suppression was calculated using the following formula.32
Average percentage parasitemia suppression = (A–B)/A where A is parasitemia in NCs and B is parasitemia in treatment groups.
Daily measurements of rectal temperature (RT), BWt, and packed cell volume (PCV) of the mice were measured. PCV was measured to predict the effectiveness of the test extracts in preventing hemolysis resulting from increasing parasitemia associated with malaria, using Wintrobe method.33
PCV = Volume of erythrocytes in a given volume of blood/Total blood volume
Data analysis
Results of the study were expressed as mean ± standard error of mean. Comparison of parasitemia suppression, BWt, PCV, and RT was determined by one-way analysis of variance (ANOVA), followed by post hoc Tukey’s test with SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) at 95% confidence interval.
Ethical clearance
The animals were handled according to the international guidelines for the use and maintenance of experimental animals,26 and the institutional review board of Wollo University has approved the protocol.
Results
This study explored the phytochemical contents, the acute toxicity, as well as the antiplasmodial activity of 80% methanol extract of leaves of B. rotundifolia against P. berghei in mice.
Phytochemical screening
Preliminary phytochemical screening of the 80% methanol leaf extract of B. rotundifolia revealed the presence of alkaloids and cardiac glycosides. Terpenoids, flavonoids, and tannins were not detected.
Acute oral toxicity
With the acute toxicity test at the limit test dose of 2,000 mg/kg, neither mortality nor changes related to behavioral, autonomic, neurological, and physical profile were observed within the first 24 h and during the 14 days’ follow-up.
Four-day suppressive test
The result of 4-day suppressive test indicated that the extract produced significant parasitemia reduction in a dose-dependent manner compared to NC. The BR400 resulted in highest percentage parasite suppression activity (67%, P<0.001), followed by BR200 (42%, P<0.01) and BR100 (37%, P<0.05). The increase in survival days of mice infected with P. berghei was significant only with BR400 treatment compared to NC, BR100, and BR200 treatments (17.3±1.61 days, P<0.001). The standard drug, CQ25, demonstrated the highest effect on percentage parasitemia, percentage parasite suppression, and survival time (Table 1).
Table 1.
Effect of Balanites rotundifolia on percentage parasitemia, and survival time of Plasmodium berghei-infected mice in the 4-day suppressive test
| Group | Parasitemia level | % parasite suppression | Survival days |
|---|---|---|---|
| NC | 27.84±2.60 | 0 | 7.71±0.29 |
| BR100 | 17.67±2.95 | 36.5a1 | 10.71±0.87 |
| BR200 | 16.11±2.30 | 42.1a2 | 10.71±0.64 |
| BR400 | 9.22±2.15 | 66.9a3 | 17.29±1.61a3b3c3 |
| CQ25 | 0.00±0.00 | 100a3b3c3 | 20.00±0.00a3b3c3 |
Notes: Data are expressed as percentage and mean ± SEM (n=7).
As compared to NC;
as compared to BR100 mg/kg;
as compared to BR200 mg/kg;
P<0.05;
P<0.01;
P<0.001.
Abbreviations: BR, Balanites rotundifolia; CQ, chloroquine; NC, negative control; SEM, standard error of mean.
Despite a decrease in parasitemia level, treatment with the crude leaf extract of B. rotundifolia did not prevent loss of BWt in P. berghei-infected mice. However, the decrease in BWt after extract treatment was not statistically significant among the different doses of B. rotundifolia. BR400 showed the smallest weight-loss effect compared to BR100, BR200, and NC.
B. rotundifolia extract treatment also prevented a significant decrease in PCV in a dose-dependent manner. The decrease in PCV was highest for BR100 (P<0.001) followed by BR200 (P<0.05) and BR400 (P>0.05) as compared to CQ25. In contrast, no difference was noted in percentage change of PCV between B100 and NC (Table 2).
Table 2.
Effect of Balanites rotundifolia on BWt and PCV of Plasmodium berghei-infected mice in the 4-day suppressive test
| Dose, mg/kg | BWt D0 | BWt D4 | % change | PCVB | PCVA | % change |
|---|---|---|---|---|---|---|
| NC | 31.79±0.69 | 29.47±0.50 | –7.3±1.717 | 62.57±0.75 | 52.43±1.29 | –16.2±2.18 |
| BR100 | 33.57±1.33 | 29.51±1.22 | –12.1±2.474 | 60.14±1.14 | 47.57±1.23 | –20.9±1.16c2d3 |
| BR200 | 28.46±1.36 | 24.94±1.23 | –12.4±1.476 | 45.43±1.48 | 40.57±1.61 | –10.7±1.86b2d2 |
| BR400 | 34.04±0.72 | 31.57±1.18 | –7.3±2.262 | 56.42±2.03 | 55.85±1.5 | –1.0±2.32a2b3c2 |
| CQ25 | 28.47±1.41 | 29.54±1.31 | 3.8±0.86a2b3c2d3 | 54.86±3.43 | 56.21±3.45 | 2.5±0.88a3b3c3 |
Notes: Data are expressed as percentage and mean ± SEM (n=7).
As compared to NC;
as compared to BR100 mg/kg;
as compared to BR200 mg/kg; and
as compared to BR400 mg/kg;
P<0.01;
P<0.001. D0, before treatment; D4, after completing treatment.
Abbreviations: BR, Balanites rotundifolia; BWt, body weight; CQ, chloroquine; NC, negative control; PCVB, packed cell volume at day 0; PCVA, packed cell volume at day 4; SEM, standard error of mean.
Evaluation of RT revealed that 80% methanolic crude extract of B. rotundifolia caused significant attenuation of reduction in RT of P. berghei-infected mice. Especially, the highest dose has demonstrated comparable effect to the standard drug (P<0.05 for B400, P<0.05 for CQ25). Similarly, the BR100 and BR200 doses prevent the decrease in RT, although the effect was not statistically significant as presented in Figure 1.
Figure 1.
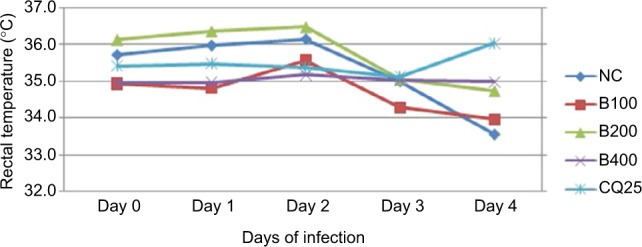
Effect of hydroalcoholic leaf extract of Balanites rotundifolia on rectal temperature of Plasmodium berghei-infected mice in 4-day suppressive test (n=7).
Abbreviations: BR, Balanites rotundifolia; CQ, chloroquine; NC, negative control.
Curative/Rane’s test
The crude extract, in a dose-dependent manner, significantly reduced parasitemia by 38%, 45%, and 69% for BR100, BR200, and BR400, respectively, as compared to the NC (P<0.001 in all treated groups). The inhibition by CQ25 was significantly higher than the inhibition by all crude extract doses and the NC (P<0.001). Unlike the 4-day suppressive test, CQ25 demonstrated significantly (P<0.001) better effect than BR400 in the curative test (Table 3).
Table 3.
Effect of Balanites rotundifolia on percentage parasitemia, and survival time of Plasmodium berghei-infected mice in Rane’s test
| Group | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | % inhibition | Survival days |
|---|---|---|---|---|---|---|---|
| NC | 20.33±1.43 | 23.33±1.54 | 28.50±1.54 | 33.33±1.83 | 34.16±1.17 | – | 7.67±0.33 |
| BR100 | 20.16±0.97 | 22.33±0.71 | 25.50±1.15 | 24.67±0.88 | 21.17±0.79 | 37.73a3c2a3 | 8.16±0.17a3 |
| BR200 | 21.17±1.30 | 21.33±0.49 | 21.50±0.34 | 19.83±0.75 | 18.67±0.72 | 45.35a3 | 8.67±0.42a3 |
| BR400 | 21.67±1.31 | 18.67±0.80 | 15.83±0.87 | 12.67±0.88 | 11.67±0.76 | 65.84a3b2a1 | 14.00±0.58a3b3c3 |
| CQ25 | 22.00±2.81 | 17.67±1.52 | 11.50±2.55 | 5.17±1.66 | 2.17±0.98 | 93.65a3b3c3d3 | 20.00±0.0a3b3c3d3 |
Notes: Data are expressed as percentage and mean ± SEM (n=7).
As compared to negative control;
as compared to BR100 mg/kg;
as compared to BR200 mg/kg;
as compared to BR400 mg/kg;
P<0.05;
P<0.01;
P<0.001.
Abbreviations: BR, Balanites rotundifolia; CQ, chloroquine; NC, negative control; SEM, standard error of mean.
Survival days of P. berghei-infected mice were slightly higher in BR100 and BR200 as compared to NC but not statistically significant. The BR400 dose, however, significantly increased the survival days when compared to NC, BR100, and BR200 (P<0.001 for all groups). CQ25 also significantly increased the survival days when compared to the remaining groups (P<0.001 for all groups) (Table 3).
For curative test, in which the parasitemia is allowed to establish, BR400 demonstrated comparable effect with CQ25 in preventing the decrease in RT in reference to NC, BR100, and BR200 (P<0.001 for all cases). However, the two lower doses failed to prevent a fall in RT posttreatment compared to pretreatment. In terms of BWt, the BR200 and BR400 prevented the decrease in BWt associated with parasitemia development as compared to NC and BR100, though statistically not significant. Especially, the BR400 increased the BWt greater than the CQ25 dose, but it was not statistically significant (Table 4).
Table 4.
Effect of Balanites rotundifolia on temperature and body weight measurements of Plasmodium berghei-infected mice in Rane’s test
| Group | Temperature
|
Body weight
|
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 4 | % change RT | BWt D4 | BWt D7 | % change BWt | |
| NC | 35.65±0.38 | 34.48±0.30 | −3.28±0.23 | 31.18±1.07 | 30.95±0.86 | −0.74±3.73 |
| BR100 | 35.38±0.36 | 34.08±0.20 | −3.67±1.05 | 33.51±0.35 | 31.55±0.86 | −5.85±2.30 |
| BR200 | 34.57±0.16 | 34.50±0.12 | −0.20±0.47 | 32.01±1.62 | 32.33±1.87 | 0.10±1.21 |
| BR400 | 34.30±0.46 | 34.95±0.32 | 1.90±0.86a3b3 | 31.82±1.31 | 32.86±1.23 | 3.27±1.98 |
| CQ25 | 35.25±0.18 | 35.65±0.22 | 1.13±0.62a2b2 | 37.91±1.01 | 38.63±1.34 | 1.90±3.37 |
Notes: Data are expressed as percentage and mean ± SEM (n=7).
As compared to negative control;
as compared to BR100 mg/kg;
P<0.01;
P<0.001.
Abbreviations: BR, Balanites rotundifolia; BWt, body weight; NC, negative control; RT, rectal temperature; SEM, standard error of mean.
Discussion
The present study explored the phytochemical contents, the acute toxicity, as well as antiplasmodial activity of B. rotundifolia against P. berghei in mice. A hydroalcoholic (80% methanol) solvent was used for extraction, as such solvent is good for extracting a wide variety of polar and moderately polar compounds.34 In vivo model was selected, since it can allow possible prodrug effect of the crude extract to be observed. Rodent models particularly have been validated through the identification of several conventional antimalarials such as chloroquine and artemisinin derivatives.35 The 4-day suppressive test is a standard test commonly used for antimalarial activity screening, and the determination of percentage inhibition of parasitemia is the most reliable parameter. It has become popular during scientific evaluation of potential phytomedicines for early investigations of the in vivo efficacy of an antimalarial activity.35
The results from this investigation show that the methanolic extracts of B. rotundifolia leaf contain antiplasmodium activity substances with both preventive and curative effects. This study also indicates that the parasite clearance ability of the extracts is concentration dependent. This was evidenced by the comparable effect with the 4-day suppressive test (BR400–67%) and curative test (BR400–66%). The extract did not show variation in the antiplasmodium activity at different levels of parasitic infection in both suppressive and curative tests. Another plant from the same genus, B. aegyptiaca, is also reported to have in vitro and in vivo antimalaria activities against other plasmodium species Plasmodium aminopeptidase that strengthens the findings of the present study.35
This study validated a promising antimalarial plant, B. rotundifolia, which reduced parasitemia by greater than 50% at 400 mg/kg. There are other plants with comparable efficacy that include extracts of Gnidia stenophylla, Vernonia bigontini, Croton myricodies, Euclea schimperi, Cissampelos mucronata, and Clerodendrum myricoides, of which three of the extracts reduced parasitemia by greater than 50% at an oral dose of 400 mg/kg/d.9,17
An in vivo antiplasmodial activity can be classified as moderate, good, and very good, if an extract displayed percentage parasite suppression equal to or greater than 50% at a dose of 500, 250, and 100 mg/kg BWt per day, respectively.36 Thus, the crude hydroalcoholic extract of B. rotundifolia exhibited a moderate in vivo antiplasmodial activity. In both 4-day suppressive and curative methods, the determination of the percentage inhibition of parasitemia is the most reliable parameter, and a mean group parasitemia level that is ≤90% of that the level in vehicle-treated control animals usually indicates that the test compound is active in standard screening studies.37 Accordingly, B. rotundifolia is considered as active. CQ25 reduced the parasitemia to undetectable level, and the finding is in agreement with other studies.17,38
Anemia, BWt loss, and body temperature reduction are the general features of malaria-infected mice,36 and hematological abnormalities too are also considered a hallmark of malaria.39 P. berghei increases erythrocyte fragility and significantly reduces PCV in mice.40
The PCV was measured to evaluate the effectiveness of the crude extract and fractions in preventing hemolysis due to a rising parasitemia level. The underlying causes of anemia in humans and mice include the clearance and/or destruction of infected RBCs, the clearance of uninfected RBC, erythropoietic suppression, and dyserythropoiesis. In untreated mice, the parasite count increased, and the hematocrit PCV decreased markedly from day to day until the death of the animal, which was also observed in previous studies.37,41
Although lesser percentage reduction in PCV and RT was observed in lower doses, the present study showed that higher dose crude plant extracts significantly prevented the decrease in PCV and RT.
In this study, remarkable suppression of parasitemia by plant extracts translated into a longer mouse survival. The longest mean survival time of the mice both in suppressive and curative tests was strongly associated with the maximum parasitemia inhibition, and this was in agreement with other in vivo antimalarial tests.42
In the 4-day suppressive test, only chloroquine treatment significantly increased BWt when compared to the other treatments. But, in the curative test, the BR200, BR400, and CQ25 treatments increased BWt of P. berghei-infected mice though statistically insignificant.
The decrease in BWt in 4-day suppressive test might be due to the possible depressing effect of the crude extract on feed intake in agreement with that of previous studies on other medicinal plant.41,43 The increase in BWt by higher doses of crude extract observed in Rane’s test might be due to the fact that the higher level of parasitemia significantly lowered the initial BWt, so the significant change was due to regain of initial weight by curative effects of higher doses of the extract.
The crude extract-treated mice in case of 4-day suppressive test and curative test had lower parasite count and survived better than those treated with respective NCs. The higher dose especially has comparative effects with the positive control, CQ25. This could largely be due to the decrease in hematocrit values with infection, the decrease in parasitemia level, or the prevention of cerebral malaria caused by the rising parasitemia in mice. In other words, NC groups suffered anemia or cerebral malaria because of RBC destruction and parasite multiplication. The anemia was evident in our observation, whereby the hematocrit level in all the groups started normally but reduced drastically as the infection progressed, and there was a clear difference between NC and other extract-treated groups.
The present study supports the earlier claims of the antimalarial activity of B. rotundifolia.16,19–21
The effect of the extract was dose dependent, and the plant may possess antiplasmodial activity even after delayed administration of the crude extract. It seems that B. rotundi-folia is one of those potential medicinal plants used in severe and complicated forms of malaria.
The plant contains alkaloids and cardiac glycosides. The former classes of compounds are reported to have antiplasmodial activity.44 Further studies are required to identify the specific phytochemicals responsible for the antiplasmodial effect as well as the mechanisms of action. It can be assumed that the extract can be safe at the dose levels used in the study with oral LD50 above 2,000 mg/kg.
Conclusion
B. rotundifolia possesses antiplasmodial activity, and the results of this study provide a basis for further studies on the plant. This includes the isolation and characterization of the bioactive principles with the ultimate objective of finding novel antimalarial compounds that can be used in the fight against drug-resistant malaria.
Acknowledgments
The financial support of Wollo University is greatly acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.WHO . World Malaria Report. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Kokwaro G. Ongoing challenges in the management of malaria. Malar J. 2009;8(Suppl 1):S2. doi: 10.1186/1475-2875-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federal Democratic Republic of Ethiopia Ministry of Health (FDRE-MOH) Country profile: overview of malaria control activity. Addis Ababa, Ethiopia: FDRE-MOH; 2005. [Google Scholar]
- 4.Federal Democratic Republic of Ethiopia Ministry of Health (FDRE-MOH) Malaria Guidelines. 3rd ed. 2012. [Google Scholar]
- 5.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2(4):209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 6.Talisuna AO, Bloland P, d’Alessandro U. History, dynamics, and public health importance of malaria parasite resistance. Clin Microbiol Rev. 2004;17(1):235–254. doi: 10.1128/CMR.17.1.235-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wongsrichanalai C, Verma JK, Juliano JJ, Kimarling ME, MacArthur JR. Extensive drug resistance in malaria & tuberculosis. Emerg Infect Dis. 2010;16(7):1063–1067. doi: 10.3201/eid1607.091840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . Global Report on Antimalarial Drug Efficacy and Drug Resistance 2000–2010. Geneva: WHO; 2014. [Google Scholar]
- 9.Assefa A, Urga K, Guta M, et al. In vivo antimalarial activities of plants used in Ethiopian traditional medicine, Delomenna, Southeast Ethiopia. Ethiop J Health Sci. 2007;17(2):1–12. [Google Scholar]
- 10.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngutaa JM, Mbariaa JM, Gakuya DW, Gathumbi PK, Kiama SG. Traditional antimalarial phytotherapy remedies used by the South Coast community, Kenya. J Ethnopharmacol. 2010;131:256–267. doi: 10.1016/j.jep.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Kunwar RM, Uprety Y, Burlakoti C, Chowdhary CL, Bussmann RW. Indigenous use and ethnopharmacology of medicinal plants in far-west Nepal. Ethnobotany Res Appl. 2009;7:5–28. [Google Scholar]
- 13.WHO . Traditional Medicine. World Health Organization; Geneva: 2008. (Factsheet No. 134). [Google Scholar]
- 14.WHO Principles of laboratory animal care. World Health Organ Chron. 1985;39:51–56. [Google Scholar]
- 15.Afar national regional state. Regional atlas, samara, Afar. 2009. pp. 65–67. [Google Scholar]
- 16.Seifu T, Asres K, Gebre-Mariam T. Ethnobotanical and ethnopharmaceutical studies on medicinal plants of Chifra District, Afar Region, North Eastern Ethiopia. Ethiopian Pharmaceutical J. 2006;24(1):41–58. [Google Scholar]
- 17.Deressa T, Mekonnen Y, Animut A. In Vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiopian J Health Dev. 24(1):27–32. [Google Scholar]
- 18.Watson L, Dallwitz MJ. The families of flowering plants: descriptions, illustrations, identification, and information retrieval. 1992. Version: 19th October 2013. Available from: http://delta-intkey.com.
- 19.Mesfin A, Giday M, Animut A, Teklehaymanot T. Ethnobotanical study of antimalarial plants in Shinile District, Somali Region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. J Ethnopharmacol. 2012;139:221–227. doi: 10.1016/j.jep.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 20.UNECA, ANRS. Sustainable Agricultural and Environmental Rehabilitation Programme The Woreda Agricultural and rural development integrated service. 1998:1–22. [Google Scholar]
- 21.Meragiaw M. Wild useful plants with emphasis on traditional use of medicinal and edible plants by the people of Aba’ala, North-eastern Ethiopia. J Med Plant Herb Ther Res. 2016;4:1–16. [Google Scholar]
- 22.Giday M, Teklehaymanot T. Ethnobotanical study of plants used in management of livestock health problems by Afar people of Ada’ar District, Afar Regional State, Ethiopia. J Ethnobiol Ethnomed. 2013;9(8) doi: 10.1186/1746-4269-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teklehaymanot and Giday Ethnobotanical study of wild edible plants of Kara and Kwego semi-pastoralist people in Lower Omo River Valley, Debub Omo Zone, SNNPR, Ethiopia. J Ethnobiol Ethnomed. 2010;6:23. doi: 10.1186/1746-4269-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanyingi MO, Mbaria JM, Lanyasunya AL, et al. Ethnopharmacological survey of Samburu district, Kenya. J Ethnobiol Ethnomed. 2008;4(1):14. doi: 10.1186/1746-4269-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusch P, Deininger S, Specht S, et al. In vitro and in vivo antimalarial activity assays of seeds from Balanites aegyptiaca: compounds of the extract show growth inhibition and activity against plasmodial aminopeptidase. J Parasitol Res. 2011:1–9. doi: 10.1155/2011/368692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute for laboratory animal research (ILAR) Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academy Press; 1996. [Google Scholar]
- 27.Trease GE, Evans WC. A Textbook of Pharmacognocy. London, UK: Bailliere Tindall Ltd; 1989. [Google Scholar]
- 28.Jones WP, Kinghorn AD. Extraction of plant secondary metabolites. Methods Mol Biol. 2012;864:341–366. doi: 10.1007/978-1-61779-624-1_13. [DOI] [PubMed] [Google Scholar]
- 29.OECD Guidelines for the Testing of Chemicals: Guideline 425: Acute Oral Toxicity. Paris, France: 2008. [Google Scholar]
- 30.Peters W, Portus JH, Robinson BL. The four-day suppressive in vivo antimalarial test. Ann Trop Med Parasitol. 1975;69:155–171. [Google Scholar]
- 31.Ryley JF, Peters W. The antimalarial activity of some quinoline esters. Ann Trop Med Parasitol. 1995;84:209–222. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 32.Kalra BS, Chawla S, Gupta P, Valecha N. Screening of antimalarial drugs: an overview. Indian J Pharmacol. 2006;38:5–12. [Google Scholar]
- 33.Gilmour D, Sykes AJ. Westergren and Wintrobe methods of estimating ESR compared. Br Med J. 1951;2(4746):1496–1497. doi: 10.1136/bmj.2.4746.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsuka H. Purification by solvent extraction using partition coefficient. In: Sarker D, Latif Z, Gray A, editors. Methods in Biotechnology Natural Products Isolation. Totowa, NJ: Human Press; 2006. pp. 269–273. [Google Scholar]
- 35.Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery efficacy models for compound screening. Nat Rev Drug Discov. 2004;3:512–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 36.Adugna M, Feyera T, Taddese W, Admasu P. In vivo antimalarial activity of crude extract of aerial part of Artemisia abyssinica against Plasmodium berghei in Mice. Global J Pharmacol. 2014;8(3):460–468. [Google Scholar]
- 37.Okokon JE, Ofodum KC, Ajibesin KK, Danladi B, Gamanil KS. Pharmacological screening and evaluation of antiplasmodial activity of Croton zambesicus against Plasmodium berghei in mice. Indian J Pharmacol. 2005;37:243–246. [Google Scholar]
- 38.Abdela J, Shibeshi W. In vivo antimalarial activity of solvent fractions of the leaves of Justicia schimperiana Hochst. Ex Nees against Plasmodium berghei in mice. Ethiopian Pharmaceutical J. 2014;30(2):95–108. [Google Scholar]
- 39.Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Malaria anemia of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 40.Iyawe HOT, Onigbinde AO. Impact of Plasmodium berghei and chloroquine on Haema antioxidant indices in mice. Asian J Biotechnol. 2009;4:30–35. [Google Scholar]
- 41.Ayodele T. Studies on Azadricha indica in malaria; Presented in: 4th OAU inter African symposium; Abijan, Ivory Cost. 1979. [Google Scholar]
- 42.Eyasu M, Shibeshi W, Giday M. In vivo antimalarial activity of hydromethanolic leaf extract of Calpurnia aurea (Fabaceae) in mice infected with chloroquine sensitive Plasmodium berghei. Int J Pharm Pharmacol. 2013;2(9):131–142. [Google Scholar]
- 43.Mengiste B, Makonnen E, Urga K. In vivo antimalarial activity of Dodonaea angustifolia seed extracts against Plasmodium berghei in mice model. MEJS. 2012;4(1):47–63. [Google Scholar]
- 44.Onguene PA, Ntie-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants. Part I: a pharmacological evaluation of alkaloids and terpenoids. Malar J. 2013;12:449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]


